Key Points
A clinical care pathway using centralized PF4-ELISA testing detected a 37% prevalence of thrombosis among patients investigated for VITT.
VITT should be confirmed by PF4-ELISA as thrombosis with thrombocytopenia can occur without PF4 antibodies after SARS-CoV-2 vaccination.
Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare complication after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adenoviral vector vaccination. In British Columbia (BC), Canada, a provincial clinical care pathway was developed to guide clinicians in evaluating for VITT among patients who present with thrombocytopenia or thrombosis symptoms within 4 to 28 days after adenoviral vector vaccine exposure. All patients had enzyme-linked immunosorbent assay (ELISA) testing for platelet factor 4 (PF4) antibodies, and all cases with positive PF4-ELISA or d-dimer levels ≥2.0 mg/L fibrinogen equivalent units (FEU) had further testing for platelet-activating PF4 antibodies using a modified serotonin release assay (SRA). Between 1 May and 30 June 2021, 37% of 68 patients investigated for VITT had thrombosis, but only 3 had VITT confirmed by PF4-ELISA and SRA. Platelet counts, d-dimer levels, and ELISA optical density values were significantly different between those with and without VITT. Three patients had thrombocytopenia and thrombosis with d-dimer levels >4.0 mg/L FEU but had negative PF4-ELISA and SRA results. Patients with VITT were treated successfully with IV immunoglobulin, nonheparin anticoagulants, and corticosteroids. Our pathway demonstrated that thrombosis is common among patients investigated for VITT and that PF4-ELISA testing is necessary to confirm VITT in those presenting with thrombosis and thrombocytopenia.
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT) can occur after adenoviral vector vaccination to prevent COVID-19.1-5 Diagnostic definitions of VITT have evolved over time but have included the presence of thrombocytopenia (platelets <150 000/mm3), thrombosis, markedly elevated d-dimer level, and platelet factor 4 (PF4) antibodies.6-8 Prompt diagnosis is critical to avoid catastrophic outcomes.6,9,10
In British Columbia (BC), a Canadian province of 5.1 million residents distributed over 944 735 km2, a multidisciplinary committee developed a clinical care pathway (CCP) to standardize the evaluation of suspected VITT. We describe the pathway, features of patients with thrombosis and thrombocytopenia, and the laboratory features of patients investigated for suspected VITT between 1 May and 30 June 2021.
Study design
Clinical care pathway
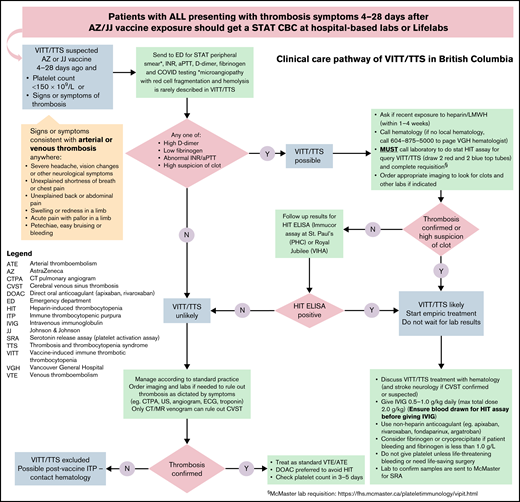
In April 2021, a provincial CCP was developed to facilitate the diagnosis and treatment of VITT (Figure 1).11 Centralized hematology consultation and follow-up and urgent transportation of samples to 1 of 2 academic sites that perform PF4-enzyme–linked immunosorbent assay (ELISA) were organized because of their restricted availability. The workflow was streamlined based on input from hematology, hematopathology, neurology, internal medicine, infectious disease, primary care, emergency medicine, critical care, transfusion medicine, pharmacy, and laboratory services. The pathway was approved and disseminated to all health care providers by the provincial COVID-19 Therapeutics Committee and its networks on 30 April 2021.12 Weekly e-mail updates and educational rounds augmented local implementation and communication.
Clinical care pathway for investigating suspected vaccine-induced immune thrombotic thrombocytopenia (VITT). All patients presenting to a health care setting with symptoms suspicious of thrombosis within 4 to 28 days after ChAdOx1 nCoV-19 vaccination were sent to the laboratory for a stat complete blood count (CBC). The patient was directed to the emergency department if VITT was suspected because of thrombocytopenia (platelets <150 000/mm3) or if thrombosis symptoms warranted further investigations. Stat CBC, blood film, INR, aPTT, d-dimer, fibrinogen, and COVID-19 testing were done. Patients who had normal results were managed according to standard practice. If any of these results were abnormal or if the emergency physician had a high suspicion of thrombosis, VITT was considered possible. This prompted hematology consultation and collection of blood samples for PF4-ELISA testing and imaging for thrombosis. Starting empiric treatment with IVIG and nonheparin anticoagulant was discussed with hematology and stroke neurology if cerebral venous thrombosis was confirmed or suspected. Samples were sent to the McMaster Platelet Immunology Laboratory in Hamilton, ON, Canada, for confirmation of platelet-activating PF4 antibodies. aPTT, activated partial thromboplastin time; ELISA, enzyme-linked immunosorbent assay; INR, international normalized ratio; PF4, platelet factor 4.
Clinical care pathway for investigating suspected vaccine-induced immune thrombotic thrombocytopenia (VITT). All patients presenting to a health care setting with symptoms suspicious of thrombosis within 4 to 28 days after ChAdOx1 nCoV-19 vaccination were sent to the laboratory for a stat complete blood count (CBC). The patient was directed to the emergency department if VITT was suspected because of thrombocytopenia (platelets <150 000/mm3) or if thrombosis symptoms warranted further investigations. Stat CBC, blood film, INR, aPTT, d-dimer, fibrinogen, and COVID-19 testing were done. Patients who had normal results were managed according to standard practice. If any of these results were abnormal or if the emergency physician had a high suspicion of thrombosis, VITT was considered possible. This prompted hematology consultation and collection of blood samples for PF4-ELISA testing and imaging for thrombosis. Starting empiric treatment with IVIG and nonheparin anticoagulant was discussed with hematology and stroke neurology if cerebral venous thrombosis was confirmed or suspected. Samples were sent to the McMaster Platelet Immunology Laboratory in Hamilton, ON, Canada, for confirmation of platelet-activating PF4 antibodies. aPTT, activated partial thromboplastin time; ELISA, enzyme-linked immunosorbent assay; INR, international normalized ratio; PF4, platelet factor 4.
All patients presenting to any health care setting with thrombosis symptoms (eg, severe headache, dyspnea) or unexplained thrombocytopenia within 4 to 28 days after ChAdOx1 nCoV-19 vaccination were investigated according to the CCP (Figure 1). Patients were sent to the closest emergency department where a stat complete blood count, blood film, international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, and d-dimer tests were done, and imaging for thrombosis was performed as appropriate for symptoms.11 If any result was abnormal, PF4-IgG ELISA (Immucor) was performed within 2 business days if the clinical suspicion for VITT was high; other samples were tested in twice-weekly batches. As the diagnostic accuracy of PF4-ELISA was uncertain and early cases of VITT might present with thrombocytopenia without thrombosis,13-15 all cases with positive PF4-ELISA (optical density [OD] ≥0.40) or d-dimer level ≥2.0 mg/L fibrinogen equivalent units (FEU) were further investigated using a modified functional platelet-activating assay (serotonin release assay [SRA] with added PF4) performed at the McMaster Platelet Immunology laboratory in Hamilton, ON, Canada.16 Empiric treatment with nonheparin anticoagulant and IV immunoglobulin (IVIG) were given prior to PF4-ELISA confirmation if VITT was considered highly likely by the treating physician. All patients had reverse transcriptase-polymerase chain reaction assay of a nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The study was approved by the institutional review board at the University of British Columbia and was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical methods
We used the Kruskal-Wallis test to compare platelet counts, d-dimer levels, and PF4-ELISA ODs at presentation among patients with VITT vs those without VITT with thrombosis, and vs those without VITT and without thrombosis.
Results and discussion
Between 15 March and 30 June 2021, 278 869 first doses and 102 337 second doses of the ChAdOx1 nCoV-19 vaccine were given to British Columbia residents ≥18 years old. As of 30 June 2021, 68 patients were investigated for suspected VITT according to the CCP; 59 and 6 of them had received a first or second dose of ChAdOx1 nCoV-19 vaccine, respectively, 1 patient had received mRNA-1273 for the second dose, and 2 patients had the first dose of BNT162b2 vaccine. The median age was 51 years (range, 30-69), and 53% were female. All 68 patients tested negative for SARS-CoV-2, and none had heparin exposure within the prior 3 months.
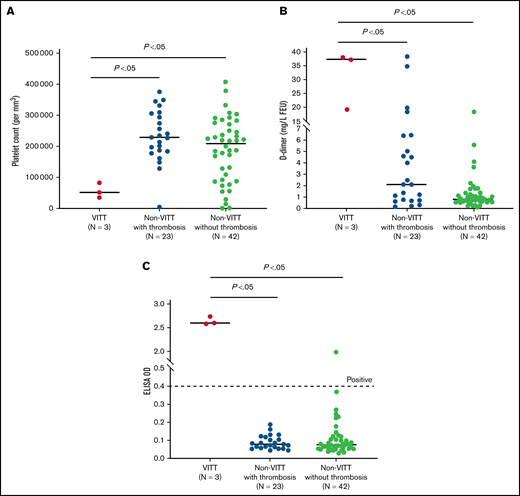
Three patients were diagnosed with VITT at 12, 14, and 17 days following the first dose of the ChAdOx1 nCoV-19 vaccine (Table 1). Their platelet counts, d-dimer levels, and PF4-ELISA OD values were significantly different from those without VITT (Figure 2); there were no differences in INR, aPTT, and fibrinogen values (data not shown). One patient, after the first dose of the ChAdOx1 nCoV-19 vaccine, had thrombocytopenia, mildly elevated d-dimer level, and positive PF4-ELISA and SRA results, but imaging was negative for thrombosis.
Patient characteristics with thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccination
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Age (yrs) | 47 | 37 | 53 | 38 | 56 | 61 | 64 |
| Sex | Female | Male | Male | Male | Male | Male | Male |
| Vaccine type | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 2) |
| Onset of symptoms after vaccination (d) | 5 | 10 | 6 | No symptoms* | 6 | 33 | 9 |
| Initial presentation after vaccination (d) | 11 | 17 | 12 | 19 | 20 | 36 | 13 |
| Laboratory parameters at presentation | |||||||
| Platelets (per mm3) | 35 000 | 83 000 | 52 000 | 88 000 | 149 000 | 129 000 | 93 000 |
| d-dimer (mg/L FEU) | 37.19 | 39.00 | 19.38 | 1.105 | 34.80 | 18.39 | 4.12 |
| PF4-ELISA (OD) | Positive (2.58) | Positive (2.56) | Positive (2.75) | Positive (1.98) | Negative (0.08) | Negative (0.12) | Negative (0.07) |
| SRA | Positive | Positive | Positive | Positive | Negative | Negative | Negative |
| Thrombotic complications | PE; DVT | SMV thrombus; PVT; SpVT; PE | PVT | None† | PE; DVT | PE; DVT; SMA thrombus | Lacunar infract |
| Treatments received‡ | IVIG; prednisone; argatroban; rivaroxaban | IVIG; argatroban; fondaparinux; small bowel resection | IVIG; dexamethason; apixaban | Rivaroxaban | Catheter-directed thrombolysis of PE; unfractionated heparin; argatroban; apixaban | IVIG; SMA thrombo-endarterectom; argatroban; unfractionated heparin; warfarin | IVIG; apixaban |
| Outcomes | Platelets normal d 29; SRA negative at d 174; PF4-ELISA positive at d 239 (OD 1.06) | Platelets normal d 202; SRA positive at d 202; PF4-ELISA positive at d 202 (OD 2.06) | Platelets normal d 16; PF4-ELISA positive d 96 (OD 1.38) | Platelets normal d 69; SRA negative d 182; PF4-ELISA positive d 182 (OD 0.65) | Platelets normal d 24 | Platelets normal d 41 | Platelets normal d 37 |
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Age (yrs) | 47 | 37 | 53 | 38 | 56 | 61 | 64 |
| Sex | Female | Male | Male | Male | Male | Male | Male |
| Vaccine type | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 1) | ChAdOx1 nCoV-19 (dose 2) |
| Onset of symptoms after vaccination (d) | 5 | 10 | 6 | No symptoms* | 6 | 33 | 9 |
| Initial presentation after vaccination (d) | 11 | 17 | 12 | 19 | 20 | 36 | 13 |
| Laboratory parameters at presentation | |||||||
| Platelets (per mm3) | 35 000 | 83 000 | 52 000 | 88 000 | 149 000 | 129 000 | 93 000 |
| d-dimer (mg/L FEU) | 37.19 | 39.00 | 19.38 | 1.105 | 34.80 | 18.39 | 4.12 |
| PF4-ELISA (OD) | Positive (2.58) | Positive (2.56) | Positive (2.75) | Positive (1.98) | Negative (0.08) | Negative (0.12) | Negative (0.07) |
| SRA | Positive | Positive | Positive | Positive | Negative | Negative | Negative |
| Thrombotic complications | PE; DVT | SMV thrombus; PVT; SpVT; PE | PVT | None† | PE; DVT | PE; DVT; SMA thrombus | Lacunar infract |
| Treatments received‡ | IVIG; prednisone; argatroban; rivaroxaban | IVIG; argatroban; fondaparinux; small bowel resection | IVIG; dexamethason; apixaban | Rivaroxaban | Catheter-directed thrombolysis of PE; unfractionated heparin; argatroban; apixaban | IVIG; SMA thrombo-endarterectom; argatroban; unfractionated heparin; warfarin | IVIG; apixaban |
| Outcomes | Platelets normal d 29; SRA negative at d 174; PF4-ELISA positive at d 239 (OD 1.06) | Platelets normal d 202; SRA positive at d 202; PF4-ELISA positive at d 202 (OD 2.06) | Platelets normal d 16; PF4-ELISA positive d 96 (OD 1.38) | Platelets normal d 69; SRA negative d 182; PF4-ELISA positive d 182 (OD 0.65) | Platelets normal d 24 | Platelets normal d 41 | Platelets normal d 37 |
Low platelets noted on routine bloodwork, no clinical symptoms present.
No thrombotic complications noted despite extensive imagine (CT head with venogram, CT chest, Doppler ultrasound both legs).
Treatment received for patients with positive PF4-ELISA. Patient 1: 3 doses of IVIG 1 g/kg and a short course of prednisone (initial dose of 1 g/kg followed by a taper). Initial anticoagulation with argatroban and then transitioned to rivaroxaban at 15 mg twice daily for 3 weeks, followed by 20 mg once daily. Patient 2: 4 doses of IVIG 1 g/kg and prolonged course of prednisone (initial dose 1 g/kg followed by a taper). Patient 2 received a short course of argatroban followed by extended anticoagulation with fondaparinux because of a high output ileostomy. Patient 3: 2 doses of IVIG 1 g/kg and dexamethasone pulse at 40 mg once daily for 4 days. Anticoagulation with apixaban at 10 mg twice daily for 6 weeks while the d-dimer level was still elevated (per physician-patient preference), then stepped down to 5 mg twice daily. Apixaban was discontinued after completing 3.5 months of treatment. Patient 4: 15 mg of rivaroxaban twice daily for 3 weeks, followed by 20 mg daily. A further step down to 10 mg daily at day 155 given persistently positive PF4-ELISA.
CT, computed tomography; DVT, deep vein thrombosis; OD, optical density; PE, pulmonary embolism; PVT, portal vein thrombosis; SMA, superior mesenteric artery; SMV, superior mesenteric vein; SpVT, splenic vein thrombus.
Distribution of platelet count, d-dimer level, and ELISA optical density (OD) for patients with VITT compared with patients without VITT, with or without thrombosis. As shown in (A), there is a significant difference in the median platelet count between the 3 groups. Similarly, median d-dimer levels (B) and median ELISA OD values (C) showed significant differences across the 3 groups. As shown in the figure, 23 patients in the non-VITT group had thrombotic events. Of these 23 patients, 3 had d-dimer levels ≥2.0 mg/L FEU and platelets <150 000/mm3. These 3 patients had negative PF4-ELISA and SRA results. Ten of the 23 patients with thrombosis had d-dimer ≥2.0 mg/L FEU, but their platelet counts were normal. These patients also had negative PF4-ELISA and SRA results. The other 10 patients in the non-VITT with thrombosis group had d-dimer <2.0 mg/L FEU and had normal platelet counts. These patients had negative PF4-ELISA results, but SRA was not performed due to a low likelihood for VITT (according to the National Institute for Health and Care Excellence guideline).6 In (C), 1 patient had a positive PF4-ELISA (OD, 1.98) confirmed by positive SRA (see Table 1).
Distribution of platelet count, d-dimer level, and ELISA optical density (OD) for patients with VITT compared with patients without VITT, with or without thrombosis. As shown in (A), there is a significant difference in the median platelet count between the 3 groups. Similarly, median d-dimer levels (B) and median ELISA OD values (C) showed significant differences across the 3 groups. As shown in the figure, 23 patients in the non-VITT group had thrombotic events. Of these 23 patients, 3 had d-dimer levels ≥2.0 mg/L FEU and platelets <150 000/mm3. These 3 patients had negative PF4-ELISA and SRA results. Ten of the 23 patients with thrombosis had d-dimer ≥2.0 mg/L FEU, but their platelet counts were normal. These patients also had negative PF4-ELISA and SRA results. The other 10 patients in the non-VITT with thrombosis group had d-dimer <2.0 mg/L FEU and had normal platelet counts. These patients had negative PF4-ELISA results, but SRA was not performed due to a low likelihood for VITT (according to the National Institute for Health and Care Excellence guideline).6 In (C), 1 patient had a positive PF4-ELISA (OD, 1.98) confirmed by positive SRA (see Table 1).
The remaining 64 patients had negative PF4-ELISA results; 16 had thrombocytopenia, 23 had confirmed thrombosis, and 3 had both. These 3 patients also had d-dimer levels >4.0 mg/L FEU and negative SRA (Table 1). Two cases had cerebral venous thrombosis, and both had normal platelet counts and d-dimer levels (<0.5 mg/L FEU). Four patients had arterial thrombosis (ischemic stroke [1], acute coronary syndrome [2], and superior mesenteric arterial thrombosis [1]). Eighteen patients had a d-dimer level ≥2.0 mg/L FEU, and SRA results were negative in 17 (SRA was not performed in 1 patient); 13 of these had thrombosis. Five patients had a d-dimer level ≥10.0 mg/L FEU: 4 had extensive clot burden, and 1 patient had sepsis without thrombosis. Twenty-two patients had a d-dimer level between 0.5 and 2.0 mg/L FEU without a clear cause. We also identified 4 patients who had new-onset immune thrombocytopenic purpura (ITP). Among patients without VITT, 6 received empiric IVIG because of critical site thrombosis or probable ITP.
Our report contributes prospective data from a dedicated CCP that systematically performed PF4-ELISA testing in unselected patients investigated for suspected VITT. Given the variable sensitivities and specificities of PF4-ELISAs in VITT diagnosis,14,15 modified SRA was performed in cases with positive PF4-ELISA or d-dimer ≥2.0 mg/L FEU to exclude false-positive and false-negative results. Prior studies did not perform PF4-ELISA testing systematically or use confirmatory SRA and are at risk for case ascertainment bias.1,3,5 Importantly, our series illustrated that not all patients presenting with thrombosis and thrombocytopenia after SARS-CoV-2 vaccination have VITT (even when the d-dimer level is >4 mg/L FEU). In fact, 6 patients presented with thrombosis and thrombocytopenia, but only 3 patients had VITT confirmed by PF4-ELISA and SRA. We also showed that thrombosis is a very common event (37%) among those investigated for VITT. Our findings emphasize the importance of PF4-ELISA testing and thrombosis investigations and support the observations of others that the risk of thrombosis is increased after SARS-CoV-2 vaccination.17,18 Our incidence of VITT at 10.8 per million after the first dose of ChAdOx1 nCoV-19 vaccine is consistent with other reports.5,19,20
Our pathway has limitations. We mandated investigations for suspected VITT if a patient presented with either thrombosis symptoms or thrombocytopenia between 4 and 28 days after vaccination.5,9,13 This time window and prudent approach reflected the initial uncertainties regarding VITT diagnosis and accuracy of PF4-ELISA testing in April 2021, when VITT was newly described. Current guidelines use a 5- to 30-day window and consider VITT unlikely in those with d-dimer <2 mg/L FEU.6 Also, unnecessary imaging and empiric treatment with IVIG could have been avoided if PF4-ELISA testing had been widely and rapidly available. We did not include long-term follow-up of all suspected cases, but there was sufficient short-term follow-up to exclude VITT. We are unable to measure adherence to the algorithm, but according to mandated adverse event reporting, there were no missed cases and no deaths from VITT identified through the BC Centre for Disease Control. The CCP was set up to identify VITT following ChAdOx1 nCoV-19 vaccine exposure but unexpectedly captured suspected cases following mRNA vaccination. Finally, although our small numbers cannot provide diagnostic accuracy assessment of the CCP, we illustrated the importance of a systemic approach and the necessity of PF4-ELISA testing to distinguish VITT from other causes of thrombosis and thrombocytopenia.6
Acknowledgments
The authors thank the members of the BC COVID-19 Therapeutics Committee, all our hematologists, hematopathologists, and clinical, laboratory, and public health colleagues, including our pharmacists, nurses, and health care providers who provided support, advice, or stewardship in the design and implementation of the clinical care pathway.
Authorship
Contribution: A.Y.Y.L. was the principal investigator, conceptualized the study, prepared Figure 1, prepared the first manuscript draft, and made critical revisions; M.A.M. prepared Figure 2 and performed data collection and statistical analysis; E.A.P. prepared Table 1; S.K.W.W., M.P.W., and H.N. processed laboratory samples and performed data collection; M.L. and M.N. provided public health data; and the above authors and R.K.M., V.C., T.S., L.J.L., C.G., B.R., S.P., K. Afra, K. Ambler, L.Y.C.C., T.S.F., H.C.L., C.L., D.M., J.P., P.R., G.S., T.W., A.Y., L.Z., D.S. contributed to the development of the care pathway and critically reviewed and approved the final manuscript.
Conflict-of-interest disclosure: A.Y.Y.L. received research funding from BMS and provided consultancy services to Bayer, BMS, LEO Pharma, Pfizer, and Servier outside the submitted work. E.A.P. reports fees for CME presentations from Pfizer and LEO Pharma outside of the submitted work. C.G. provided advisory boards participation for Sevier Canada and reports fees for CME presentations for Pfizer, Servier, and Bayer outside the submitted work. S.P. reports honoraria and consulting fees from Celgene and Janssen outside the submitted work. T.S.F. reports in-kind study medication from Bayer Canada outside the submitted work. T.W. reports honoraria from Bayer and BMS for CME presentations outside the submitted work. P.R. reports honoraria from Bayer, Pfizer, and BMS and received research funding from Servier outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Agnes Y. Y. Lee, Division of Hematology, 2775 Laurel Street, 10th Floor, Vancouver, BC, Canada V5Z 1M9; e-mail: alee14@bccancer.bc.ca.
References
Author notes
Requests for data sharing may be submitted to Agnes Y. Y. Lee (alee14@bccancer.bc.ca).