TO THE EDITOR:
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma (NHL).1,2 Despite advances in chemoimmunotherapy and autologous stem cell transplantation (SCT) in the past decades, outcomes of MCL remain inferior when compared with most other NHL histologies.2,3 Patients with advanced age have particularly poor outcomes with standard chemoimmunotherapy. Due to comorbidities and frailty, many older patients do not tolerate intensive therapies (particularly high-dose cytarabine and SCT),4 which typically offer the longest progression-free interval.5 Further, before the emergence of targeted therapy (2013), treatment options had been relatively limited for relapsed/refractory (r/r) MCL patients, especially in the older population, a subgroup more likely to experience early relapses following less intensive frontline management.6,7 Early relapse itself has also been shown to correlate with poorer long-term outcomes in MCL.8
Ibrutinib was the first Bruton tyrosine kinase inhibitor (BTKi) evaluated for r/r MCL and led to an overall response rate of 67% and a median duration of response of 17.5 months in a heavily pretreated study population.9 This led to the approval of ibrutinib for ≥second line use for MCL in 2013,9 followed by the approval of acalabrutinib10 and zanubrutinib11 for similar indications in 2017 and 2019, respectively. The approval of BTKis provided well-tolerated and efficacious options for older and frail patients with r/r MCL. We hypothesized that outcomes in MCL have improved in the BTKi era, with early postapproval survival benefit greatest in older patients who are less likely to be candidates for aggressive frontline treatment.
To evaluate this hypothesis, we used the SEER (Surveillance, Epidemiology, and End Results) database, which covers ∼50% of the population in the United States.12 We included 7625 adult patients (age ≥18 years) diagnosed with MCL between the years 2007 and 2018 (excluding 2012-2013) (Appendix). Most patients were men (71%) and White (90%), 49% were diagnosed at the age ≥70 years, and 69% were diagnosed at an advanced stage. We defined the pre-BTKi era as the years of diagnosis between 2007 and 2011 and the BTKi era as the years between 2014 and 2018. We considered the years 2012 and 2013 as a “washout” period to allow practice change related to BTKi approval. Among all patients included, 3424 and 4201 were diagnosed during pre-BTKi and BTKi eras, respectively. Besides lower likelihood of advanced stage at diagnosis (64% vs 76%; P < .001), patients diagnosed in the BTKi era had similar baseline characteristics to those in the pre-BTKi period (Appendix).
Our study examined all-cause mortality and mortality from MCL (MFM) and followed the patients from the date of MCL diagnosis to the end of 2018 or death, whichever occurred first. We applied a multivariable Cox proportional hazards regression model for all-cause mortality and reported an adjusted hazard ratio (HR) with 95% confidence interval (CI). Multivariable competing risk analyses were used for MFM, considering all other causes of death as competing events. We adjusted for age, sex, race, stage, and median household income at the census level in all models. As age plays an important role in treatment decisions, including whether to use consolidative transplantation, we performed subgroup analyses based on age at diagnosis (<60, 60-69, 70-79, and ≥80 years). To reduce potential confounding by the duration of follow-up among patients diagnosed in different periods, we used up to 3-year follow-up data for the primary analysis. In sensitivity analyses, we included patients diagnosed between 2008 and 2011 and 2014 and 2017 for pre-BTKi and BTKi eras, respectively, so that the median follow-up for the BTKi cohort reaches 3 years. We also changed the defined duration of follow-up to 2 and 4 years, respectively.
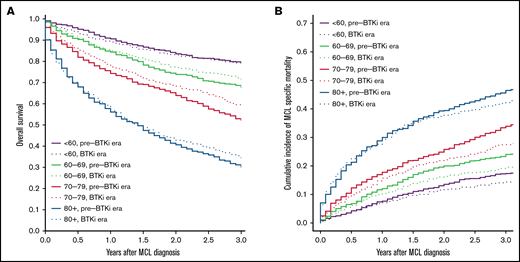
The median follow-up was 9.2 and 2.4 years for patients diagnosed during pre-BTKi and BTKi eras, respectively. The 3-year all-cause mortality and 3-year MFM were 39.8% and 27.3% in the overall population. Both the 3-year all-cause mortality and MFM increased with age at diagnosis. The 3-year all-cause mortality was lower in the BTKi era among most age groups, except patients <60 years old, and the 3-year MFM was lower in the BTKi era among all age groups. The numeric difference of 3-year outcomes was most substantial in patients aged 70 to 79 for both all-cause mortality (pre-BTKi era: 47.8%; BTKi era: 40.4%) and MFM (pre-BTKi era: 33.9%; BTKi era: 27.5%) (Table 1; Figure 1A-B). In the multivariable analyses, the risk of death was significantly lower during the BTKi era in the 60 to 69 (HR, 0.85; 95% CI, 0.72-1.00) and 70 to 79 (HR, 0.80; 95% CI, 0.70-0.92) age groups. MFM was also significantly lower during the BTKi era in these 2 age groups (60-69: sub-HR, 0.78; 95% CI, 0.64-0.94; 70-79: sub-HR, 0.76; 95% CI, 0.65-0.90) (Table 1). The results were largely unchanged (not shown) for sensitivity analyses involving only patients diagnosed between 2008 and 2011 (pre-BTKi) and 2014 and 2017 (BTKi) and using 2 and 4 years as the cutoff for follow-up, respectively.
Three-year all-cause mortality and mortality from MCL based on age and year of diagnosis
| . | 3-y all-cause mortality . | 3-y mortality from MCL . | ||
|---|---|---|---|---|
| % . | Adjusted HR (95% CI) . | % . | Adjusted sub-HR (95% CI) . | |
| Overall | 39.8 | 27.3 | ||
| <60, pre-BTKi era | 20.5 | 1.00 | 17.3 | 1.00 |
| <60, BTKi era | 21.0 | 1.04 (0.82-1.31) | 14.3 | 0.80 (0.62-1.04) |
| 60-69, pre-BTKi era | 32.3 | 1.00 | 24.0 | 1.00 |
| 60-69, BTKi era | 28.4 | 0.85 (0.72-1.00) | 19.5 | 0.78 (0.64-0.94) |
| 70-79, pre-BTKi era | 47.8 | 1.00 | 33.9 | 1.00 |
| 70-79, BTKi era | 40.4 | 0.80 (0.70-0.92) | 27.5 | 0.76 (0.65-0.90) |
| ≥80, pre-BTKi era | 69.9 | 1.00 | 46.5 | 1.00 |
| ≥80, BTKi era | 65.8 | 0.91 (0.80-1.04) | 42.2 | 0.87 (0.75-1.02) |
| . | 3-y all-cause mortality . | 3-y mortality from MCL . | ||
|---|---|---|---|---|
| % . | Adjusted HR (95% CI) . | % . | Adjusted sub-HR (95% CI) . | |
| Overall | 39.8 | 27.3 | ||
| <60, pre-BTKi era | 20.5 | 1.00 | 17.3 | 1.00 |
| <60, BTKi era | 21.0 | 1.04 (0.82-1.31) | 14.3 | 0.80 (0.62-1.04) |
| 60-69, pre-BTKi era | 32.3 | 1.00 | 24.0 | 1.00 |
| 60-69, BTKi era | 28.4 | 0.85 (0.72-1.00) | 19.5 | 0.78 (0.64-0.94) |
| 70-79, pre-BTKi era | 47.8 | 1.00 | 33.9 | 1.00 |
| 70-79, BTKi era | 40.4 | 0.80 (0.70-0.92) | 27.5 | 0.76 (0.65-0.90) |
| ≥80, pre-BTKi era | 69.9 | 1.00 | 46.5 | 1.00 |
| ≥80, BTKi era | 65.8 | 0.91 (0.80-1.04) | 42.2 | 0.87 (0.75-1.02) |
Pre-BTKi era is defined as the year of diagnosis between 2007 and 2011; BTKi era is defined as the year of diagnosis between 2014 and 2018.
All the above analysis was adjusted for age, sex, race, stage, and median household income at the census level.
HRs or sub-HRs that are statistically significant (P < .05) are in bold.
Overall survival and cumulative incidence of lymphoma-specific death. (A) Overall survival based on age and year of diagnosis. (B) Cumulative incidence of death from MCL based on age and year of diagnosis. Pre-BTKi era is defined as the year of diagnosis between 2007 and 2011, BTKi era is defined as the year of diagnosis between 2014 and 2018.
Overall survival and cumulative incidence of lymphoma-specific death. (A) Overall survival based on age and year of diagnosis. (B) Cumulative incidence of death from MCL based on age and year of diagnosis. Pre-BTKi era is defined as the year of diagnosis between 2007 and 2011, BTKi era is defined as the year of diagnosis between 2014 and 2018.
Overall and lymphoma-specific survival in individuals diagnosed with MCL has improved in the BTKi era, as evidenced by this large, population-based cohort study. At a median follow-up of 2.4 years in our BTKi cohort, significant survival benefits were observed in those ⩾60 but <80 years of age, and the observed benefits were greatest in the 70 to 79 age group. This difference may be explained by the heterogeneous frontline treatment approaches based on age. Many patients <60 receive aggressive chemoimmunotherapy followed by consolidative SCT in the frontline setting.7 This approach provides a median progression-free survival of 8.5 years.5 With a duration of follow-up of 2.4 years, most younger patients likely have not relapsed and have not started deriving benefit from BTKis. In contrast, many patients in the age groups of 60 to 79 often receive less intensive regimens, and relapse tends to occur much sooner after less intensive frontline treatment. Before BTKis became available, treatment options were limited for r/r MCL, and outcomes were generally dismal after the first relapse.13,14 Among those ⩾80, minimal improvement in survival may be attributed to significant competing risks of death from other comorbidities and limited life expectancy.
There are several limitations in our retrospective analysis. We were unable to adjust potential confounders not included in the SEER database (eg, biological features of MCL). Evidence on the efficacy of several other therapies for MCL emerged around the time of ibrutinib approval, including the use of bendamustine as frontline therapy,15,16 lenalidomide,17,18 proteasome inhibitors,19 and maintenance rituximab.20 Therefore, the improvement in survival observed in our study may not be solely attributable to BTKis. Our analysis used the SEER database, which does not contain details of lymphoma treatment and limited our ability to estimate the benefit specific to BTKis. In addition, the duration of follow-up in our BTKi cohort remains relatively short (2.4 years). The survival benefit in the current analysis unlikely reflects the improvement in outcomes among younger and fit patients following standard, intensive frontline therapies. This needs to be reexamined as longer follow-up data become available.
In this large, population-based study, we observed significant improvement in overall and lymphoma-specific survival in MCL patients 60 to 79 years old in the immediate period following BTKi approval, with greater benefit shown in the 70 to 79 age group. Future real-world studies should examine the impact of novel agents on treatment patterns and outcomes of MCL over a longer follow-up period.
Contribution: M.D. and S.F.H. contributed to the conception and design of the study; M.D. and C.C. provided data accrual and analyses; M.D. and S.F.H. wrote the manuscript; and M.D., S.F.H., C.C., S.K.K., A.M.Z., N.A.P., N.N., R.M.S., R.W., and X.M. contributed to data interpretation, editing and reviewing the manuscript.
Conflict-of-interest disclosure: A.M.Z. reports consultancy and research funding from AbbView, Acceleron, Amgen, Aprea, Boehringer Ingelheim, Cardiff Oncology, BMS, Incyte, and Novartis; research funding from ADC Therapeutics, Astex, and Pfizer; consultancy from Agios, Astellas, BeyondSpring, Daiichi Sankyo, Epizyme, Genentech, Gilead, Kura, Ionis, Loxo Oncology, Janssen, AstraZeneca, Jasper, and Jazz; serving on clinical trial committees at AbbVie, BioCryst, BMS, Geron, Gilead, Kura, Loxo Oncology, and Novartis; travel support from Cardiff Oncology, Novartis, and Pfizer. N.A.P. reports honoraria from Pfizer, Blueprint Medicines, Incyte, Novartis, Celgene, Bristol-Myers Squib, CTI BioPharma, PharmaEssentia, AbbVie, and Constellation Pharmaceuticals; serving on independent data monitoring committee at Cogent biosciences. N.N. reports research funding from Janssen and GlaxoSmithKline; membership on an entity’s board of directors or advisory committees at Eidos Therapeutics. R.M.S. reports divested equity in a private or publicly-traded company in the past 24 months at Curis. SKK reports consultancy and honoraria from Incyte and Karyopharm. X.M. reports research funding from Celgene/Bristol Myers Squibb and consultancy with Bristol Myers Squibb. S.F.H. reports consultancy from Janssen, Pharmacyclics, AbbVie, AstraZeneca, Flatiron Health Inc., Novartis, SeaGen, Genetech, Merck, TG Therapeutics, ADC Therapeutics, Epizyme, Servier, and Thyme Inc.; research funding from Celgene, DTRM Biopharm, and TG Therapeutics; honoraria form Pharmacyclics and AstraZeneca, Bayer. The remaining authors declare no competing financial interests.
Correspondence: Scott F. Huntington, Section of Hematology, Department of Internal Medicine, Yale University School of Medicine, 37 College St, Rm 124, New Haven, CT 06520; e-mail: scott.huntington@yale.edu.
References
Author notes
The study was performed using the SEER database, which is publicly available upon request to NCI.
This work was presented at the 63rd American Society of Hematology Annual Meeting & Exposition, Atlanta, GA, 10-14 December 2021.
The full-text version of this article contains a data supplement.