Key Points
This paper is an evaluation of perceptions, facilitators, and barriers to clinical trial participation for SCD.
This paper contains recommendations by persons directly affected by SCD to improve clinical trials and standard of care.
Abstract
Sickle cell disease (SCD) is the most common inherited red blood cell disorder in the United States, affecting 70 000 to 100 000 Americans and causing a range of serious medical complications. Although the cause of SCD was established decades ago, existing therapies have varied effectiveness and side effects, and development of novel therapies has been slow. The limitations of existing treatment options highlight the need for new therapies that are aligned with the desires of the community. To date, little has been done to systematically seek and report the opinions and experiences of people with SCD regarding clinical research. In 2019, the American Society of Hematology Research Collaborative conducted 8 community workshops across the United States engaging 472 people, including persons with SCD and caregivers of those living with the disease. The workshop goals included assessing understanding, awareness, and perceptions of clinical research; and identifying the most critical clinical trial considerations of this community. Participants were asked about their experiences living with SCD and their satisfaction with treatment options. Pain and fatigue were reported as symptoms requiring better therapies. Although few participants reported being asked to enroll in a clinical trial, they expressed conditional willingness to participate. A majority were willing to share personal health information to further research and improve health outcomes. To actively engage the SCD community and increase enrollment and retention in clinical trials, researchers should address the treatment priorities of this population and ensure they have access to trusted information about clinical research and opportunities for participation.
Introduction
Sickle cell disease (SCD) is the most common inherited red blood cell disorder in the United States, affecting 70 000 to 100 000 Americans.1 The signature sickled cells of this disease can obstruct blood flow to specific organs, leading to stroke, acute chest syndrome, organ damage (particularly renal and cardiovascular), other disabilities, and in many cases premature death.2-7 People with SCD also experience anemia, which often leaves them feeling fatigued and weak. A prominent hallmark of the disease is vaso-occlusion causing ischemic pain, which manifests with varying degrees of severe, episodic bone pain or abdominal pain (referred to as vaso-occlusive crisis). These debilitating symptoms and the complex treatment needs of those living with SCD can limit educational achievement, career opportunities, and quality of life.8 Although SCD was once associated primarily with early childhood mortality, currently in the United States most people with SCD live into adulthood, which poses new treatment issues and research challenges as the population of individuals living with SCD ages.9
Although the molecular basis of SCD is established, it has been challenging to translate this knowledge into the development of novel therapies. Moreover, the limitations of existing options highlight the need for new treatments that are more aligned with the health outcomes desired by the community. Dissatisfaction with current treatment options and strategies can also influence perspectives about the value of research, either negatively or positively. As such, it is critical that the SCD research community is aware of the concerns of those living with SCD and that these subjects with SCD remain actively engaged in clinical research to ensure therapies are being developed that meet their needs. However, several factors create barriers to participation, which must be recognized and addressed to advance SCD research.
Critically, the disproportionate impact of this rare disease on the African-American community is an important factor in assessing barriers. The Centers for Disease Control and Prevention estimates that SCD occurs in ∼1 in every 365 Black or African-American births.10 As such, the heavy toll of SCD on this population, combined with the lag in new treatments, is especially significant given the historical causes of mistrust of the health-care system and the research community among some African-American individuals.
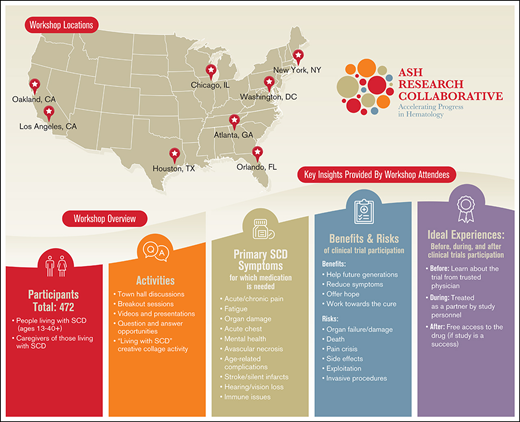
To date, little has been done to systematically seek and report the opinions, thoughts, and experiences of people with SCD regarding clinical research. The American Society of Hematology Research Collaborative (ASH RC) is a nonprofit organization established by the ASH in 2018 to improve the lives of those living with blood disorders and inform the medical field as it works toward improved outcomes in hematologic care. Recognizing the lack of patient-driven SCD research and treatments to date, ASH RC launched an initiative to accomplish the following: increase awareness and understanding of clinical research; build trust and connections with the SCD community; foster stronger relationships with the broader medical and research communities; and ultimately ensure that therapies for this disease meet the needs of this population. Involving members of the SCD community in this initiative was the first step in this process. To achieve this goal, ASH RC, working with the independent nonprofit organization CISCRP (Center for Information and Study on Clinical Research Participation), conducted 8 community workshops across the United States between April and September 2019 (Table 1). The goals of the workshops were to: (1) obtain a comprehensive and coherent understanding of what it is like to live with and care for someone with SCD; (2) gain insights into the community’s satisfaction with currently available treatments; (3) uncover opportunities to improve clinical care; (4) determine desired clinical outcomes; (5) assess understanding, awareness, and perceptions related to clinical research; and (6) identify the most critical clinical trial considerations of this community. The current report focuses on results related to the 2 most closely related themes that emerged during the multifaceted workshops: the SCD community’s views related to treatment needs, and the community’s awareness and perceptions of clinical research.
Overview of workshops
| Participants | Populations
|
| Locations | Chicago, IL Los Angeles, CA Atlanta, GA Houston, TX Orlando, FL New York, NY Oakland, CA Washington, DC |
| Activities | Large group town hall discussions Small group breakout sessions Informative educational videos and presentations Question-and-answer opportunities Living with SCD creative collage activity Interactive “ideal” clinical trial journey mapping activity |
| Participants | Populations
|
| Locations | Chicago, IL Los Angeles, CA Atlanta, GA Houston, TX Orlando, FL New York, NY Oakland, CA Washington, DC |
| Activities | Large group town hall discussions Small group breakout sessions Informative educational videos and presentations Question-and-answer opportunities Living with SCD creative collage activity Interactive “ideal” clinical trial journey mapping activity |
Methods
ASH RC and CISCRP employed principles of a community-engaged research approach using mixed methods to conduct 8 community-based workshops across the United States (Table 1).11 Community-engaged research principles emphasize the need to be clear about the purposes of engagement, establish relationships and partnerships, recognize and respect the engaged community, and permit flexibility to meet changing needs, among others.12,13 The workshops used an adapted community-based participatory research method, which has been shown to be an effective strategy for sensitive health topics and varied research objectives.14
Based on previous research showing that different age groups perceive health and clinical research differently depending on knowledge, prior experiences, and expectations,15 representatives from 4 distinct SCD community populations were recruited for participation. These populations were as follows: (1) parents of children living with SCD; (2) adolescents and teens with SCD (aged 13-17 years); (3) young adults living with SCD (aged 18-39 years); and (4) older adults with SCD (aged ≥40 years) (Table 1).
The 8 workshop locations were selected based on their sizable populations of people living with SCD and their geographic diversity. Organizers leveraged existing relationships with community partners and local medical providers to publicize the workshops. Outreach was conducted through dissemination of flyers via e-mail and surface mail, as well as through telephone calls to SCD community-based organizations (CBOs), faith-based organizations, minority-based organizations, hematologists, local and regional public health departments, infusion clinics and departments focused on benign hematology, and clinical researchers. CBOs, clinical research coordinators, and clinical staff assisted with recruitment of participants by distributing flyers and announcing the workshops at support group meetings and other SCD-focused meetings and events. Expanding outreach efforts to ensure that the workshop findings included the perspectives of a more ethnically diverse population was a priority. To address this goal, outreach was conducted with community organizations and individuals representing the Latinx, Middle Eastern, and Indian communities. Social media outreach on Facebook and Twitter consisted of both organic and paid postings to target populations in the 8 cities and surrounding locations.
To increase accessibility and reduce barriers to participation, incentives were provided, including transportation assistance (mass transportation, chartered buses, Lyft rides, and travel reimbursement), lunch, snacks, and free child care. Additional incentives included a $50 gift card and gift bags. Because those living with SCD often experience vaso-occlusive crises as a result of being cold and dehydrated, efforts were made to ensure that the venues were appropriately heated and resourced with hot beverages and blankets.
Eligibility and consent to participate
To ensure each workshop obtained feedback from the appropriate targeted audiences, potential participants were screened by CISCRP to determine their status, including: connection to SCD, age, sex, race, ethnicity, and prior participation in clinical research. This information was used to assign participants to the appropriate groups.
An institutional review board reviewed the project and deemed it exempt from the regulatory requirements. Nevertheless, consent was obtained from most participants via an online screening and registration tool. Parents and guardians of adolescents with SCD were asked to consent to their child’s participation. Walk-in registration was also made available, and those individuals were able to provide written informed consent.
Workshop activities and data collection
Workshops were conducted on weekends at a convenient time midday to avoid conflicts with school or work and to allow for adequate travel time for participants. At each workshop, experienced moderators conducted large town hall sessions and breakout sessions. Each workshop began with a review of meeting objectives and the agenda. Four CISCRP employees trained as moderators were at all sites except Washington, DC and Los Angeles (where there were 5), and 3 to 4 ASH RC employees were at each site.
Diverse workshop activities allowed for rapport to be built among the collective groups while still obtaining unique findings specific to different ages and SCD status. A collage activity was an effective and creative method of describing the participant journey in a visual manner and was effective at articulating emotional experiences. A mapping activity was designed to identify gaps in experiences and visualize the ideal clinical trial experience.
In addition to a note taker being present, sessions were digitally recorded. A semi-structured discussion and moderator guide was developed and used to facilitate activities at each workshop, with minor refinements made in the initial version based on feedback from early workshops. The moderator guide was developed to capture the following information from participants: goals for participating in the workshop, experiences living with SCD, attitudes toward their physician or hematologist, attitudes toward clinical trials, decision-making processes, reasons for participation/nonparticipation, clinical trial barriers, and recommendations to improve research participation.
Using instant participant feedback technology from polleverywhere.com, live polling of all participants was conducted for 3 questions chosen to best identify the priorities of those sampled in the SCD community and to inform future trials supported by ASH RC. Those questions are:
“Outside of a cure, what SCD symptoms would the ideal medication address (think about the symptom that bothers you the most)?” (Available choices were acute pain [SCD crisis], general pain, fatigue, organ damage, acute chest, mental health, avascular necrosis of the hip, age-associated complications, stroke and silent infarcts, hearing and vision loss, immune issues, and other.)
“Do you think that a cure for SCD should be the number one priority for researchers?” (Available choices were strongly agree, agree, disagree, and strongly disagree.)
“How willing would you be to share your personal medical information so that doctors can better understand SCD and improve health outcomes?” (Available choices were very willing, somewhat willing, not very willing, and not at all willing.)
Staff provided devices for use by attendees without access to smartphones or tablets who were interested in participating in the live polling exercise.
Large group town hall discussions included presentations on the clinical trial process and the stages of participation (ie, recruitment and enrollment, screening, informed consent process, treatment period, safety follow-up), with an opportunity for questions and answers. Participants were split into 2 breakout sessions according to their assignment to 1 of the 4 subgroups to discuss clinical research perceptions and to participate in mapping the ideal clinical trial experience.
Clinical research perceptions.
In the focus group breakout sessions, facilitators led discussions in response to the following questions:
What do you think of when you hear the term “clinical research”?
What do you know about clinical research?
What are the perceived risks/benefits?
Have you ever participated in a clinical trial?
Do you know of any clinical trials for SCD? If yes, where did you learn about this clinical trial? What are your general impressions of these trials?
Mapping the ideal clinical trial experience.
The facilitator led the group through a map that outlined the key steps in a participant’s clinical trial journey before, during, and after participation. An emphasis was placed on the “before participation” phase to identify initiatives that might best raise awareness of and inform the SCD community about clinical research.
Participants were asked how they would prefer to learn about clinical trials, from whom, and the types of information they need to make a decision about participation. During the clinical trial experience, participants were asked about their preferences for screening, providing informed consent, the amount and types of visits, and time between visits, as well as communication with study staff and their personal health-care provider. They were asked about their preferences after participation regarding follow-up, transition back to standard of care, and learning about the research results.
Based on their responses to these questions, maps were completed, and the facilitator asked the group to identify one ideal experience from each section of the journey map that they felt was most critical to the participant experience.
Analysis
Live polling was conducted for the 3 questions asked using smartphones and tablets and polleverywhere.com in the town hall discussion. However, live polling was not conducted at all sites. Responses from the breakout sessions were recorded and transcribed for qualitative analysis. Qualitative content analysis was used to identify themes and report results.16 Analyses were conducted by 2 readers based on recordings, notes, and transcripts. Because some questions were not relevant to all workshop attendees, the total number of responses varies; therefore, frequency counts differ. In addition, not all participants had access to smartphones or tablets, or they opted to forgo the polling exercise. These factors were also reflected in the polling response numbers. Descriptive statistics were used to summarize demographic and survey data.
Results
The outcomes of the workshops described in this report can be broadly categorized into 4 topics: addressing treatment needs, willingness to share medical information, awareness and perceptions of clinical research, and experiences living with SCD and implications for research. These themes are closely interrelated when considering clinical research participation and priorities. Demographic and other characteristics of those who registered for the workshops are presented in Table 2. Note that data are provided for registrants, not those who actually attended.
Workshop registrant characteristics (registrants, N = 589/attendees, N = 472)
| Characteristic . | Value . |
|---|---|
| Connection to SCD Adult with SCD Parent/guardian of someone with SCD Partner/spouse of someone with SCD | 308 (52%) 240 (41%) 41 (7%) |
| Adult living with SCD (n = 308) | |
| Female sex Race Black/African American White Asian Other Age “I have participated in a clinical trial” | 70% 94% 1% 1% 4% Median, 34 y; range, 18-78 y 132 (43%) |
| Parent/guardian of someone with SCD (n = 240) “My child has participated in a clinical trial” | 79 (33%) |
| Characteristic . | Value . |
|---|---|
| Connection to SCD Adult with SCD Parent/guardian of someone with SCD Partner/spouse of someone with SCD | 308 (52%) 240 (41%) 41 (7%) |
| Adult living with SCD (n = 308) | |
| Female sex Race Black/African American White Asian Other Age “I have participated in a clinical trial” | 70% 94% 1% 1% 4% Median, 34 y; range, 18-78 y 132 (43%) |
| Parent/guardian of someone with SCD (n = 240) “My child has participated in a clinical trial” | 79 (33%) |
Addressing treatment needs
Workshop participants reported factors that leave them dissatisfied with current treatment options, underscoring the need for novel and more effective therapies that are responsive to patient needs. Lack of access to treatment is a primary source of frustration. Many participants reported having access only to the treatments they can afford and that their insurance will cover. Furthermore, participants noted that although a potential cure for SCD is available (bone marrow/stem cell transplantation), it is an expensive and high-risk procedure and is only an option for a small proportion of the SCD population because donor matches are difficult to find.
Although individuals living with SCD experience similar symptoms, the severity and prevalence of these symptoms and the long-term complications of the disease vary by person. As such, treatment effective for one person may not work for another, and responses to different therapies may change over time. Participant-reported dissatisfaction with treatment is compounded by the length of time it can take to find one that works. Many felt there was a substantial amount of trial-and-error involved in finding the right medication. In addition, participants reported that treatment options are focused primarily on pain reduction. Persons with SCD and their families expressed concern that pain medications pose risks, especially when used regularly and for long periods of time. At the time of the workshops, crizanlizumab and voxelotor had not yet been approved by the US Food and Drug Administration (FDA); however, there was discussion of their potential use.
Participants also described the challenging relationship they have with pain medication, particularly the persistent risk of addiction or the development of tolerance to medications over time. Some participants also described experiencing stigma in seeking treatment during a painful crisis, with some even being turned away by clinic personnel who did not understand or appreciate the underlying cause of their pain. Further organ damage and long-term side effects resulting from the use of such treatments were also a major concern. Many felt research on the long-term implications of these treatments is limited and that better pain management options are needed. As a result, individuals living with SCD who attended the workshops reported a growing interest in preventing a crisis and managing their disease by modifying diet and lifestyle and would also prefer to seek alternative pain management options during a crisis rather than taking narcotics or other opiates.
The workshops offered an opportunity to survey the community about its most bothersome symptoms, whether a cure should be the top priority for research, and how willing participants were to share personal health information to further research and improve health outcomes. Of 262 participants at the workshop (including those living with SCD and their caregivers or parents) who responded to the live polling question about their top priority, a majority would like a cure (73% strongly agree; 18% agree). Those who did not agree (9% disagree; 1% strongly disagree) that finding a cure should be the top priority stated that there is a possibly more realistic opportunity to improve quality of life for people living with SCD by reducing the symptoms that bother them most, which should be a research priority. One participant mentioned, “We do want a cure, it should be a top priority, but there should be [clinical trial options] for things that make life better for us …” Some members of the community offered follow-up commentary stating that there should be more research opportunities available for those living longer with SCD, as current exclusion criteria prevent many otherwise healthy adults living with SCD from participating in clinical research, especially those studies with the potential to deliver curative therapies.
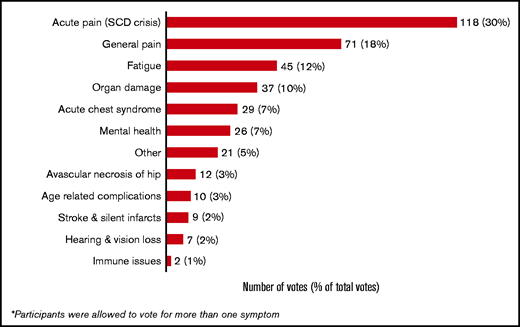
To query what mattered most to the workshop participants (those living with SCD and their caregivers or parents), the following question was posed: “Outside of a cure, what SCD symptoms would the ideal medication address? (think about the symptom that bothers you the most).” Of the 387 responses provided via a smartphone or tablet, nearly 30% (n = 118) indicated that acute pain/crisis was the most bothersome, followed by general pain (n = 70 [18%] and fatigue (n = 45 [12%]) (Figure 1).
Willingness to share medical information
In an attempt to further research and improve health outcomes for others with SCD, the majority of participants responding (133 of 203 [66%]) were very willing to share their personal medical information; 29% were somewhat willing. One participant said, “I’m very willing to share my information. I realized a long time ago and I’ve accepted that I’m not going through this for myself anymore. I’m going through this for someone else to have a better life.” Only 6% were not very willing or not at all willing to share their personal medical information.
Many hoped that sharing this information would allow researchers to look across a wide variety of SCD experiences and provide more insight into how the disease differentially affects individuals. However, among those who had reservations about sharing such information, the story of Henrietta Lacks and her immortalized cell line, the legacy of the Tuskegee Study of Untreated Syphilis in the African American Male, and other unfortunate historical events involving the mistreatment of minorities in research were cited as reasons for their hesitancy.
Regardless of how willing members of the community were to share medical information, transparency about the collection of information was critical. One participant said, “We need to have the company’s history, experience with sickle cell, their credibility and previous trials.” Before consenting to share information, participants said they would want to know who will have access to it, what it might be used for, why it was being collected, how it will be stored, and whether they had the right to withdraw their consent for such sharing. Being provided with the answers to these questions was cited as critical to building trust.
Awareness and perceptions of clinical research
Few workshop attendees were familiar with what is involved with clinical trial participation and the regulatory agencies involved in the research process. Many members of the SCD community had limited exposure to clinical research, had not discussed research participation with their health-care providers, or were hesitant to participate. One participant said, “I just thought clinical trials were for people with cancer, and that’s a lack of education on what clinical trials are, what they’re for, their purpose, and all of that.”
At each workshop, a few individuals reported participation in SCD clinical trials. Most recalled first learning about the opportunity from their physician or hematologist. A few participants shared that they had previously tried to enroll in clinical trials but were told they were not eligible. Some deemed eligible to participate mentioned that they had discussed enrolling with their health-care professional and were discouraged from participating. Also, several who had participated said that they never received any follow-up information or learned of the outcome of the trial.
Although many participants recognized the importance of clinical research in developing new treatments and stated that research offers a sense of hope, the fear of worsening symptoms and concerns around safety leave some wary of experimental interventions (Table 3). One participant said, “I’d rather battle sickle cell than battle sickle cell and whatever side effect comes from a clinical trial. I feel like clinical trials can help, but I don’t want to go through the uncertainty.”
Perceived benefits and risks of clinical trial participation as identified by workshop participants
| Benefits . | Risks . |
|---|---|
| Help future generations | Organ failure/damage |
| Feel better, alleviate pain, reduce symptoms | Death |
| Be part of the cure, work toward the cure | Pain crisis |
| Receive better health care during the study | Become sicker with medical complications |
| Compensation | Short- and long-term effects |
| Offers hope | Exploitation |
| Travel reimbursement | Need to take time off from work |
| Close monitoring of my condition | Invasive procedures |
| Gain access to new treatments, as available options for patients are currently limited | Poor communication, not being fully informed of purpose, side effects |
| Need to travel from home | |
| Receiving a placebo |
| Benefits . | Risks . |
|---|---|
| Help future generations | Organ failure/damage |
| Feel better, alleviate pain, reduce symptoms | Death |
| Be part of the cure, work toward the cure | Pain crisis |
| Receive better health care during the study | Become sicker with medical complications |
| Compensation | Short- and long-term effects |
| Offers hope | Exploitation |
| Travel reimbursement | Need to take time off from work |
| Close monitoring of my condition | Invasive procedures |
| Gain access to new treatments, as available options for patients are currently limited | Poor communication, not being fully informed of purpose, side effects |
| Need to travel from home | |
| Receiving a placebo |
Despite having reservations about volunteering for research, several participants expressed interest in learning more about current research and ways to become involved. As one adult person living with SCD said, “When you look back, the story of African Americans has been experimental. It is difficult for us to even want to trust a clinical trial after you’ve had bad experiences, but I’m still hopeful.” Some expressed concern that their guardedness might be interpreted as disinterest or unwillingness to enroll. During the workshops, the SCD community mapped out their “ideal” clinical trial journey by describing the experiences, information, and communication channels they would desire before, during, and after participating in a clinical trial. The results are presented in Table 4.
Ideal experiences, key information, and preferred communication methods before, during, and after clinical trial participation
| Timing . | Ideal experiences . | Key information . | Communication preferences . |
|---|---|---|---|
| Before participation | Learn about the trial from trusted source (eg, physician) Personal physician supports participation All study information accessible and in plain language Less strict eligibility criteria (especially regarding age) to allow more individuals to participate Time to consider participation Trial is sponsored or conducted by a trusted organization | All relevant information about the study intervention(s) (known benefits, side effects, method of administration) Source of research funding and its history with SCD Study protocol and policies (purpose, phase of the study, procedures involved, inclusion/exclusion criteria, volunteer expectations, duration of the trial, number of visits, compensation, reimbursed expenses) | Ability to speak with personal physician to discuss risks and benefits of the study before enrollment and with past participants about their previous experiences Connect with the study personnel by telephone and in-person to address questions or concerns Text messages, e-mails, telephone calls, and face-to-face conversations |
| During participation | Treated as a partner by study personnel Lodging and travel expenses covered if needed Study personnel keep their regular physician informed of their progress and health Participation has little to no impact on personal/day-to-day life Study clinic environments are welcoming and have amenities such as television and Wi-Fi Those enrolled can stay on their existing medication(s) Compensation provided throughout the study as opposed to just at the end Only minimally invasive procedures are required Access to mental health support, support groups, and other resources | Materials to explain the study to others Progress updates (individual and study) Who to contact with questions How patients will be kept safe must be made clear What data are being collected and what is being done with it What role they play in the process | Access to a 24/7 hotline for questions Meet regularly with the study physician as well as their personal physician Mobile apps and patient platforms could help communicate important information Text message reminders for appointments Visual aids to explain procedures Follow-up telephone calls from the study staff to check in |
| After participation | Free access to the drug (if study is a success) Access to long-term care and follow-up Trial sponsor re-invests in the SCD community by sponsoring events or offering scholarships Receive support during the transition to regular standard clinical care Access to counseling sessions Receive recognition for participation Results of the study are communicated to personal physician | Results of the study and personal results/progress in plain language Understand how their participation made a difference What was learned about any new side effects Drug approval status and next steps Information about future trial opportunities How privacy will be maintained in the future | Study results and personal results are e-mailed or sent via surface mail Facetime, in-person, and telephone call follow-ups Public service announcement or media broadcasts of results In-person meetings or conferences to learn more about results and connect with others |
| Timing . | Ideal experiences . | Key information . | Communication preferences . |
|---|---|---|---|
| Before participation | Learn about the trial from trusted source (eg, physician) Personal physician supports participation All study information accessible and in plain language Less strict eligibility criteria (especially regarding age) to allow more individuals to participate Time to consider participation Trial is sponsored or conducted by a trusted organization | All relevant information about the study intervention(s) (known benefits, side effects, method of administration) Source of research funding and its history with SCD Study protocol and policies (purpose, phase of the study, procedures involved, inclusion/exclusion criteria, volunteer expectations, duration of the trial, number of visits, compensation, reimbursed expenses) | Ability to speak with personal physician to discuss risks and benefits of the study before enrollment and with past participants about their previous experiences Connect with the study personnel by telephone and in-person to address questions or concerns Text messages, e-mails, telephone calls, and face-to-face conversations |
| During participation | Treated as a partner by study personnel Lodging and travel expenses covered if needed Study personnel keep their regular physician informed of their progress and health Participation has little to no impact on personal/day-to-day life Study clinic environments are welcoming and have amenities such as television and Wi-Fi Those enrolled can stay on their existing medication(s) Compensation provided throughout the study as opposed to just at the end Only minimally invasive procedures are required Access to mental health support, support groups, and other resources | Materials to explain the study to others Progress updates (individual and study) Who to contact with questions How patients will be kept safe must be made clear What data are being collected and what is being done with it What role they play in the process | Access to a 24/7 hotline for questions Meet regularly with the study physician as well as their personal physician Mobile apps and patient platforms could help communicate important information Text message reminders for appointments Visual aids to explain procedures Follow-up telephone calls from the study staff to check in |
| After participation | Free access to the drug (if study is a success) Access to long-term care and follow-up Trial sponsor re-invests in the SCD community by sponsoring events or offering scholarships Receive support during the transition to regular standard clinical care Access to counseling sessions Receive recognition for participation Results of the study are communicated to personal physician | Results of the study and personal results/progress in plain language Understand how their participation made a difference What was learned about any new side effects Drug approval status and next steps Information about future trial opportunities How privacy will be maintained in the future | Study results and personal results are e-mailed or sent via surface mail Facetime, in-person, and telephone call follow-ups Public service announcement or media broadcasts of results In-person meetings or conferences to learn more about results and connect with others |
Finally, a portion of the SCD community that remains active and interested in clinical research efforts reported “research fatigue,” in that they find themselves answering the same questions with the same answers every time they are asked to participate in focus groups or the initiation of a new clinical trial.
Experiences living with SCD and implications for research
Participants reported several ways in which SCD affects their lives, including anxiety, stress, depression, and financial burden. Participants also provided feedback on the wide array of physical symptoms they experience (Figure 1) for which they are seeking better or new treatments. Among the adults with SCD, some reported being negatively judged or even turned away when they were in the most critical need of care. They suggested that negative experiences in the clinical setting can affect their views of clinical research. One participant said, “If we are treated in a racist way in the emergency rooms, we begin to think that all of medical care is racist. That’s one of the big reasons I believe the attitude is you can’t trust those folks. Unless the big drug companies and pharmaceuticals can really focus on improving emergency room care, they’re going to have a hard time finding people to be part of their studies.”
Discussion
This effort to better understand the treatment and research needs of persons living with SCD and those who care for them included a diverse group of individuals: persons with SCD representing various disease genotypes and disease symptoms, ages, and sexes, and caregivers, parents, and family members of persons with SCD. Its focus was to examine the SCD community’s attitudes, beliefs, and barriers to and facilitators of clinical trial participation to better identify for clinicians and investigators where the needs are the greatest.
The workshops revealed both concerning and promising insights. First, lack of access to trusted information about clinical trials has left some individuals living with SCD with a limited understanding of the critical role research has in the development of new treatments and therapies.
Second, to effectively improve health outcomes for those living with SCD through research, it is crucial to first understand how living with this chronic illness affects all aspects of life. A seminal effort in 2014 through the FDA’s Patient-Focused Drug Development Initiative convened people living with SCD, caretakers, and other SCD community representatives to hear directly about their experiences with SCD and its treatment.17 The findings of that effort underscored the troubling symptoms of SCD, the complexity of treatment, and the challenges individuals living with SCD face in receiving needed care and support. These issues were also voiced in these workshops, as participants provided feedback on the emotional, social, and physical impacts of their disease. These reports, directly from the community, are instructive when developing new treatments or treatment approaches and setting research priorities.
Third, historical events, discrimination, and health inequities have been shown to be a major barrier to participation by African-American subjects in clinical research.18 A 2017 survey of minority populations regarding clinical trials found that lack of trust and information or awareness about clinical research lead to low levels of participation.19 However, despite these barriers, this survey also found a high level of willingness among minority populations to participate in research under certain conditions. The findings reported here are consistent with the 2017 survey results. Despite the distrust and fear many individuals living with SCD have about research and their frustration with duplicated efforts, there remains significant interest among the workshop attendees in becoming more informed and engaged in SCD research.
Consistent with findings in a Research!America survey20 and the FDA’s patient engagement efforts,17 participants in the workshops reported here overwhelmingly expressed support for and willingness to participate in clinical trials, recognizing that is how better treatments will emerge. However, they also expressed several conditions for such support, including the need to trust the source of information, the requirement for full disclosure and transparency, and concerns about risk and potential benefit. In contrast to what has been reported elsewhere, workshop participants did not express overwhelming distrust of clinical research. Notably, 66% of respondents said they would be willing to share personal medical information for research purposes. This aligns with the findings of Haywood et al,19 who surveyed attitudes toward clinical trials among people living with SCD. Their sample of people living with SCD expressed favorable attitudes about clinical trials, with 77% to 92% agreeing with a series of positive statements about clinical trials. The Research!America survey similarly found that 78% of the minority population surveyed would share such information.20
Also similar to the findings of the FDA and Research!America, respondents in the workshops reported here expressed concerns about the potential of receiving placebo instead of the experimental drug, safety, efficacy, reimbursements or compensation for time or injuries, and burden of participation.17,20 These are not necessarily unique concerns for persons contemplating clinical research participation and have been documented elsewhere. For example, Patterson et al21 studied factors involved in clinical trial decision-making in pediatric disease and found that concerns of potential harm most affected the decision to participate while secondary factors were potential benefit, study demands, and trust in the medical staff.
Importantly, workshop participants provided feedback on priority areas for research. Although a cure would be welcomed by all, many said that better treatments for the most troubling symptoms, primarily pain, are desperately needed, as are studies on the long-term impact of SCD as more persons living with the disease survive well into adulthood. Similar to the findings of the FDA, individuals living with SCD are seeking a more holistic approach to treatment as limited drug regimens do not always work and can cause downstream complications. Given dissatisfaction with currently available therapies, poor treatment often received in the ED, and a lack of adult health-care providers familiar with their disease, participants reported turning to alternative ways to manage their disease and symptoms.
With new attention and research funding being directed toward SCD, it is critical to ensure that these therapies are aligned with the needs and desired health outcomes of this community. Understanding the experiences of people living with SCD is imperative to informing and improving health outcomes. The workshops also emphasized the need to better educate the SCD community about clinical research as many have limited understanding of the clinical trial process and the regulatory agencies with oversight. More than one-half (57%) of adults and 67% of those responding on behalf of their child with SCD who attended the workshops reported never participating in clinical trials. This is despite there being, on average, >200 active or enrolling clinical trials for SCD listed with ClinicalTrials.gov at any given time. In comparison, 65% of the Research!America survey respondents reported never having participated.20 Increasing knowledge about what clinical research is, raising awareness of ongoing clinical trials, incorporating participant priorities into the research agenda, and building trust by listening to their needs and acknowledging painful events in history will help engage the community as a key stakeholder.
Limitations of this study include the inability of the sickest members of the community to travel and/or participate, no virtual participation was provided, and the workshop locations were in urban environments where access to health care might differ from that in more rural settings. As such, this population may not be representative of a more geographically dispersed population because the workshops were all held in major metropolitan areas, and transportation and awareness may have been contributing barriers to participation.
Other methodologic limitations should be noted. Some aspects of the workshops evolved as the team moved from city to city learning what contributed to the success of a workshop session and what could be improved. For example, child care was provided after a workshop was attended by participants who brought toddlers and small children and were then distracted and unable to fully engage. In addition, the workshops were conducted in an iterative process, where questions were modified in subsequent workshops based on lessons learned. However, the basic flow and substance of the agenda and questions were consistent. Finally, not all participants had access to smartphones or tablets for some activities, such as live polling, which limited full participation.
Regardless of these limitations, this effort builds on a few previous efforts to understand aspects of current treatment options that are most troubling for individuals living with SCD, identify barriers to clinical trial participation, and assess research priorities of the SCD community.
In conclusion, it is hoped that the insights participants provided will influence research priorities and improve research participation. These insights will be used as ASH RC launches its research network and will be provided as guidance to sponsors and researchers. The ASH RC will continue to engage those living with SCD and their support networks to update these findings and ensure that the voice of the community is incorporated into building and maintaining a network of clinical trials constructed with a shared vision for community-centric research.
Acknowledgments
The authors thank the members of the SCD community who gave their time and shared their thoughts and opinions during the workshops. They also thank the SCD CBOs, clinicians, faith-based organizations, minority-based organizations, local and regional public health departments, and clinical researchers who assisted with recruitment efforts. Special thanks to the CISCRP team, who spent numerous weekends with the authors as they traveled around the country for these workshops. Medical writing and editorial support were provided by Kathi E. Hanna, the contracted science writer.
The ASH RC Sickle Cell Disease Community Workshops were supported by the ASH RC Sickle Cell Disease Clinical Trials Network.
Authorship
Contribution: L.H.L. wrote the paper, contributed to research design, analyzed data, and performed research; L.H.W. performed research, wrote the paper, and contributed to research design; S.L. wrote the paper and analyzed data; and J.B. contributed to the paper, contributed to research design, analyzed data, and performed research. All listed authors have been involved in the writing of the manuscript and have read and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: LaShanta H. Whisenton, ASH Research Collaborative, 2021 L Street, NW, Suite 900, Washington, DC 20036; e-mail: swhisenton@ashrc.org.
References
Author notes
Requests for data sharing may be submitted to LaShanta H. Whisenton (swhisenton@ashrc.org).