Key Points
90Y-antiCD25 aTac-BEAM AHCT was safe and tolerable in patients with high-risk R/R HL.
aTac-BEAM AHCT was associated with favorable outcomes in high-risk R/R HL without the use of posttransplant consolidation.
Abstract
High-risk relapsed or refractory (R/R) classical Hodgkin lymphoma (HL) is associated with poor outcomes after conventional salvage therapy and autologous hematopoietic cell transplantation (AHCT). Post-AHCT consolidation with brentuximab vedotin (BV) improves progression-free survival (PFS), but with increasing use of BV early in the treatment course, the utility of consolidation is unclear. CD25 is often expressed on Reed-Sternberg cells and in the tumor microenvironment in HL, and we hypothesized that the addition of 90Y-antiCD25 (aTac) to carmustine, etoposide, cytarabine, melphalan (BEAM) AHCT would be safe and result in a transplantation platform that is agnostic to prior HL-directed therapy. Twenty-five patients with high-risk R/R HL were enrolled in this phase 1 dose-escalation trial of aTac-BEAM. Following an imaging dose of 111In-antiCD25, 2 patients had altered biodistribution, and a third developed an unrelated catheter-associated bacteremia; therefore, 22 patients ultimately received therapeutic 90Y-aTac-BEAM AHCT. No dose-limiting toxicities were observed, and 0.6 mCi/kg was deemed the recommended phase 2 dose, the dose at which the heart wall would not receive >2500 cGy. Toxicities and time to engraftment were similar to those observed with standard AHCT, though 95% of patients developed stomatitis (all grade 1-2 per Bearman toxicity scale). Seven relapses (32%) were observed, most commonly in patients with ≥3 risk factors. The estimated 5-year PFS and overall survival probabilities among 22 evaluable patients were 68% and 95%, respectively, and non-relapse mortality was 0%. aTac-BEAM AHCT was tolerable in patients with high-risk R/R HL, and we are further evaluating the efficacy of this approach in a phase 2 trial. This trial was registered at www.clinicaltrials.gov as #NCT01476839.
Introduction
Standard treatment for relapsed/refractory (R/R) classical Hodgkin lymphoma (HL) is salvage therapy followed by autologous hematopoietic cell transplantation (AHCT) in patients who are chemosensitive.1-3 While most patients are able to proceed to AHCT, less than half of patients with high-risk R/R HL achieve long-term durable remissions with the procedure.2 Efforts to improve outcomes in patients with R/R HL have focused on improving salvage regimens by intensifying chemotherapy,4-6 incorporating novel immunotherapies like brentuximab vedotin (BV) or anti-PD1 antibodies,7-12 or by using these novel agents as consolidation after AHCT.2,13 Although post-AHCT BV consolidation improves progression-free survival (PFS) in BV-naïve patients undergoing AHCT, with increasing use of BV and PD-1 blockade in frontline and salvage therapy,14-20 the utility of post-AHCT consolidation with these agents becomes less clear. Radiation therapy (RT) has a long-established track record as an effective treatment of HL and remains a key part of HL management.21 However, RT can be associated with toxicities and late effects.21,22 Radioimmunotherapy (RIT) is a targeted approach for delivery of RT that has been shown to be safe and effective as a treatment of non-Hodgkin lymphoma (NHL) and has been incorporated into AHCT conditioning regimens.23-34 CD25 is the interleukin-2 receptor α-subunit (IL-2αR) and is expressed on Reed-Sternberg (RS) cells and lymphocytes in the surrounding tumor microenvironment in HL. There have been several RIT trials evaluating radio-labeled antibodies directed against CD25 in patients with lymphoma, including HL, which have demonstrated tolerability and antitumor activity.35-38
We hypothesized that adding CD25-directed RIT into the AHCT conditioning regimen would be safe and allow the targeted delivery of a potent anti-HL therapy as part of a transplant approach that is agnostic to the prior HL-directed treatment. We also hypothesized that using RIT as part of the AHCT conditioning regimen may limit the potential risk of secondary myelodysplastic syndrome (MDS), as the bone marrow (BM) will be ablated after RIT and replaced with hematopoietic stem cells (HSC) collected prior to the radiation exposure. In this manuscript, we report the results of our phase 1 trial evaluating 90Y labeled anti-CD25 antibody combined with carmustine, etoposide, cytarabine, melphalan (aTac-BEAM) conditioning for AHCT in patients with R/R HL.
Methods
Patients
This was a single-center, phase 1, dose-escalation trial of aTac-BEAM 18 to 70 years and were required to have high-risk R/R HL, defined as one of the following: primary refractory HL, relapse within 12 months of completing frontline treatment, less than complete response (CR) to salvage therapy, or second or subsequent relapse regardless of disease status after salvage therapy. Additional criteria for inclusion were: Karnofsky performance status ≥70%, cardiac ejection fraction ≥50% by echocardiogram or multigated acquisition scan, FEV1 >65% of predicted or DLCO ≥50% of predicted, bilirubin ≤1.5x upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤2 x ULN unless due to liver involvement with HL, serum creatinine ≤1.5 mg/dL, and creatinine clearance ≥60 mL/min. Recovery from non-hematologic toxicities (to ≤grade 2 per CTCAE v4.0) related to salvage therapy was required. Patients were also required to have received at least 2 cycles of salvage therapy and have collected at least 3.0 × 106 CD34 cells/kg autologous hematopoietic progenitor cells by apheresis.
Patients with nodular lymphocyte predominant HL, HIV infection, active hepatitis B or C infection, or >10% persistent BM involvement by HL were ineligible. Patients with MDS or any cytogenetic abnormality in the BM known to be associated with MDS were also ineligible. Prior AHCT or allogeneic HCT was not allowed. Any significant external beam radiation to a critical organ, including >20 Gy to any portion of the lung, >5 Gy to any portion of the kidney, or any prior radiation to the heart rendered a patient ineligible. All patients provided informed consent for participation in the clinical trial. The study was approved by the City of Hope National Medical Center Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki. This trial was registered at www.clinicaltrials.gov as #NCT01476839.
Radiolabeling, biodistribution, dosimetry, and pharmacokinetics (PK)
The anti-CD25 monoclonal antibody, basiliximab, was conjugated with 1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) and radiolabeled with either 111In (111In-basiliximab/DOTA) for imaging/biodistribution assessment and/or with 90Y (90Y-basiliximab/DOTA) for therapeutic dosing (supplemental Methods).
On day −21, 5 mg unlabeled “cold” basiliximab was administered intravenously to block circulating soluble antigen and improve the radioimmunoconjugate biodistribution and tumor targeting of the radiolabeled basiliximab.37 Premedication with acetaminophen and diphenhydramine was given 30 to 60 minutes prior to the cold antibody infusion, and basiliximab was infused over 20 to 30 minutes within 1 to 2 hours prior to the administration of the radiolabeled antibody. Then, a day −21 imaging dose of 5 mCi of 111In-basiliximab/DOTA was administered for biodistribution assessment. Whole-body planar scans were acquired along with a calibrated source of 111In-basiliximab/DOTA placed adjacent to the patients’ legs for verification of scanner sensitivity at 2, 24, 48, 72 to 120, and 144 to 168 hours post-111In-basiliximab/DOTA injection. A single-photon emission computerized tomography (SPECT) scan was also acquired at the 48- or 72-hour time point. Organ dosimetry and biodistribution assessment criteria are described in the supplemental Methods.39,40 Patients with altered biodistribution were considered ineligible to receive the therapeutic 90Y-basiliximab/DOTA. The 111In-basiliximab/DOTA biodistribution data were used to estimate the 90Y-basiliximab/DOTA radiation dose to the red marrow and the residual activity in the body.41 Whole blood, serum, and urine samples were collected at pre-basiliximab (Simulect), pre-111In-basiliximab/DOTA, 2, 4 to 6 hours, days 1, 2, 3 to 4, 5, and 6 postadministration of 111In-basilixiamb/DOTA.
Study treatment and autologous hematopoietic cell transplantation (HCT)
On day −14, 5 mg of cold basiliximab was administered according to the same protocol and with the same premedication regimen as on day −21. The day −14 therapeutic 90Y-basiliximab/DOTA dose was then administered. Prior dosimetry studies of 90Y conjugated anti-CD2042-44 had established that a 90Y dose of 0.4 mCi/kg was the maximum tolerated dose (MTD) for marrow toxicity with predictable full marrow recovery and no organ radiation doses beyond safe levels. Therefore, the protocol initially evaluated 2 90Y-basiliximab/DOTA dose levels, 0.3 mCi/kg and 0.4 mCi/kg. However, after acceptable safety was established and no MTD was observed, 2 additional dose levels were added through a protocol amendment: 0.5 mCi/kg and 0.6 mCi/kg. The maximum total dose of 90Y-labeled radioimmunoconjugate was set at 40 mCi for the 0.4 mCi/kg dose level, 50 mCi for the 0.5 mCi/kg dose level, and 60 mCi for the 0.6 mCi/kg dose level.
BEAM was administered according to institutional practice. Carmustine 150 mg/m2 was administered intravenously on days −7 and −6 based on adjusted ideal body weight (IBW). Cytarabine and etoposide were administered twice daily on days −5, −4, −3, and −2 each at a dose of 100 mg/m2 IBW for a total of 8 doses. Melphalan 140 mg/m2 was administered on day −1. AHCT occurred on day 0, according to institutional practice. G-CSF was administered (5 μg/kg/d) starting on day +5 and continued daily until ANC >500 for 3 consecutive days. Supportive care, including premedications, antiemetics, and infectious prophylaxis, was administered according to institutional practices. Post-HCT consolidative radiation (20 Gy) was permitted at the discretion of the treating radiation oncologist and hematologist.
Study assessments and endpoints
Safety was monitored continuously, with toxicities assessed using the CTCAEv4 and the Bearman toxicity scale.45 The window for dose-limiting toxicity (DLT) assessment was day −21 through day +30 post-AHCT. The Bearman scale was used to define nonhematologic DLTs, and the CTCAE 4.0 scale was used to define hematological and infusion-related/hypersensitivity DLTs. Nonhematologic toxicity ≥grade 3, grade 4 neutropenia lasting beyond 42 days, infusion-related reaction grade ≥4, and any death were considered DLTs. BM biopsies for disease assessment and post-RIT cytogenetic assessment were performed at day +100, day +180, 1 year, and yearly until 5 years after AHCT. Anti-basiliximab antibodies were assessed at day +100, day +180, and 1 year after AHCT. Pulmonary function tests and cardiac echocardiogram or multigated acquisition scan were performed on day +100 and 1 year after AHCT. Positron emission tomography-computed tomography (PET-CT) scans were performed at day +30, day +100, day +180, 1 year, and yearly until 5 years after AHCT. After CR was documented, either PET-CT or computed tomography (CT) were allowed to assess disease response. Response assessment was performed by investigators according to the 2007 Revised Response Criteria for Malignant Lymphoma.46 The primary endpoints were safety, feasibility, and selection of the recommended phase 2 dose (RP2D) of aTac-BEAM AHCT. Secondary endpoints included time to neutrophil and platelet engraftment, as well as estimates of overall response rate after AHCT, PFS, overall survival (OS), cumulative incidence of nonrelapse mortality (NRM), and relapse-progression (CIR). Estimation of the radiation doses to the whole body and organs through serial imaging studies and definition of the biodistribution/extended PK of 111In-basiliximab/DOTA and 90Y- basiliximab/DOTA including terminal elimination, serum half-life (t1/2), and area under the curve (AUC) were also secondary endpoints.
Correlative studies
Immunohistochemistry for CD25 (cat# 760-4439, clone 4C9; Cell Marque) was performed on 3 to 4 micron sections of formalin-fixed, paraffin-embedded tissue and stained on the Ventana Discovery XT platform (Ventana, Tucson, AZ). Tumor cells were scored 0, 1, or 2 if 0%, 1% to 49%, or 50% or more were RS cells, respectively, in a high-powered field (HPF) demonstrated CD25 expression. Lymphocytes in the tumor microenvironment (TME) were scored 0, 1, or 2 if 0%, 1% to 9%, or 10% or more lymphocytes, respectively, in a HPF expressed CD25.
Statistical considerations
This phase 1 study assessed 4 dose levels: level 1, 0.3 mCi/kg; level 2, 0.4 mCi/kg; level 3, 0.5 mCi/kg; and level 4, 0.6 mCi/kg. Escalation was limited to 0.6 mCi/kg, the dose estimated by our radiophysicist to be the highest dose that would deliver less than the maximum allowable absorbed radiation to the heart. Thus, we did not seek to find the MTD of 90Y-basiliximab/DOTA, but rather determine the higher of the 4 doses with an acceptable toxicity profile and at which the heart wall would receive no more than 2500 cGy. Survival estimates were calculated based on the Kaplan-Meier product-limit method, and 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate. Cumulative incidence curves were generated for NRM and CIR in the competing risk setting, given that death and relapse/progression events were in competition. All calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC) or R v3.6.3. Trial data were locked for analysis on 09/28/2020.
Trial design
Initially, the study followed a standard 3 + 3 phase 1 dose-escalation design. However, with the addition of dose levels 3 and 4, the trial was amended to employ a modified rolling 6 design (see supplemental Methods).47 Like the standard 3 + 3 design, under this design, at most, 3 patients were under observation for DLT on dose level under evaluation at any time. Patients who were not evaluable for DLT were replaced. Intrapatient dose escalation was not permitted.
Patients who did not receive therapeutic aTac-BEAM due to altered biodistribution were replaced but monitored for adverse events (AEs) for a minimum of 30 days after the imaging/biodistribution dose or until resolution of study intervention-related AE.
Results
Patients
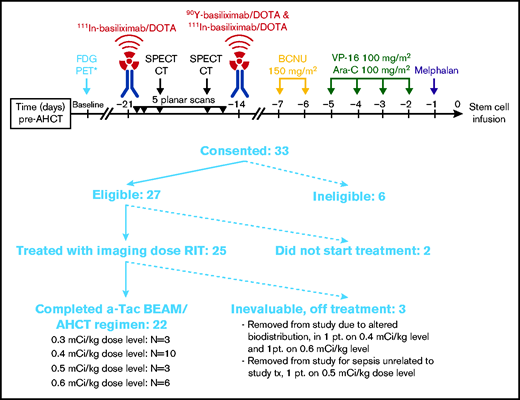
Twenty-five patients were enrolled in the study between February 2013 and September 2018 (Figure 1). All 25 patients received an imaging dose of cold basiliximab and 111In-basiliximab-DOTA. Two patients had altered biodistribution and did not proceed to receive a therapeutic dose of 90Y-basiliximab-DOTA. One patient developed catheter-associated gram-negative rod sepsis unrelated to study intervention and received only the imaging dose and not the therapeutic dose to minimize further delay in transplantation. A total of 22 patients received the therapeutic dose of 90Y-basiliximab-DOTA and proceeded to aTac-BEAM AHCT. Baseline characteristics of the 22 evaluable patients are provided in Table 1. The most common salvage therapies (supplemental Table 1) were BV monotherapy (13/22, 59%) and ifosfamide, carboplatin, and etoposide (ICE, 10/22, 45%). In total, 17 (77%) patients had prior BV exposure, and 4 (18%) had received prior PD-1 blockade (all nivolumab). Sixteen (73%) patients were in CR at the time of AHCT. The median size of the largest residual mass at AHCT in patients not in CR was 2 cm (range, 0.8-2.5 cm). At the time of AHCT, all patients would have met the eligibility criteria for the AETHERA trial (all either primary refractory or early relapse). Including the expanded set of AETHERA risk factors (ie, primary refractory HL or relapse within 1 year of completing initial therapy, extranodal disease at relapse, B symptoms at relapse, receiving >1 salvage regimen, or less than CR at HCT), there were 5 (23%) patients with 1 risk factor, 10 (45%) patients with 2 risk factors, 6 (27%) patients with 3 risk factors, and 1 (5%) patient with 4 risk factors. In total, 17 (77%) patients had ≥2 risk factors, and 7 (32%) patients had ≥3 risk factors.
Baseline characteristics
| Characteristic . | n (%), median (or range) . |
|---|---|
| Gender | |
| Female | 11 (50) |
| Male | 11 (50) |
| Age at transplant in years (range) | 34 (19-60) |
| Race/ethnicity | |
| Hispanic | 8 (36) |
| White | 10 (77) |
| Asian | 2 (9) |
| Black | 2 (9) |
| Stage at diagnosis | |
| I-II | 11 (50) |
| III-IV | 11 (50) |
| Disease status at transplant | |
| CR | 16 (73) |
| PR | 5 (23) |
| PD | 1 (5) |
| More than 1 line of salvage therapy | 9 (41) |
| Primary refractory to frontline therapy | 15 (68) |
| Relapse after frontline therapy | 7 (32) |
| ≤1 y | 7 |
| >1 y | 0 |
| Stage at relapse | |
| I-II | 15 (68) |
| III-IV | 7 (32) |
| B symptoms at relapse | 3 (14) |
| Extranodal disease at relapse | 8 (36) |
| Median number of high-risk factors | 2 (1-4) |
| 1 | 5 (23) |
| 2 | 10 (45) |
| 3 | 6 (27) |
| 4 | 1 (5) |
| Prior BV | 17 (77) |
| Prior PD-1 blockade | 4 (18) |
| Prior radiation therapy | 3 (14) |
| Characteristic . | n (%), median (or range) . |
|---|---|
| Gender | |
| Female | 11 (50) |
| Male | 11 (50) |
| Age at transplant in years (range) | 34 (19-60) |
| Race/ethnicity | |
| Hispanic | 8 (36) |
| White | 10 (77) |
| Asian | 2 (9) |
| Black | 2 (9) |
| Stage at diagnosis | |
| I-II | 11 (50) |
| III-IV | 11 (50) |
| Disease status at transplant | |
| CR | 16 (73) |
| PR | 5 (23) |
| PD | 1 (5) |
| More than 1 line of salvage therapy | 9 (41) |
| Primary refractory to frontline therapy | 15 (68) |
| Relapse after frontline therapy | 7 (32) |
| ≤1 y | 7 |
| >1 y | 0 |
| Stage at relapse | |
| I-II | 15 (68) |
| III-IV | 7 (32) |
| B symptoms at relapse | 3 (14) |
| Extranodal disease at relapse | 8 (36) |
| Median number of high-risk factors | 2 (1-4) |
| 1 | 5 (23) |
| 2 | 10 (45) |
| 3 | 6 (27) |
| 4 | 1 (5) |
| Prior BV | 17 (77) |
| Prior PD-1 blockade | 4 (18) |
| Prior radiation therapy | 3 (14) |
BV, brentuximab vedotin; CR, complete response; PD, progressive disease; PD1, programmed death receptor-1; PR, partial response.
Treatment disposition and safety
Of the 22 patients who received a therapeutic dose of 90Y-basiliximab-DOTA, there were 3 patients treated at a dose of 0.3 mCi/kg, 10 patients treated at 0.4 mCi/kg (the highest dose in the initial design of the trial), 3 patients treated at 0.5 mCi/kg, and 6 patients treated at 0.6 mCi/kg (Figure 1). Administered activity ranged from 23.8 to 60 mCi. No DLTs were observed at any dose level, and 0.6 mCi/kg was determined to be the RP2D. No patients experienced graft failure. The median time to neutrophil engraftment was 10 days (range, 9-14 days), and the median time to platelet engraftment was 12 days (range, 9-21 days) (supplemental Table 2). One patient received subsequent post-AHCT radiation therapy.
There were no clinically meaningful differences in the AEs observed among patients treated at the different dose levels or in the patient who received post-AHCT radiation. Treatment-related AEs are summarized according to the Bearman Toxicity Criteria in Table 2 and the CTCAE v4.0 in supplemental Table 3. Per the Bearman scale, all patients experienced at least 1 grade ≥1 AE; grade 3 to 4 AEs were not observed. The most common toxicities (Bearman scale, all grades) were: 21 stomatitis (95%), 6 GI (27%), and 5 hepatic (23%). Per the CTCAE v4.0, the most common nonhematologic AEs observed (all grades) were fatigue, hyponatremia, anemia, neutropenia, leukopenia, lymphopenia, thrombocytopenia, nausea, and vomiting, which were experienced by all patients. The most common CTCAE v4.0 grade ≥3 AEs were: lymphopenia (100%), neutropenia (100%), thrombocytopenia (100%), leukopenia (100%), anemia (86%), febrile neutropenia (59%), and oral mucositis (41%) (supplemental Table 4).
Adverse events per Bearman scale
| . | Grade . | |||||||
|---|---|---|---|---|---|---|---|---|
| DL1: 0.3 mCi/kg (n = 3) . | DL2: 0.4 mCi/kg (n = 10) . | DL3: 0.5 mCi/kg (n = 3) . | DL4: 0.6 mCi/kg (n = 6) . | |||||
| Organ | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Bladder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Central nervous system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 0 |
| Hepatic | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Pulmonary | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| Renal | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Stomatitis | 3 | 0 | 4 | 5 | 3 | 0 | 3 | 3 |
| . | Grade . | |||||||
|---|---|---|---|---|---|---|---|---|
| DL1: 0.3 mCi/kg (n = 3) . | DL2: 0.4 mCi/kg (n = 10) . | DL3: 0.5 mCi/kg (n = 3) . | DL4: 0.6 mCi/kg (n = 6) . | |||||
| Organ | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Bladder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Central nervous system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 0 |
| Hepatic | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Pulmonary | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| Renal | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Stomatitis | 3 | 0 | 4 | 5 | 3 | 0 | 3 | 3 |
DL, dose level.
Between baseline and 1-year post-AHCT, there were no appreciable differences in cardiac or pulmonary parameters (supplemental Table 5). At 3-years post-AHCT, a cytogenetic abnormality ([46,XY,del20 (q11.2q13.3)]) was detected in 1 patient without evidence of MDS or other abnormality observed in the BM. This abnormality persisted in years 4 and 5. At the time of last follow-up, no patients had developed MDS or acute leukemia (AL). Two patients developed secondary malignancies. One patient developed diffuse large B-cell lymphoma 35 months after AHCT, and another developed a tonsillar squamous cell carcinoma 3 months after AHCT. The only other severe AEs in patients who received a therapeutic dose of 90Y-basiliximab-DOTA were: a grade 3 lung infection in 1 patient that occurred 7 months after AHCT, a readmission for grade 1 fever in another patient, and a grade 2 nausea on day +28 after AHCT. There were no other notable toxicities, and no cardiac toxicities or veno-occlusive disease was observed. There were no treatment-related deaths.
Outcomes
Following aTac-BEAM AHCT, the best overall response and CR rates among evaluable patients were 95% (21/22) and 91% (20/22), respectively. Seven (32%) patients relapsed at a median of 3.7 months (range, 2.7-11.3) after AHCT. There were 4 relapses among the 16 evaluable patients in CR at the time of AHCT and 3 relapses among 6 patients who were not in CR at the time of AHCT. There was 1 relapse among the 5 patients with 1 high-risk factor, 2 relapse events among the 10 patients with 2 risk factors, and 4 relapses among the 7 patients with ≥3 risk factors (Table 3). The median follow-up time in surviving patients was 59.2 months (range, 18.3-65.7 months). Among the 22 evaluable patients who received a therapeutic dose of 90Y-basiliximab-DOTA, the estimated 2- and 5-year PFS and OS probabilities were 68% (95% CI: 45-83) and 95% (95% CI: 69-99), respectively. The NRM was 0% at 5 years, and the cumulative incidence of relapse was 32% (95% CI: 17-59) at 2 years and 5 years. At the time of analysis, 1 patient had died due to disease progression 24.4 months after AHCT.
Patient summary
| UPN . | Dose level (mCi/kg) . | Age at HCT . | Frontline status . | Prior lines . | Disease status at HCT . | Risk factors . | Response after HCT . | CD25 expression score . | Current status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.3 | 60 | Primary ref | 2 | PD | 3 | PR | RS: 0 Lymph: 2 | Relapsed at day +100, still alive ∼5 y after HCT |
| 2 | 0.3 | 24 | Primary ref | 2 | CR | 1 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 3 | 0.3 | 37 | Primary ref | 3 | PR | 4 | CR | RS: 2 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 4 | 0.4 | 35 | Primary ref | 3 | CR | 2 | CR | RS: NA Lymph: NA | Alive without relapse ∼5 y after HCT |
| 5 | 0.4 | 60 | Primary ref | 2 | CR | 2 | CR | RS: 2 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 6 | 0.4 | 40 | Early relapse | 2 | CR | 1 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 7 | 0.4 | 42 | Early relapse | 2 | CR | 1 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 8 | 0.4 | 32 | Primary ref | 3 | CR | 3 | CR | RS: 1 Lymph: 2 | Relapsed at day +144, died of HL ∼2 y after HCT |
| 9 | 0.4 | 32 | Primary ref | 3 | PR | 4 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 10 | 0.4 | 28 | Primary ref | 2 | CR | 2 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 11 | 0.4 | 26 | Primary ref | 2 | PR | 2 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 12 | 0.4 | 19 | Primary ref | 3 | CR | 3 | CR | RS: 0 Lymph: 2 | Post-HCT radiation, Relapsed at day +90, still alive ∼5 y after HCT |
| 13 | 0.4 | 33 | Early relapse | 2 | CR | 1 | CR | RS: 0 Lymph: 2 | Relapsed at day +262, still alive ∼5 y after HCT |
| 14 | 0.5 | 24 | Early relapse | 2 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 15 | 0.5 | 38 | Primary ref | 3 | CR | 2 | CR | RS: NA Lymph: NA | Alive without relapse ∼3.5 y after HCT |
| 16 | 0.5 | 32 | Primary ref | 3 | PR | 3 | CR | RS: 1 Lymph: 2 | Relapsed at day +114, still alive ∼3 y after HCT |
| 17 | 0.6 | 42 | Early relapse | 2 | CR | 1 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼3.5 y after HCT |
| 18 | 0.6 | 40 | Early relapse | 2 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼3.5 y after HCT |
| 19 | 0.6 | 21 | Primary ref | 2 | CR | 2 | CR | RS: NA Lymph: NA | Relapsed at day +345, still alive ∼3 y after HCT |
| 20 | 0.6 | 22 | Primary ref | 2 | PR | 2 | PR | RS: 2 Lymph: 1 | Relapsed at day +81, still alive ∼3 y after HCT |
| 21 | 0.6 | 49 | Primary ref | 3 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼2 y after HCT |
| 22 | 0.6 | 44 | Early relapse | 3 | CR | 3 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼1.5 y after HCT |
| UPN . | Dose level (mCi/kg) . | Age at HCT . | Frontline status . | Prior lines . | Disease status at HCT . | Risk factors . | Response after HCT . | CD25 expression score . | Current status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.3 | 60 | Primary ref | 2 | PD | 3 | PR | RS: 0 Lymph: 2 | Relapsed at day +100, still alive ∼5 y after HCT |
| 2 | 0.3 | 24 | Primary ref | 2 | CR | 1 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 3 | 0.3 | 37 | Primary ref | 3 | PR | 4 | CR | RS: 2 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 4 | 0.4 | 35 | Primary ref | 3 | CR | 2 | CR | RS: NA Lymph: NA | Alive without relapse ∼5 y after HCT |
| 5 | 0.4 | 60 | Primary ref | 2 | CR | 2 | CR | RS: 2 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 6 | 0.4 | 40 | Early relapse | 2 | CR | 1 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 7 | 0.4 | 42 | Early relapse | 2 | CR | 1 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 8 | 0.4 | 32 | Primary ref | 3 | CR | 3 | CR | RS: 1 Lymph: 2 | Relapsed at day +144, died of HL ∼2 y after HCT |
| 9 | 0.4 | 32 | Primary ref | 3 | PR | 4 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 10 | 0.4 | 28 | Primary ref | 2 | CR | 2 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 11 | 0.4 | 26 | Primary ref | 2 | PR | 2 | CR | RS: 2 Lymph: 1 | Alive without relapse ∼5 y after HCT |
| 12 | 0.4 | 19 | Primary ref | 3 | CR | 3 | CR | RS: 0 Lymph: 2 | Post-HCT radiation, Relapsed at day +90, still alive ∼5 y after HCT |
| 13 | 0.4 | 33 | Early relapse | 2 | CR | 1 | CR | RS: 0 Lymph: 2 | Relapsed at day +262, still alive ∼5 y after HCT |
| 14 | 0.5 | 24 | Early relapse | 2 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼5 y after HCT |
| 15 | 0.5 | 38 | Primary ref | 3 | CR | 2 | CR | RS: NA Lymph: NA | Alive without relapse ∼3.5 y after HCT |
| 16 | 0.5 | 32 | Primary ref | 3 | PR | 3 | CR | RS: 1 Lymph: 2 | Relapsed at day +114, still alive ∼3 y after HCT |
| 17 | 0.6 | 42 | Early relapse | 2 | CR | 1 | CR | RS: 1 Lymph: 2 | Alive without relapse ∼3.5 y after HCT |
| 18 | 0.6 | 40 | Early relapse | 2 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼3.5 y after HCT |
| 19 | 0.6 | 21 | Primary ref | 2 | CR | 2 | CR | RS: NA Lymph: NA | Relapsed at day +345, still alive ∼3 y after HCT |
| 20 | 0.6 | 22 | Primary ref | 2 | PR | 2 | PR | RS: 2 Lymph: 1 | Relapsed at day +81, still alive ∼3 y after HCT |
| 21 | 0.6 | 49 | Primary ref | 3 | CR | 2 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼2 y after HCT |
| 22 | 0.6 | 44 | Early relapse | 3 | CR | 3 | CR | RS: 0 Lymph: 2 | Alive without relapse ∼1.5 y after HCT |
CR, complete response; Lymph, lymphocytes; PD, progressive disease; PR, partial response; Primary ref, primary refractory; SD, stable disease; UPN, unique patient number.
Biodistribution, dosimetry, and PK studies
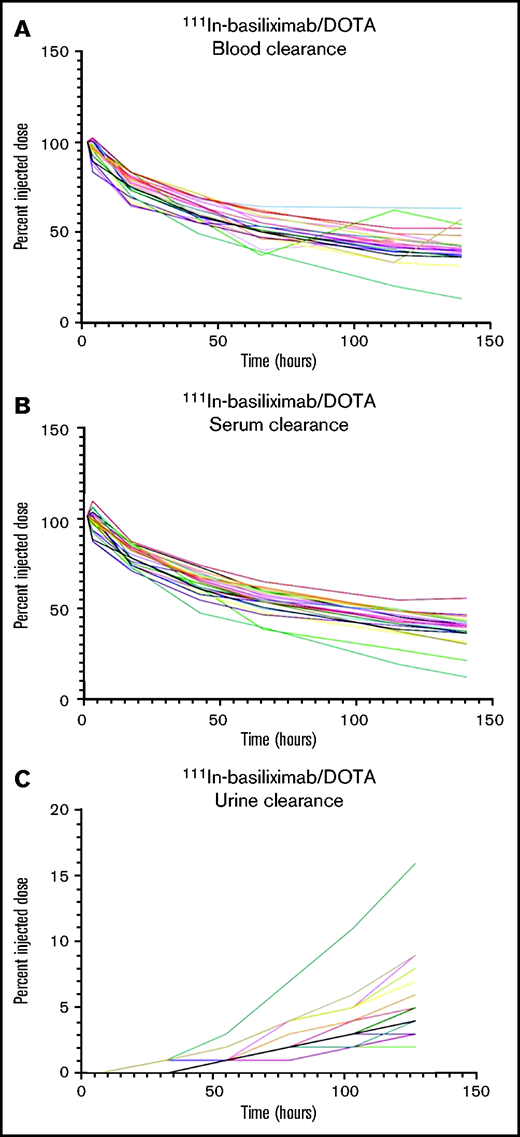
Twenty-five patients received an imaging dose of cold basiliximab and 111In-basiliximab-DOTA. Two patients had altered biodistribution and did not proceed to receive a therapeutic dose. One of those patients had increased blood pool activity in their 144-hour 111In scan, and 1 patient had increased left posterior-lung activity compared with the heart. Organ dosimetry was evaluated for 3 patients treated at each dose level and the 2 patients who had altered imaging biodistribution. Cumulative absorbed doses to key organs from the 3 patients analyzed at each therapeutic dose level are shown in supplemental Table 6. The patients with altered biodistribution would not have had significantly higher organ doses of 90Y compared with the average dose across all patients evaluated. For the 2 patients with altered biodistribution, the heart wall and the spleen received the highest doses: 11.6 and 9.6 mGy/MBq (heart wall) and 8.6 and 9.9 mGy/MBq (spleen), which were not appreciably different than the average for all patients of 10.3 ± 1.7 mGy/MBq in the heart wall and 13.1 ± 4.3 mGy/MBq in the spleen. The lungs of the 2 patients with altered biodistribution received 6.1 and 5.2 mGy/MBq while the average dose for all patients on the 0.6 mCi/kg dose was 6.3 ± 1.4 mGy/MBq, and their red marrow dose was 1.0 and 0.9 mGy/MBq, which was similar to the average dose for patients on all dose levels (0.9 ± 0.1 mGy/MBq). We examined plasma half-life of basiliximab in the two-compartment model for all dose levels: the mean ± standard deviation T1/2 for the α and β phases was 13.2 ± 13.9 hours and 168.7 ± 118.3 hours, respectively. PK curves of the 111In-basiliximab clearance in the blood and serum and accumulation of 111In-basiliximab in the urine are shown in Figure 2. No delayed blood or serum clearance or increased urine accumulation was observed.
PK of 111In-basiliximab/DOTA in all patients. (A) Clearance of 111In-basiliximab/DOTA from blood over time. (B) Clearance of 111In-basiliximab/DOTA from serum over time. (C) Clearance of 111In-basiliximab/DOTA from urine over time.
PK of 111In-basiliximab/DOTA in all patients. (A) Clearance of 111In-basiliximab/DOTA from blood over time. (B) Clearance of 111In-basiliximab/DOTA from serum over time. (C) Clearance of 111In-basiliximab/DOTA from urine over time.
Biodistribution of 111In-basiliximab/DOTA at 144 hours in UPN-1 (left) and 18F-FDG-PET (right).
Biodistribution of 111In-basiliximab/DOTA at 144 hours in UPN-1 (left) and 18F-FDG-PET (right).
Correlative studies
Nineteen patients had tumor tissue evaluable for CD25 expression by immunohistochemistry. Eleven (58%) patients expressed CD25 on RS cells, with 6 (32%) patients exhibiting CD25 expression on >50% of tumor cells. CD25 was expressed on lymphocytes in the tumor microenvironment in all evaluable patients, with CD25 expression on >10% of lymphocytes in 15 (79%) patients.
Discussion
The addition of CD25-directed RIT to BEAM AHCT, aTac-BEAM, was safe and tolerable in patients with high-risk R/R HL. The dose of radiation was escalated safely to 0.6 mCi/kg without DLT, and the toxicity profile was similar to standard BEAM AHCT. As was seen with RIT-based AHCT conditioning in NHL, the rate of mucositis seemed higher than standard BEAM, although the number of patients treated at the RP2D was small, and this requires confirmation.33 Although the phase 1 nature of our study with a small number of patients and the heterogeneity of CD25 RIT dosing precludes definitive conclusions, the efficacy we observed was promising in patients with high-risk HL who received no posttransplantation consolidation.
Modern efforts to improve outcomes in patients with R/R HL have primarily focused on pretransplant salvage therapy, posttransplant consolidation, or novel therapies for patients with relapse after AHCT. Novel combination chemotherapy salvage regimens and incorporation of BV and PD-1 blockade have yielded highly active salvage regimens that produce CR and bridge a majority of patients to AHCT.7-9,11,12 Despite these advances, a sizable proportion of patients with high-risk HL will relapse after AHCT. Post-AHCT BV consolidation is associated with improved PFS in patients with high-risk R/R HL,2 but was only studied in patients who were naïve to BV. The use of BV as part of frontline or salvage therapy is increasingly common, and BV consolidation is of unclear utility in patients with prior BV exposure. Furthermore, BV consolidation is a long course of therapy associated with toxicities that often lead to early discontinuation of therapy and can impact quality of life.48,49 PD-1 blockade has also been studied as part of post-AHCT consolidation with promising efficacy, but requires further study and may result in additional inconvenience and toxicities, just as in BV consolidation.13
We sought to improve on the AHCT conditioning regimen by incorporating RIT directed against CD25, which can be present on RS cells and lymphocytes in the HL TME. The appeal of this approach is that it is agnostic to the patient’s prior therapies, in contrast to BV or PD-1 blockade as part of salvage or consolidation therapy. Admittedly, this approach is not applicable when patients have received higher doses of radiation, which is a key component of frontline therapy for patients with early-stage HL. However, many patients with HL will have advanced-stage disease, and most would not receive frontline radiotherapy. In the evolving landscape of HL therapy, PET-adapted chemotherapy approaches and regimens that incorporate BV and PD-1 blockade often omit radiotherapy. Thus, an increasing proportion of patients who would be eligible for radiation do not receive it in the modern era. HL is an exquisitely radiosensitive tumor, and thus aTac-BEAM is a potential way to incorporate more “systemic” radiotherapy in patients with high-risk R/R HL. An improved AHCT conditioning regimen could potentially abrogate the need for costly and potentially toxic post-AHCT consolidation therapies. Other groups have also studied modifications to AHCT conditioning for lymphoma, including anti-CD45 RIT,50,51 novel chemotherapy combinations inclusive of alternative chemotherapies (eg, gemcitabine, vinorelbine, or bendamustine), or inclusion of novel drugs like histone deacteylase inhibitors or hypomethylating agents.52-56 Although these single-center approaches have demonstrated promising results, none have been widely adopted. In patients with B-cell NHL, CD20-directed RIT was evaluated as part of AHCT conditioning with acceptable, albeit increased, toxicity. A randomized phase 3 study evaluating CD20-RIT AHCT in patients with R/R diffuse large B-cell lymphoma showed no improvement in outcomes compared with rituximab-BEAM, but patients in both study arms received CD20-directed therapy (either RIT or rituximab) as part of transplant conditioning.33 An RIT conditioning approach may be useful in patients with HL, a disease in which CD25 is not targeted with standard therapies. Targeting CD25 with RIT can deliver not only targeted radiation to the tumor but also to CD25+ immune cells in the HL TME, which may disrupt the complex feedback signaling in the TME that promotes HL survival.
Intended to evaluate the safety and feasibility of our CD25-RIT AHCT approach, our study has inherent limitations. It evaluated a small number of patients treated at various dose levels of radiation, with only 6 patients treated at the RP2D. Our results require confirmation, and a larger phase 2 study evaluating this approach in patients with high-risk R/R HL is ongoing at our institution. Many patients enrolled in the study received BV monotherapy as opposed to the more active BV-based combination regimens as their first salvage treatment, which is not a commonly used approach and “added” a risk factor in some patients who required second salvage before transplantation. The incorporation of novel agents in HL therapy is rapidly evolving, and since all patients had primary refractory HL or early relapse with a majority having 2 risk factors independent of the number of prior therapies, the cohort remains a representative high-risk cohort. A majority of patients were in PET-based CR at the time of AHCT, an important prognostic factor for outcome, and suggests that outcomes may have been favorable with a standard approach. Given the small number of patients and multiple dose levels in this phase 1 study designed to evaluate safety, we are unable to draw conclusions about the efficacy or the contribution of the anti-CD25 RIT to the outcomes. Similarly, it was also not possible to draw conclusions from the CD25 expression data, especially since there were similar degrees of CD25 expression across the cohort and among patients who remained in durable remission. Evaluation of late effects after RIT is an important part of evaluating safety, and although the number of patients is small, the median follow-up time is 5 years, and we have observed abnormal cytogenetics in only 1 patient with no secondary MDS or leukemia events. In this phase 1 study, we infused an imaging dose of 111In-basiliximab/DOTA with multiple whole-body planar scans to carefully evaluate the biodistribution and organ doses of radiation to critical organs. This introduced additional time into the transplantation process compared with standard AHCT. Based on the favorable dosimetry observed in this study, we have removed a week from the phase 2 trial regimen by administration of imaging and therapeutic RIT doses on the same day. Finally, the administration of RIT requires that a site have radiopharmaceutical expertise and is relatively labor-intensive compared with standard AHCT. The prior experience with RIT AHCT in B-cell NHL, in which a randomized phase 3 multicenter study was performed successfully, suggests that an RIT AHCT approach could be generalizable in HL if it proves to be effective.
A potential future direction for anti-CD25 RIT could be labeling with α-emitters, which have higher linear energy transfer characteristics and shorter particle path length; therefore, delivering a more concentrated and targeted radiation dose and resulting in more effective tumor cell kill. Encouraging preclinical and early clinical results have been reported with antibodies (including anti-CD25) labeled with α-emitters as well as with targeted small molecules in patients with a range of malignancies, including lymphoma.57-78
In conclusion, aTAC-BEAM was found to be a safe and tolerable AHCT regimen that allowed delivery of targeted RIT as part of transplantation conditioning in patients with high-risk R/R HL. aTAC-BEAM was associated with promising post-AHCT outcomes in our cohort of patients with high-risk R/R HL without the requirement for additional posttransplant consolidation. The safety and preliminary efficacy we observed support further exploration of the aTAC-BEAM conditioning regimen; we are currently conducting a phase 2 trial (ClinicalTrials.gov Identifier: NCT04871607) to evaluate the efficacy of this approach.
Acknowledgments
The authors would like to acknowledge Andrew Raubitschek’s contributions to the development and design of the research, including his leadership with the City of Hope Division of Cancer Immunotherapeutics and Tumor Immunology until his retirement and his key role in initiating this clinical trial. This research was supported by the National Cancer Institute of the National Institutes of Health R21CA185875-02 (ES) and P50 CA107399-11A1 (ES, AFH). AFH was supported by the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award.
Authorship
Contributions: E.S., J.P., S.H.T., and D.C. provided conceptualization and design; E.S., J.P., N.-C.T., A.F.H., J.S., J.Y.S., V.A., D.Y., E.K.P., J.B., and P.M. collected and assembled data; E.S., J.P., N.-C.T., A.F.H., V.A., and E.K.P. provided data analysis; A.F.H., J.P., V.A., D.Y., E.K.P., J.B., P.Y., S.D., M.M., R.C., T.C., N.K., P.M., A.N., L.P., F.S., J.E.S., J. Simpson, D.L.S., J. Song, R.S., N.T., S.H.T., S.J.F., D.C., A.M.W., J.W., and E.S. provided data interpretation and manuscript revision; and A.F.H. prepared the first draft of the manuscript.
Conflict-of-interest disclosure: A.F.H. reports research funding from BMS, Merck, Genentech, Inc./F, Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, AstraZeneca, ADC Therapeutics, and consultancy for BMS, Merck, Genentech, Inc./F. Hoffmann-La Roche Ltd, Kite Pharma/Gilead, Seattle Genetics, Karyopharm, Takeda, Tubulis, AstraZeneca. S.D. reports research funding from Bayer. M.M. reports research funding from TG Therapeutics, Epizyme, BMS, and consultancy with Morphosys, GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Eileen Smith, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: esmith@coh.org.
References
Author notes
For primary data, please contact the corresponding author at esmith@coh.org.
The full-text version of this article contains a data supplement.