Abstract
von Willebrand disease (VWD) is the most common inherited bleeding disorder. The management of patients with VWD who are undergoing surgeries is crucial to prevent bleeding complications. We systematically summarized the evidence on the management of patients with VWD who are undergoing major and minor surgeries to support the development of practice guidelines. We searched Medline and EMBASE from inception through October 2019 for randomized clinical trials (RCTs), comparative observational studies, and case series that compared maintaining factor VIII (FVIII) levels or von Willebrand factor (VWF) levels at >0.50 IU/mL for at least 3 days in patients undergoing major surgery, and those with options for perioperative management of patients undergoing minor surgery. Two authors screened and abstracted data and assessed the risk of bias. We conducted meta-analyses when possible. We evaluated the certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. We included 7 case series for major surgeries and 2 RCTs and 12 case series for minor surgeries. Very-low-certainty evidence showed that maintaining FVIII levels or VWF levels of >0.50 IU/mL for at least 3 consecutive days showed excellent hemostatic efficacy (as labeled by the researchers) after 74% to 100% of major surgeries. Low- to very-low-certainty evidence showed that prescribing tranexamic acid and increasing VWF levels to 0.50 IU/mL resulted in fewer bleeding complications after minor procedures compared with increasing VWF levels to 0.50 IU/mL alone. Given the low-quality evidence for guiding management decisions, a shared-decision model leading to individualized therapy plans will be important in patients with VWD who are undergoing surgical and invasive procedures.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder and is the result of either quantitative (Type 1 and Type 3) or qualitative defects (Type 2) in von Willebrand factor (VWF).1 Patients with VWD are at particular risk of hemorrhage in the perioperative setting, given the key role of VWF in both hemostasis2 and wound healing.3 This risk depends on the severity of the patient’s bleeding phenotype and the type of surgery or procedure being performed. Treatment options for patients with VWD who are undergoing surgery include administration of VWF-containing concentrates (both plasma derived and recombinant), desmopressin to induce release of stored endogenous VWF from the vascular endothelium, and adjunctive antifibrinolytic therapy such as tranexamic acid (TXA).4
In 2017, the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis (ISTH), the National Hemophilia Foundation (NHF), and the World Federation of Hemophilia (WFH) convened a working group to define the scope and priority areas of focus for updated guidelines on VWD. As part of the guideline development process, an international survey to define key questions in the management of VWD revealed both “Management of bleeding… prior to invasive procedures,” and “Treatment options for surgical patients” as highly rated by patients, caregivers, and health care providers.5
The aim of this article is to describe the methods and the results of the systematic reviews conducted to inform the 2 recommendations relevant to the treatment of patients with VWD who are undergoing surgery about the VWD management guidelines.6
Methods
We did not register a protocol, but we did follow prespecified methods for synthesizing evidence that are standard to ASH,7 and we established eligibility criteria for which studies to include on the basis of the recommendation questions prioritized by the panel. This article addresses 2 systematic review questions:
- 1.
In patients with VWD who are undergoing major surgery, what are the comparative effects of keeping the factor VIII (FVIII) level ≥0.50 IU/mL for at least 3 days after the surgery or the VWF level ≥0.50 IU/mL for at least 3 days after the surgery? (SR1)
- 2.
In patients with VWD who are undergoing minor surgery or minor invasive procedures, what are the comparative effects of increasing the VWF level to ≥0.50 IU/mL by using VWF concentrate or desmopressin, increasing the VWF level to ≥0.50 IU/mL by using VWF concentrate or desmopressin in conjunction with TXA, or prescribing TXA alone? (SR2)
Eligibility criteria
We included randomized clinical trials (RCTs) and comparative observational studies of any design for the outcomes of interest. When neither of these study designs were available, we included case series. We included studies in which researchers enrolled patients with all types of VWD or hemophilia and studies that addressed any of the clinical outcomes of interest listed below. We excluded studies that provided information only about physiological outcomes such as VWF levels and those published only as conference abstracts. We excluded patients with acquired forms of VWF deficiency (such as acquired von Willebrand syndrome that results from aortic valve stenosis, mechanical circulatory support, or autoantibodies to VWF). Specific eligibility criteria for each of the questions are described below.
SR1.
We included patients undergoing any type of major surgery (defined as procedures in which a mesenchymal barrier is opened), procedures requiring surgical opening into the large body cavities, procedures in which severe hemorrhage was possible, interventions involving joints, third molar extractions, and interventions in which the patient’s life was at risk. We also considered surgeries as major if the researchers characterized them as such. Final categorization of a surgery as major was confirmed by the VWD guideline panel. We included studies that provided information on mortality, major bleeding, need for additional surgical procedures, transfusions, serious adverse events (AEs), hospitalization, and thrombotic events.
SR2.
We included patients undergoing any type of minor surgery (defined as any invasive operative procedure in which only skin or mucosal membranes and connective tissue are resected). We also included surgeries characterized as minor by the researchers. Final categorization of a surgery as minor was confirmed by the VWD guideline panel. We included studies that provided information on major bleeding, need for additional hemostatic agents, need for additional surgical procedures, serious AEs, mortality, hospitalization, transfusion, or inability to perform the surgery.
Information sources
We searched for RCTs and comparative observational studies in Medline (OVID) and EMBASE from inception (1946 and 1974, respectively) to October 2019. We conducted an umbrella search that encompassed all recommendation questions addressed in the guidelines (supplemental Data 1). We did not limit by date or language of publication. We also searched the reference lists of studies we included and contacted the panel of experts to obtain relevant studies. We searched for gray literature using OpenGrey.8 We conducted a targeted search for case series based on the results of screening for other types of studies, and the lists of references for those studies. For SR1, because these were the most relevant types of evidence for addressing the question of interest, we included case series in which patients with VWD were undergoing major surgery, and in which researchers reported both their FVIII levels and VWF activity levels at postoperative day 3 or after.
Study selection and data abstraction
Pairs of independent reviewers screened the titles and abstracts of all citations for both SRs. We included all studies identified as potentially relevant and performed duplicate screening of the full texts for each of the SRs separately. Reviewers resolved disagreements by discussion with the help of a third reviewer or the clinical experts. We abstracted data in duplicate. For each study, we abstracted information about the setting, participant characteristics (mean age, distribution according to type of bleeding disorder), interventions received (specific agent and regimen), and outcome data for all clinical outcomes reported, with any method of measurement, at any timepoint. Reviewers used standardized, piloted data abstraction forms and underwent training and calibration at all stages.
Data synthesis
For dichotomous outcomes, we used the risk ratio and its 95% confidence interval (CI) as the effect measure in comparative studies, and proportions and their 95% CIs in single-arm studies. For continuous outcomes, we used the mean difference and its 95% CI for comparative studies and the mean and standard deviation for non-comparative studies. When studies did not report enough data to present the effect estimates using these measures, we used what was available. We pooled results across studies using random-effects meta-analyses when it was possible. We used Review Manager 5.39 and R software10 to conduct meta-analyses. When it was not possible to conduct meta-analyses, we performed a narrative synthesis of the results at the outcome level.
Assessment of quality of the evidence
We assessed the quality of the evidence for each of the outcomes using the Grading of Recommendations Assessments, Development, and Evaluation (GRADE) approach.11 The quality of the evidence assessment considered the study design, and risk of bias, inconsistency, indirectness, imprecision, publication bias, presence of large effects, dose-response gradient, and residual confounding. We assessed risk of bias using the Cochrane Risk of Bias tool12 for RCTs and the Risk of Bias in Non-Randomized Studies of Interventions tool13 (ROBINS) for comparative observational studies. Because of the lack of a comparison group in single-arm studies, which we used to make inferences about how treatments compare, their risk of bias was judged as high by default. We assessed inconsistency by comparing the point estimates and CIs across studies, and we used statistical measures (χ2 and I2). We assessed indirectness by focusing on characteristics of the population, particularly the proportion of participants who had VWD instead of other bleeding disorders. We assessed imprecision using a non-contextualized approach and the null effect as the threshold of interest, as well as the optimal information size.14 We planned to assess publication bias using funnel plots if a meta-analysis had ≥10 studies.
We constructed Summary of Findings tables using GRADEpro.15 Whenever possible, we present absolute and relative estimates of effects. We calculated absolute estimates using the results from the studies included. In the tables, we included all outcomes for which there was evidence and outcomes that were considered critical or important for decision-making by the guideline panel but for which there was no information. We planned to conduct subgroup analyses based on the risk of bias of the studies and the populations included. We did not plan any sensitivity analyses.
Results
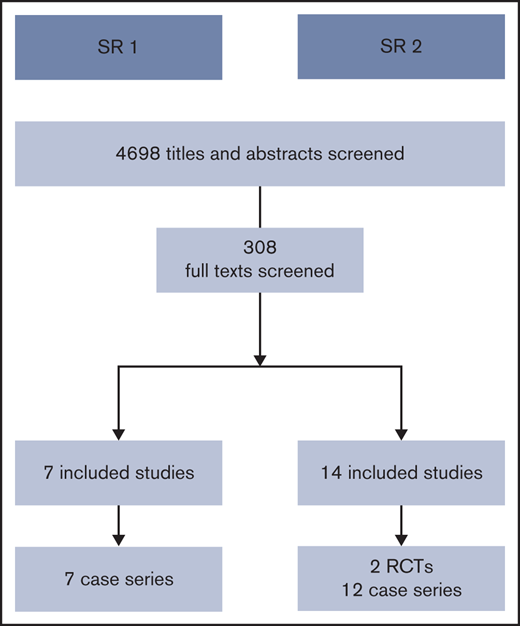
We screened a total of 4698 titles and abstracts. Figure 1 summarizes the results of the search and study selection process. We present the results of each of the SRs below.
SR1: factor levels for patients undergoing major surgery
After reviewing 308 full texts, we did not find any comparative studies addressing this question. On the basis of methodologic and clinical considerations, we conducted a targeted search for case series in which patients with VWD were undergoing major surgery, and researchers reported both their FVIII levels and VWF activity levels at postoperative day 3 or after. We included evidence from 7 case series.16-22 Because these case series reported the outcomes of a single group of patients for whom both FVIII levels and VWF activity levels were available, there were no data to compare the outcomes after each intervention. Supplemental Data 2 presents the characteristics of the included studies. Because of our method of analysis and reporting of the results across studies, we could not conduct meta-analyses. Tables 1 and 2 group the studies according to outcome and summarize the factor levels in each of the studies.
Summary of outcomes and factor levels in a study that reported information at the patient level
| Outcome . | Study . | FVIII levels (IU/mL) . | VWF levels (IU/mL) . |
|---|---|---|---|
| Hemostatic efficacy: Excellent, 92%; good, 4%; poor, 4% | Khair et al18 | Mean max, 1.34 | Mean max, 0.92 |
| — | Dunkley et al17 | Median, 1.15 (IQR, 0.97-1.34) | Median, 0.85 (IQR, 0.67-1.03) |
| Outcome . | Study . | FVIII levels (IU/mL) . | VWF levels (IU/mL) . |
|---|---|---|---|
| Hemostatic efficacy: Excellent, 92%; good, 4%; poor, 4% | Khair et al18 | Mean max, 1.34 | Mean max, 0.92 |
| — | Dunkley et al17 | Median, 1.15 (IQR, 0.97-1.34) | Median, 0.85 (IQR, 0.67-1.03) |
The Khair and Dunkley studies reported no postoperative bleeding complications, AEs, or thrombotic events.
Summary of outcomes and factor levels in studies that reported information at the procedure level
| Outcomes . | Study . | FVIII levels (IU/mL) . | VWF levels (IU/mL) . | ||
|---|---|---|---|---|---|
| Mean (range) . | Median (IQR) . | Mean (range) . | Median (IQR) . | ||
| Hemostatic efficacy | |||||
| Excellent, 74%; good, 11%; fair, 5%; poor, 11%* | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Excellent, 84%; good, 16%† | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| 100%‡ | Dunkley et al17 | 1.15 (0.97-1.34) | 0.85 (0.67-1.03) | ||
| Major bleeding | |||||
| 5%* | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| 0§ | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| Hgb decreased to ≥1.24 mmol/L and/or RBC transfusion | |||||
| 6.70%§ | Srivastava et al20 | 0.92 (0.82-1.02) | 0.41 (0.32-0.50) | ||
| 3% RBC transfusionǁ | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| 20% RBC transfusionǁ | Dunkley et al17 | 1.15 (0.97-1.34) | 0.85 (0.67-1.03) | ||
| Symptomatic VTE | |||||
| 0.00% | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| 0% | Srivastava et al20 | 0.92 (0.82-1.02) | 0.41 (0.32-0.50) | ||
| Wound infection | |||||
| 0.00% | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Received ≥2 units RBCs | |||||
| 58.00%¶ | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Estimated blood loss | |||||
| Mean, 427 mL (SD, 70-1500 mL) | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Duration of hospitalization | |||||
| Mean, 5 days (range, 3-13 days) | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Outcomes . | Study . | FVIII levels (IU/mL) . | VWF levels (IU/mL) . | ||
|---|---|---|---|---|---|
| Mean (range) . | Median (IQR) . | Mean (range) . | Median (IQR) . | ||
| Hemostatic efficacy | |||||
| Excellent, 74%; good, 11%; fair, 5%; poor, 11%* | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Excellent, 84%; good, 16%† | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| 100%‡ | Dunkley et al17 | 1.15 (0.97-1.34) | 0.85 (0.67-1.03) | ||
| Major bleeding | |||||
| 5%* | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| 0§ | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| Hgb decreased to ≥1.24 mmol/L and/or RBC transfusion | |||||
| 6.70%§ | Srivastava et al20 | 0.92 (0.82-1.02) | 0.41 (0.32-0.50) | ||
| 3% RBC transfusionǁ | Borel-Derlon et al16 | 2.40 (1.00-3.14) | 0.94 (0.48-1.36) | ||
| 20% RBC transfusionǁ | Dunkley et al17 | 1.15 (0.97-1.34) | 0.85 (0.67-1.03) | ||
| Symptomatic VTE | |||||
| 0.00% | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| 0% | Srivastava et al20 | 0.92 (0.82-1.02) | 0.41 (0.32-0.50) | ||
| Wound infection | |||||
| 0.00% | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Received ≥2 units RBCs | |||||
| 58.00%¶ | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Estimated blood loss | |||||
| Mean, 427 mL (SD, 70-1500 mL) | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
| Duration of hospitalization | |||||
| Mean, 5 days (range, 3-13 days) | Rugeri et al19 | 1.74 (1.53-2.20) | 2.10 (0.87-2.10) | ||
Hgb, hemoglobin; IQR, interquartile range; RBC, red blood cell; SD, standard deviation; VTE, venous thromboembolism.
Outcomes definitions based on International Society on Thrombosis and Haemostasis definitions.
Excellent: bleeding during surgery and the postoperative period similar to that expected for normal individuals; good: slightly excessive bleeding.
Excellent: hemostasis achieved and cessation of bleeding; good: partial but adequate control of bleeding and did not require additional product for unplanned treatment; moderate: moderate control of bleeding and required additional product for unplanned treatment; none: severe uncontrolled bleeding.
The author did not provide information on the outcome definition.
Includes intraoperative (allogeneic and cell saver) transfusion and postoperative allogeneic transfusion.
Outcome data and factor levels at day 3 from studies reporting results at the patient level.
Two case series analyzed results at the patient level.17,18 These 2 studies included data from 28 patients undergoing 35 surgical procedures. Hemostatic efficacy was excellent (as labeled by the researchers) in 92% of the patients.18 No postoperative bleeding complications,18 AEs,18 or thrombotic events were reported.17,18 The mean of the maximum FVIII level in patients from the study that reported the most outcomes was 1.34 IU/mL, whereas the VWF level was 0.92 IU/mL (Table 1). The certainty of the evidence for the comparative effects of keeping the FVIII levels >0.50 IU/mL for at least 3 days after the surgery or the VWF level >0.50 IU/mL for at least 3 days after the surgery is very low because of risk of bias, indirectness, and imprecision (very few patients).
Outcome data and factor levels at day 3 from studies reporting results at the procedure level.
Four case series analyzed results at the procedure level.16,17,19,20 The studies included data from 86 patients undergoing 150 procedures. Hemostatic efficacy was excellent (as labeled by the researchers) in 74% to 100% of the procedures across studies.16,17,19 Major bleeding occurred during 0% to 5% of the procedures.16,19 Hemoglobin decrease or blood transfusion occurred in 3% to 20% of the procedures.16,17,20 Symptomatic venous thromboembolism and wound infection did not occur in any of the procedures.19,20 Median FVIII levels varied from 1.15 to 2.40 IU/mL across studies, whereas median VWF levels varied from 0.85 to 2.10 IU/mL (Table 2). The certainty of the evidence was very low because of the risk of bias, indirectness, and imprecision (very few patients). From the other 2 studies that met eligibility criteria, 1 reported factor levels in a graph from which we could not extract aggregate data (mean or median),22 and the other did not specify whether the analysis was done at the patient or procedure level but reported a success rate of 95.2%.21
SR2: management of patients undergoing minor surgery
After reviewing 308 full texts, we found 2 RCTs comparing the use of an increase of VWF plus TXA vs an increase of VWF alone,23,24 8 case series in which patients undergoing transvenous and percutaneous liver biopsies, gastroscopies, colonoscopies, cystoscopies, circumcisions, and dental extractions received factor replacement therapy alone,25-33 and 4 case series in which patients undergoing gastroscopies, colonoscopies, sigmoidoscopies, and dental extractions received TXA alone.34-37 Supplemental Data 3 presents the characteristics of the included studies.
Comparison: Increasing VWF levels to ≥0.50 IU/mL vs increasing VWF levels to ≥0.50 IU/mL plus TXA.
Low-certainty evidence suggested fewer AEs (absolute risk reduction, −30 per 1000 patients; 95% CI, −130 to 80 per 1000 patients),23,24 and no important difference in major bleeding (absolute risk reduction, 0 per 1000 patients; 95% CI, −120 to 120 per 1000 patients)24 when increasing VWF levels to 0.50 IU/mL only vs increasing VWF levels to 0.50 IU/mL and prescribing TXA. Very-low certainty evidence suggested that increasing VWF levels to 0.50 IU/mL only increases the risk of postoperative bleeding (risk ratio, 6.29; 95% CI, 2.12-18.65) when compared with increasing VWF levels to 0.50 IU/mL plus prescribing TXA (Table 3).23,24
Summary of findings of RCTs that compared increasing VWF levels to 0.50 IU/mL alone vs increasing VWF levels to 0.50 IU/mL and prescribing TXA for patients with VWD undergoing minor surgery
| Outcomes . | No. of participants . | No. of follow-up RCTs . | Certainty of the evidence . | GRADE . | RR (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|---|
| Risk with increasing VWF to 0.50 IU/mL + TXA . | Difference in risk with increasing VWF level to 0.50 IU/mL . | ||||||
| Postoperative bleeding | 59 | 2 | ⨁◯◯◯ | Very low*†‡ | 6.29 (2.12-18.65) | 103/1000 | 547 more per 1000 (116-1826 more) |
| AEs requiring withdrawal | 59 | 2 | ⨁⨁◯◯ | Low*‡ | Not estimable | 34/1000 | 34 fewer per 1000 (34-34 fewer) |
| Major bleeding requiring transfusion | 31 | 1 | ⨁⨁◯◯ | Low*† | Not estimable | 0/1000 | 0 fewer per 1000 (0-0 fewer) |
| Postoperative blood loss, mL § | 28 | 1 | ⨁◯◯◯ | Very low*†ǁ | |||
| Outcomes . | No. of participants . | No. of follow-up RCTs . | Certainty of the evidence . | GRADE . | RR (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|---|
| Risk with increasing VWF to 0.50 IU/mL + TXA . | Difference in risk with increasing VWF level to 0.50 IU/mL . | ||||||
| Postoperative bleeding | 59 | 2 | ⨁◯◯◯ | Very low*†‡ | 6.29 (2.12-18.65) | 103/1000 | 547 more per 1000 (116-1826 more) |
| AEs requiring withdrawal | 59 | 2 | ⨁⨁◯◯ | Low*‡ | Not estimable | 34/1000 | 34 fewer per 1000 (34-34 fewer) |
| Major bleeding requiring transfusion | 31 | 1 | ⨁⨁◯◯ | Low*† | Not estimable | 0/1000 | 0 fewer per 1000 (0-0 fewer) |
| Postoperative blood loss, mL § | 28 | 1 | ⨁◯◯◯ | Very low*†ǁ | |||
The following outcomes were not reported: serious AEs, mortality, need for additional hemostatic agents, need for additional surgical procedures, and inability to perform the surgery. The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence: Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the estimate of effect. The true effect is likely to be substantially different from the estimate of effect.
RR, risk ratio.
Randomization and allocation concealment were at unclear or high risk of bias in both trials.
The panel judged that there were serious applicability concerns because all the patients had hemophilia.
Small number of patients and events overall and very wide CIs.
Increasing FVIII level to 0.50 IU/mL: mean blood loss per participant, 84.1 mL (range, 4-323 mL) (n = 14). Increasing FVIII level to 0.50 IU/mL + TXA: mean blood loss per participant, 61.2 mL (range, 1-749 mL) (n = 14). P = .02.
Meta-analysis was performed in Risk Difference (RD) because there were no AEs in either arm in 1 trial.
Intervention: Increasing VWF levels to 0.50 IU/mL only.
Meta-analysis showed that the proportion of surgeries in which there were bleeding complications was 11% (95% CI, 6%-19%),26,27,29,31-33 the proportion of procedures in which hemostasis was judged as appropriate was 98% (95% CI, 91%-99%),28,30,33 the proportion of participants who needed transfusions was 2% (95% CI, 0%-50%),26,29,32 and no thrombotic events occurred.26,28,33 Because these are all case series, and for some of the outcomes there were very few patients providing information, the certainty of the evidence of how increasing VWF levels to 0.50 IU/mL compares with other options is very low for all outcomes (Table 4).
Summary of findings of studies in which clinicians increased VWF levels to 0.50 IU/mL in patients with VWD undergoing minor surgery
| Outcome . | No. of participants . | No. of observational studies . | Total No. of surgeries . | Certainty of the evidence . | GRADE . | Impact . |
|---|---|---|---|---|---|---|
| Bleeding complications: hemorrhagic, postoperative bleeding | 278 | 6 | 281 | ⨁◯◯◯ | Very low*† | Proportion of surgeries in which there were bleeding complications, 11% (95% CI, 6%-19%). |
| Hemostasis during surgery: excellent or good; adequate, as judged by clinician | 88 | 3 | ⨁◯◯◯ | Very low* | Proportion of procedures in which hemostasis was judged as appropriate, 98% (95% CI, 91%-99%). | |
| No. of patients with need for additional hemostatic agents (postoperative factor replacement) | 13 | 1 | ⨁◯◯◯ | Very low*‡ | Proportion of participants who required postoperative factor replacement, 54% (7 of 13). Proportion who required continuous replacement, 38% (5 of 13). | |
| Hospitalization needed for performing the procedure | 13 | 1 | ⨁◯◯◯ | Very low* | In 1 study in which researchers report outcomes of 13 liver or percutaneous biopsies, all 13 patients had to be hospitalized for the procedure. | |
| No. of patients who needed transfusion | 51 | 3 | 54 | ⨁◯◯◯ | Very low*§ | Proportion of participants who needed transfusions, 2% (95% CI, 0%-50%). |
| Thrombotic serious AEs | 76 | 3 | 94 | ⨁◯◯◯ | Very low* | Three studies reported this outcome; no thrombotic events occurred in any of the 3 studies. |
| No. of patients who developed inhibitors or AEs | 39 | 2 | ⨁◯◯◯ | Very low*† | Proportion of patients who developed inhibitors, 2% (95% CI, 0%-21%). | |
| Several definitions provided for AEs | 133 | 4 | ⨁◯◯◯ | Very low* | Four studies reported AEs; 3 reported no allergic reactions (0 of 28 surgeries), no wound infections (0 of 11 surgeries), and no AEs (0 of 29 surgeries); 1 study reported a vasovagal episode that required hospitalization for observation in 1 of 65 patients. |
| Outcome . | No. of participants . | No. of observational studies . | Total No. of surgeries . | Certainty of the evidence . | GRADE . | Impact . |
|---|---|---|---|---|---|---|
| Bleeding complications: hemorrhagic, postoperative bleeding | 278 | 6 | 281 | ⨁◯◯◯ | Very low*† | Proportion of surgeries in which there were bleeding complications, 11% (95% CI, 6%-19%). |
| Hemostasis during surgery: excellent or good; adequate, as judged by clinician | 88 | 3 | ⨁◯◯◯ | Very low* | Proportion of procedures in which hemostasis was judged as appropriate, 98% (95% CI, 91%-99%). | |
| No. of patients with need for additional hemostatic agents (postoperative factor replacement) | 13 | 1 | ⨁◯◯◯ | Very low*‡ | Proportion of participants who required postoperative factor replacement, 54% (7 of 13). Proportion who required continuous replacement, 38% (5 of 13). | |
| Hospitalization needed for performing the procedure | 13 | 1 | ⨁◯◯◯ | Very low* | In 1 study in which researchers report outcomes of 13 liver or percutaneous biopsies, all 13 patients had to be hospitalized for the procedure. | |
| No. of patients who needed transfusion | 51 | 3 | 54 | ⨁◯◯◯ | Very low*§ | Proportion of participants who needed transfusions, 2% (95% CI, 0%-50%). |
| Thrombotic serious AEs | 76 | 3 | 94 | ⨁◯◯◯ | Very low* | Three studies reported this outcome; no thrombotic events occurred in any of the 3 studies. |
| No. of patients who developed inhibitors or AEs | 39 | 2 | ⨁◯◯◯ | Very low*† | Proportion of patients who developed inhibitors, 2% (95% CI, 0%-21%). | |
| Several definitions provided for AEs | 133 | 4 | ⨁◯◯◯ | Very low* | Four studies reported AEs; 3 reported no allergic reactions (0 of 28 surgeries), no wound infections (0 of 11 surgeries), and no AEs (0 of 29 surgeries); 1 study reported a vasovagal episode that required hospitalization for observation in 1 of 65 patients. |
The following outcomes were not reported in the studies: need for additional surgical procedures, mortality, inability to perform the surgery. The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grade of evidence: Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
These are case series, and there are no comparisons with other groups.
The CIs show that the proportion can be very small or not so small.
Very small number of patients.
The CIs are very wide and suggest that the proportion can be very small to very large.
Intervention: TXA alone.
Meta-analysis showed that the proportion of patients or surgeries in which there is bleeding is 14% (95% CI, 9%-20%).34-37 One study showed that the mean number of days in hospital per surgery performed was 4 (no CI provided; Table 5).37
Summary of findings of studies in which clinicians prescribed TXA only for patients with VWD undergoing minor surgery
| Outcome . | No. of participants . | No. of observational studies . | Certainty of evidence . | GRADE . | Impact . |
|---|---|---|---|---|---|
| Several definitions for bleeding; No. of events and total No. of patients or surgeries | 119 | 4 | ⨁◯◯◯ | Very low* | Pooled analysis showed that the proportion of patients or surgeries with bleeding was 14% (95% CI, 9%-20%). |
| Hospitalization days per surgery | 22 | 1 | ⨁◯◯◯ | Very low* | Mean, 4 (no 95% CI provided). |
| Outcome . | No. of participants . | No. of observational studies . | Certainty of evidence . | GRADE . | Impact . |
|---|---|---|---|---|---|
| Several definitions for bleeding; No. of events and total No. of patients or surgeries | 119 | 4 | ⨁◯◯◯ | Very low* | Pooled analysis showed that the proportion of patients or surgeries with bleeding was 14% (95% CI, 9%-20%). |
| Hospitalization days per surgery | 22 | 1 | ⨁◯◯◯ | Very low* | Mean, 4 (no 95% CI provided). |
The following outcomes were not reported in the studies: Need for additional hemostatic agents, need for additional surgical procedures, serious AEs, mortality, transfusion, inability to perform the surgery. GRADE Working Group grades of evidence: Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Discussion
We conducted 2 systematic reviews to inform the development of the ASH ISTH NHF WFH Guidelines on surgical management for patients with VWD. The questions were chosen based on what the guideline panel prioritized as the most important questions to be addressed. Two other systematic reviews were conducted to tackle the gynecologic treatment of women with VWD38 and the long-term prophylaxis treatment of VWD.39 For SR1, we relied on case series (very-low certainty evidence). The evidence suggests that, among patients undergoing major procedures, keeping the FVIII level >0.50 IU/mL for at least 3 days after the surgery or the VWF level >0.50 IU/mL for at least 3 days after the surgery showed efficient hemostasis and low risk of complications, but there is high uncertainty in this evidence. A meta-analysis, published in 2021 of >200 studies including >125 000 patients revealed 1020 (2.1%) total thromboembolic events in the TXA and 900 (2%) in the control group.40 For SR2, we relied on 2 RCTs for directly comparing an increase in VWF plus TXA vs an increase in VWF alone. The RCTs suggest that using an increase in VWF alone showed higher risk of postoperative bleeding but a lower risk of AEs than using an increase of VWF plus TXA, but there is high uncertainty in this evidence. In addition, we reported results from case series, but we rated the evidence as very low quality because of the lack of comparative data. It should be noted that if desmopressin is used to increase VWF levels, there is the potential for tachyphylaxis after several doses, in addition to the potential for more significant hyponatremia.
Overall, the certainty of the evidence for both systematic reviews was low to very low. Only 2 RCTs provided information for direct comparison of the 2 interventions. We down-rated the evidence from these RCTs because of the low number of patients and the potential indirectness of the evidence since all the patients had hemophilia.
Given the lack of high-quality evidence such as RCTs, future research should investigate the importance of maintaining FVIII and/or VWF levels after surgery stratified by type of procedure (eg, dental, mucosal, orthopedic), VWD type or subtype, history of bleeding, and baseline VWF levels. Future studies should account for number of infusions required to achieve hemostatic FVIII and VWF levels and provide sufficient timepoints for detailed analysis of efficacy. If desmopressin is used to increase VWF levels, the number of infusions should also be noted. In addition, research priorities should include the utility of TXA across a variety of procedures including anatomic site, TXA formulation, and VWD type and subtype. Future research should also focus on AEs (eg, thrombotic events) especially in high-risk patients such as elderly patients. The advent of new VWF formulations containing VWF alone without concomitant FVIII will make this knowledge even more important going forward with VWD treatment. Given the high cost of factor, cost effectiveness should be analyzed in future studies.
This systematic review directly informs clinical practice guidelines for the management of VWD and includes recommendations (1) to target both FVIII and VWF activity levels of ≥0.50 IU/mL for at least 3 days after major surgery, (2) to increase VWF activity levels to ≥0.50 IU/mL with desmopressin or VWF concentrate with the addition of TXA after minor surgery or invasive procedures, and (3) to give TXA monotherapy for minor mucosal procedures in patients with type 1 VWD and baseline VWF activity levels >0.30 IU/mL and a mild bleeding phenotype.6 Given the low-quality evidence to guide management decisions, a shared-decision model leading to individualized therapy plans will be important in patients with VWD who are undergoing surgical and invasive procedures.
Acknowledgments
The authors thank Angela Weyand, Rezan Abdul-Kadir, Susie Cooper, Peter Kouides, Michelle Lavin, Margareth Castro Ozelo, and J. Evan Sadler for their invaluable assistance and ASH, ISTH, NHF, and WFH for their support of the guideline process, with specific thanks to Jenny Castano, Cary Clark, Rob Kunkle, Ellen Riker, Fiona Robinson, and Mark Skinner.
Authorship
Contribution: R.A.M., R.B.-P., and N.H. helped design the study, select studies, extract data, analyze the statistics, and interpret the results; A.E.A., S.S., Y.A., A.B., M.K., H.A., H.E.-K., S.M., J.R., A.D., O.A., and B.M. helped select the studies and extract data; N.H., A.E.A., R.B.-P., and R.A.M. helped draft the report; and J.M.G., P.K., M.L., A.A., F.W.G.L., S.H.O., A.T., P.D.J., N.T.C., and V.F. helped interpret the results and critically revise the report.
This systematic review was conducted to support the development of the ASH ISTH NHF WFH 2020 Guidelines for Management of VWD. The entire guideline development process was funded by ASH, ISTH, NHF, and WFH (the 4 collaborating organizations). A.E.A., N.H., R.B.-P., and R.A.M. received salary or grant support from the Outcomes and Implementation Research Unit at the University of Kansas Medical Center and the McMaster GRADE center; others participated to fulfill the requirements of an academic degree or program or volunteered their time.
Conflict-of-interest disclosure; All authors completed a disclosure-of-interest form that was reviewed by ASH and is available in supplemental Appendices 4 and 5 (all authors were members of the guideline panel or members of the systematic review team or both).
Correspondence: Reem A. Mustafa, University of Kansas Medical Center, 3901 Rainbow Blvd, MS3002, Kansas City, KS 66160; e-mail: rmustafa@kumc.edu.
References
Author notes
For original data, please contact Reem A. Mustafa via email at rmustafa@kumc.edu.
The full-text version of this article contains a data supplement.