Abstract
Von Willebrand disease (VWD) can be associated with significant morbidity. Patients with VWD can experience bruising, mucocutaneous bleeding, and bleeding after dental and surgical procedures. Early diagnosis and treatment are important to minimize the risk of these complications. Several bleeding assessment tools (BATs) have been used to quantify bleeding symptoms as a screening tool for VWD. We systematically reviewed diagnostic test accuracy results of BATs to screen patients for VWD. We searched Cochrane Central, MEDLINE, and EMBASE for eligible studies, reference lists of relevant reviews, registered trials, and relevant conference proceedings. Two investigators screened and abstracted data. Risk of bias was assessed using the revised tool for the quality assessment of diagnostic accuracy studies and certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation framework. We pooled estimates of sensitivity and specificity. The review included 7 cohort studies that evaluated the use of BATs to screen adult and pediatric patients for VWD. The pooled estimates for sensitivity and specificity were 75% (95% confidence interval, 66-83) and 54% (29-77), respectively. Certainty of evidence varied from moderate to high. This systematic review provides accuracy estimates for validated BATs as a screening modality for VWD. A BAT is a useful initial screening test to determine who needs specific blood testing. The pretest probability of VWD (often determined by the clinical setting/patient population), along with sensitivity and specificity estimates, will influence patient management.
Introduction
Von Willebrand factor (VWF) is a hemostatic protein that facilitates platelet adhesion and aggregation in addition to stabilizing coagulation factor VIII (FVIII).1-4 Qualitative or quantitative abnormalities in VWF can lead to von Willebrand disease (VWD).5 The reported prevalence of VWD is up to 1% in the general population6,7 with a symptomatic prevalence of ∼1 in 1000 at the level of primary care.8,9 This prevalence may be up to 15% in women with chronic heavy menstrual bleeding, making VWD the most common inherited bleeding disorder.10,11
Patients with VWD may experience easy bruising and bleeding, especially mucocutaneous bleeding such as epistaxis, oral cavity, and heavy menstrual bleeding as well as bleeding after childbirth and dental and surgical procedures. The clinical presentation varies greatly and the bleeding phenotype may change throughout a person’s life, leading to different management plans, depending on the type and subtype of VWD.12,13 Three types of VWD have been defined depending on the type of abnormality in VWF. Type 1 VWD occurs because of partial quantitative deficiency in VWF as a result of a deficit in synthesis or increased clearance, type 2 VWD is commonly divided into 4 major qualitative variants (types 2A, 2B, 2M, and 2N), and in type 3 VWD there is an absence of VWF production.4,7,10
In addition to variation in VWD management, there is limited awareness within the VWD community on the best clinical practice for screening and diagnosis.14 The aim of this systematic review is to determine the accuracy of bleeding assessment tools (BATs) and other nonstandard bleeding inventories as screening tests for VWD, which can be used to inform a combined strategy for diagnosis. Test accuracy estimates obtained in this systematic review were used to inform evidence-based recommendations on diagnostic strategies for the recently published clinical practice guidelines on VWD, developed by a combined effort from the American Society of Hematology, the International Society on Thrombosis and Haemostasis (ISTH), the National Hemophilia Foundation, the World Federation of Hemophilia, and the University of Kansas Medical Center.15 The guidelines aim to inform all stakeholders on essential issues in which there is variation or uncertainty in clinical practice and will support decision-making in the context of patients’ values and preferences.
Methods
Search strategy and data sources
We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from inception through August 2019. We also manually searched the reference lists of relevant articles and existing reviews. The search was limited to studies reporting data for accuracy of diagnostic tests. The complete search strategy is available in supplement 1. The prespecified protocol for this review is registered with PROSPERO (CRD42020147977). This review is reported in accordance with Preferred Reporting Items for Systematic reviews and Meta-Analyses for diagnostic test accuracy guidelines.16
Study selection
We used the following eligibility criteria.
Studies.
We included studies reporting data on diagnostic test accuracy (cohort studies, cross-sectional studies) for VWD.
Participants.
Patients suspected of having VWD of any age, presenting to inpatient or outpatient settings.
Index tests for diagnosis.
BATs and nonstandardized testing. We did not exclude studies based on the timing of when the index test was conducted.
Reference standards.
If a reference diagnostic test was not conducted, we accepted clinical follow-up as a reference standard.
Exclusion criteria.
Although studies reporting on patients with VWD as well as other bleeding disorders were eligible for inclusion, we excluded studies in which >80% of the study population included a different bleeding disorder. When possible, we extracted data separately for patients with VWD from these studies. We also excluded studies that did not provide sufficient data to determine test accuracy (sensitivity and specificity), studies only available as an abstract, studies with sample size fewer than 10 patients, and studies that used an unsuitable reference standard.
Screening and data extraction
Independent reviewers conducted title and abstract screening and full-text review in duplicate to identify eligible studies. Two reviewers completed data extraction independently and in duplicate and data were verified by a third reviewer (M.A.K.). Disagreements were resolved by discussion to reach consensus, in consultation with 2 expert clinician scientists (N.C. and P.J.). We extracted data about general study characteristics (authors, publication year, country, study design), diagnostic index test and reference standard, prevalence of VWD, and parameters to determine test accuracy (ie, sensitivity and specificity of the index test).
Risk of bias and certainty of evidence
We conducted the risk of bias assessment for diagnostic test accuracy studies using the Quality Assessment of Diagnostic Accuracy Studies-2 revised tool.17 We used the Grading of Recommendations Assessment, Development and Evaluation framework to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias.18,19
Data synthesis
When feasible, we combined the accuracy estimates from individual studies quantitatively (ie, pooled) for each test using OpenMetaAnalyst.20 We conducted a bivariate analysis for pooling sensitivity and specificity for each of the test comparisons to account for variation within and between studies. Forest plots were created for each comparison. The Breslow-Day test was used to measure the percentage of total variation across studies because of heterogeneity (I2); however, the results did not influence our judgment about inconsistency from the known methodological limitations of I2 in test accuracy reviews.21
Diagnostic strategies for VWD are based on assessment of the pretest probability for individual patients, which provides an estimate of the expected prevalence of VWD at a population level. We calculated the absolute differences in effects for each comparison as true positives, true negatives, false positives, and false negatives. Here, we present the results for the low, intermediate, and high pretest probability groups.
Results
Description of studies
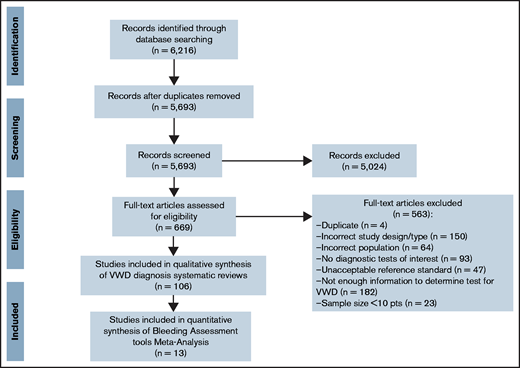
The initial search retrieved 5693 nonduplicate studies, of which 669 were included for full-text review. Following full-text review, we identified 106 studies eligible for data abstraction, of which 13 answered the questions addressed in this systematic review. A list of excluded studies is provided in supplement 2. Reasons for exclusion at full-text review were ineligible study design, study population, or diagnostic test, sample size <10 patients, and not enough information to determine diagnostic test accuracy for VWD (Figure 1).
All the included studies reported on the use of BATs in VWD.22-34 Table 1 summarizes general characteristics of included studies, as well as the index and reference tests. The most common BATs used were the Molecular and Clinical Markers for the Diagnosis and Management of Type 1 (MCMDM-1) VWD Bleeding Questionnaire; the ISTH-BAT; the self-administered bleeding assessment tool (Self-BAT), which is the ISTH-BAT converted to a grade 4 reading level; the Vicenza score; questionnaires based on the bleeding symptoms especially in women; and the Pediatric Bleeding Questionnaire, which is a modified version of the MCMDM-1VWD Bleeding Questionnaire.
Characteristics of included studies
| First author . | Year . | Study design . | No. of patients . | Patient selection . | Index test . | Reference standard . |
|---|---|---|---|---|---|---|
| Bowman | 2008 | Cohort with DTA results for adults | 217 | Unrelated adults (age 20-88 y) recruited from primary care clinics investigated for VWD type 1 (35 male, 65 female) | MCMDM-1 VWD Bleeding Questionnaire. A bleeding score ≥4 was considered abnormal | Laboratory workup including ABO blood group, VWF:Ag, VWF:RCo, and FVIII:C |
| Deforest | 2015 | Cohort with DTA results for adults | 64 | Adult patients (age 18-73 y) referred for the first time to a hematologist because of a problem with bleeding or bruising (11 male, 53 female) | Self-BAT: ISTH-BAT was converted to a grade 4 reading level to produce the first version of the Self-BAT, which was then optimized to ensure agreement with the ISTH-BAT. A normal bleeding score was 0 to +5 for females and 0 to +3 for males | Laboratory workup including CBC, INR/PT/PTT, thrombin time, fibrinogen, ferritin, ABO blood group, VWF:Ag, VWF:RCo, FVIII:C, and VWF multimers |
| Philipp | 2008 | Cohort with DTA results for adults | 146 | Females (age 13-55 y) receiving a physician diagnosis of heavy menstrual bleed at the faculty gynecology practice of UMDNJ-Robert Wood Johnson Medical School or collaborating community gynecology and pediatric practices | 12-page questionnaire based on the bleeding symptoms found significant in women with WWD. A screening tool was positive if 1 of 4 criteria were met: severity of heavy menstrual period, history of treatment of anemia, excess bleeding after challenges including dental surgery, surgery and delivery, family history of bleeding disorder | Laboratory workup including VWF:Ag and VWF:RCo |
| Bidlingmaier | 2012 | Cohort with DTA results for children | 100 | Children (age 1-17 y), 44 with a positive bleeding history, 29 referred because of an isolated APTT prolongation, and 27 because of a positive family history of bleeding | Quantitative ISTH child bleeding score and the qualitative ITEM analysis. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers |
| Bowman | 2009 | Cohort with DTA results for children | 151 | Children (age <18 y) from the waiting room of the Childrens Outpatient Centre, the Hotel Dieu Hospital in Kingston, Ontario, investigated for VWD because of a personal history of hemorrhagic symptoms and/or a family history of VWD and/or for preoperative screening | PBQ: The MCMDM-1VWD Bleeding Questionnaire was modified by including pediatric-specific bleeding symptoms in the “other” category. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers, genetic testing |
| Malec | 2016 | Cohort with DTA results for children | 193 | Children (age <11 y) referred to an outpatient bleeding disorders clinic for evaluation of VWD and/or other bleeding disorders | Composite score that was considered positive when 2 of 4 criteria were positive: Tosetto bleeding score Z1; family history of VWD or bleeding; personal history of iron deficiency anemia; and positive James early bleeding score | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers |
| Marcus | 2011 | Cohort with DTA results for children | 104 | Children (age <17 y) referred for evaluation of bleeding symptoms, family history of a bleeding disorder, and/or abnormal coagulation studies | Modified Vicenza score to include an “other” category with pediatric-specific bleeding questions. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo and VWF:Ag |
| Belen, B. | 2015 | Case control | 84 | Children (age <8 y) with VWD (46) and control group (32) with bleeding symptoms but had normal prothrombin time, APTT, PFA 100, VWF:Ag, VWF:RCo, and platelet function tests | PBQ administration. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Faiz | 2017 | Case control | 53 | Women (age 14-53 y): 41 previously untested family members of VWD patients, 26 previously diagnosed VWD patients, and 27 healthy controls | Modified screening tool considered positive if 1 of 3 criteria were met: severity of heavy menstrual period, history of treatment of anemia, excess bleeding after challenges including dental surgery, and surgery and delivery | Laboratory workup including CBC, ferritin, FVIII:C, VWF:Ag, and VWF:RCo |
| Mittal | 2015 | Case control | 1316 | Healthy children (age <18 y) without a diagnosis of a chronic medical condition presenting to a general pediatrician’s office for routine or sick visits, and 35 children (21 male, 14 female) with a known diagnosis of VWD | PBQ. Children with total bleeding questionnaire scores ≥3 were predicted to have VWD | Laboratory workup including VWF:Ag, VWF:RCo, and multimer analysis |
| Pathare | 2018 | Case control | 96 | 46 patients with type 1 VWD; 46 and 50 healthy subjects with no known history of bleeding or bruising (ages 7-49 y) | MCMDM-1 VWD questionnaire. Bleeding score >2 considered significantly abnormal | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Bujnicki | 2011 | Case control | 160 | 80 children (age <11 y) with VWF:RCo <0.50 IU/mL, and 80 controls without VWD | Pediatric bleeding score modified for children based on the PBQ. A bleeding score ≥1 was predictive of VWD | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Rodeghiero | 2005 | Case control | 341 | 42 adults that are obligatory carriers of VWD type I, 84 affected with VWD type 1, and 215 controls | A standardized questionnaire, using a bleeding score ranging from 0 (no symptom) to 3 (hospitalization, replacement therapy, blood transfusion) | Laboratory workup including VWF:Ag, VWF:RCo, FVIII:C, and APTT |
| First author . | Year . | Study design . | No. of patients . | Patient selection . | Index test . | Reference standard . |
|---|---|---|---|---|---|---|
| Bowman | 2008 | Cohort with DTA results for adults | 217 | Unrelated adults (age 20-88 y) recruited from primary care clinics investigated for VWD type 1 (35 male, 65 female) | MCMDM-1 VWD Bleeding Questionnaire. A bleeding score ≥4 was considered abnormal | Laboratory workup including ABO blood group, VWF:Ag, VWF:RCo, and FVIII:C |
| Deforest | 2015 | Cohort with DTA results for adults | 64 | Adult patients (age 18-73 y) referred for the first time to a hematologist because of a problem with bleeding or bruising (11 male, 53 female) | Self-BAT: ISTH-BAT was converted to a grade 4 reading level to produce the first version of the Self-BAT, which was then optimized to ensure agreement with the ISTH-BAT. A normal bleeding score was 0 to +5 for females and 0 to +3 for males | Laboratory workup including CBC, INR/PT/PTT, thrombin time, fibrinogen, ferritin, ABO blood group, VWF:Ag, VWF:RCo, FVIII:C, and VWF multimers |
| Philipp | 2008 | Cohort with DTA results for adults | 146 | Females (age 13-55 y) receiving a physician diagnosis of heavy menstrual bleed at the faculty gynecology practice of UMDNJ-Robert Wood Johnson Medical School or collaborating community gynecology and pediatric practices | 12-page questionnaire based on the bleeding symptoms found significant in women with WWD. A screening tool was positive if 1 of 4 criteria were met: severity of heavy menstrual period, history of treatment of anemia, excess bleeding after challenges including dental surgery, surgery and delivery, family history of bleeding disorder | Laboratory workup including VWF:Ag and VWF:RCo |
| Bidlingmaier | 2012 | Cohort with DTA results for children | 100 | Children (age 1-17 y), 44 with a positive bleeding history, 29 referred because of an isolated APTT prolongation, and 27 because of a positive family history of bleeding | Quantitative ISTH child bleeding score and the qualitative ITEM analysis. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers |
| Bowman | 2009 | Cohort with DTA results for children | 151 | Children (age <18 y) from the waiting room of the Childrens Outpatient Centre, the Hotel Dieu Hospital in Kingston, Ontario, investigated for VWD because of a personal history of hemorrhagic symptoms and/or a family history of VWD and/or for preoperative screening | PBQ: The MCMDM-1VWD Bleeding Questionnaire was modified by including pediatric-specific bleeding symptoms in the “other” category. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers, genetic testing |
| Malec | 2016 | Cohort with DTA results for children | 193 | Children (age <11 y) referred to an outpatient bleeding disorders clinic for evaluation of VWD and/or other bleeding disorders | Composite score that was considered positive when 2 of 4 criteria were positive: Tosetto bleeding score Z1; family history of VWD or bleeding; personal history of iron deficiency anemia; and positive James early bleeding score | Laboratory workup including VWF:RCo, VWF:Ag, FVIII:C, VWF multimers |
| Marcus | 2011 | Cohort with DTA results for children | 104 | Children (age <17 y) referred for evaluation of bleeding symptoms, family history of a bleeding disorder, and/or abnormal coagulation studies | Modified Vicenza score to include an “other” category with pediatric-specific bleeding questions. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:RCo and VWF:Ag |
| Belen, B. | 2015 | Case control | 84 | Children (age <8 y) with VWD (46) and control group (32) with bleeding symptoms but had normal prothrombin time, APTT, PFA 100, VWF:Ag, VWF:RCo, and platelet function tests | PBQ administration. A bleeding score ≥2 was considered abnormal | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Faiz | 2017 | Case control | 53 | Women (age 14-53 y): 41 previously untested family members of VWD patients, 26 previously diagnosed VWD patients, and 27 healthy controls | Modified screening tool considered positive if 1 of 3 criteria were met: severity of heavy menstrual period, history of treatment of anemia, excess bleeding after challenges including dental surgery, and surgery and delivery | Laboratory workup including CBC, ferritin, FVIII:C, VWF:Ag, and VWF:RCo |
| Mittal | 2015 | Case control | 1316 | Healthy children (age <18 y) without a diagnosis of a chronic medical condition presenting to a general pediatrician’s office for routine or sick visits, and 35 children (21 male, 14 female) with a known diagnosis of VWD | PBQ. Children with total bleeding questionnaire scores ≥3 were predicted to have VWD | Laboratory workup including VWF:Ag, VWF:RCo, and multimer analysis |
| Pathare | 2018 | Case control | 96 | 46 patients with type 1 VWD; 46 and 50 healthy subjects with no known history of bleeding or bruising (ages 7-49 y) | MCMDM-1 VWD questionnaire. Bleeding score >2 considered significantly abnormal | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Bujnicki | 2011 | Case control | 160 | 80 children (age <11 y) with VWF:RCo <0.50 IU/mL, and 80 controls without VWD | Pediatric bleeding score modified for children based on the PBQ. A bleeding score ≥1 was predictive of VWD | Laboratory workup including VWF:Ag, VWF:RCo, and FVIII:C |
| Rodeghiero | 2005 | Case control | 341 | 42 adults that are obligatory carriers of VWD type I, 84 affected with VWD type 1, and 215 controls | A standardized questionnaire, using a bleeding score ranging from 0 (no symptom) to 3 (hospitalization, replacement therapy, blood transfusion) | Laboratory workup including VWF:Ag, VWF:RCo, FVIII:C, and APTT |
APTT, activated partial thromboplastin time; CBC, complete blood count; DTA, diagnostic test accuracy; INR, international normalized ratio; ITEM, Test Question Analysis; PBQ, Pediatric Bleeding Questionnaire; PFA 100, Platelet Function Assay; PT, prothrombin time; PTT, partial thromboplastin time; UMDNJ, University of Medicine and Dentistry of New Jersey.
Use of BATs as a screening tool for VWD
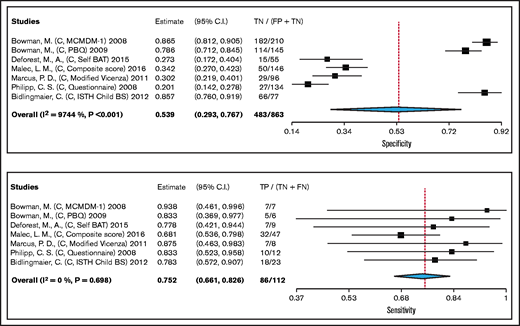
We pooled test accuracy of BATs when used as a screening tool for VWD from 7 cohort studies, including 112 participants. Studies used laboratory testing (VWF:Ag, VWF:RCo, FVIII:C) as a reference standard for confirming VWD, with some studies also including historic clinical diagnosis. The pooled estimates for sensitivity and specificity were 75% (95% confidence interval, 66-83) and 54% (29-77), respectively (high certainty in the sensitivity results and moderate certainty in the specificity results). Figure 2 shows the forest plot displaying the sensitivity and specificity from individual studies and the pooled estimates for BAT when used as a screening tool for VWD. The complete risk of bias assessment for individual studies is included in supplement 3.
Forest plots for sensitivity and specificity for individual studies and the pooled estimates of BAT when used as a screening tool for VWD.
Forest plots for sensitivity and specificity for individual studies and the pooled estimates of BAT when used as a screening tool for VWD.
BATs results were illustrated for 1000 patients from a low prevalence population undergoing the test (3% prevalence, which is typically seen in patients investigated for VWD because of a personal history of abnormal laboratory test [eg, increased partial thromboplastin time]), intermediate prevalence (20% prevalence which is typically seen in patients investigated for VWD because of a personal history of bleeding symptoms [eg, mucocutaneous bleeding]), and high prevalence (50% prevalence, which is typically seen in patients investigated for VWD as a first-degree relative for a patient with VWD); absolute differences indicate a low (<20%) proportion of false negative. Overall, the test was shown to be highly sensitive and moderately specific and the certainty of evidence was moderate to high. Table 2 shows Grading of Recommendations Assessment, Development and Evaluation test accuracy evidence summary for BAT when used as a screening test for VWD. The interactive summary of findings can be accessed using the following link: https://gdt.gradepro.org/presentations/#/isof/isof_c5b33e22-a646-4654-9f09-b820aff36c5c-1569520689536?_k=eump67.
GRADE test accuracy evidence summary for BAT when used as a screening test for VWD
| . | Sensitivity . | 0.75 (95% CI, 0.66-0.83) . | . | Prevalences . | 3% . | 20% . | 50% . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Specificity . | 0.54 (95% CI, 0.29-0.77) . | . | . | . | ||||||
| . | Factors that may decrease certainty of evidence . | Effect per 1000 patients tested . | . | ||||||||
| Outcome . | No. of studies (No. of patients) . | Study design . | Risk of bias . | Indirectness . | Inconsistency . | Imprecision . | Publication bias . | Pretest probability of 3%* . | Pretest probability of 20%† . | Pretest probability of 50%‡ . | Test accuracy CoE . |
| True positives (patients with suspected patients) | 7 studies, 112 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Not serious | Not serious | None | 23 (20-25) | 150 (132-165) | 376 (331-413) | ⨁⨁⨁⨁ HIGH |
| False negatives (patients incorrectly classified as not having suspected patients) | 7 (5-10) | 50 (35-68) | 124 (87-169) | ||||||||
| True negatives (patients without suspected patients) | 7 studies, 863 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious§ | Not serious | None | 523 (284-744) | 431 (234-614) | 270 (147-384) | ⨁⨁⨁◯ MODERATE |
| False positives (patients incorrectly classified as having suspected patients) | 447 (226-686) | 369 (186-566) | 230 (116-353) | ||||||||
| . | Sensitivity . | 0.75 (95% CI, 0.66-0.83) . | . | Prevalences . | 3% . | 20% . | 50% . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Specificity . | 0.54 (95% CI, 0.29-0.77) . | . | . | . | ||||||
| . | Factors that may decrease certainty of evidence . | Effect per 1000 patients tested . | . | ||||||||
| Outcome . | No. of studies (No. of patients) . | Study design . | Risk of bias . | Indirectness . | Inconsistency . | Imprecision . | Publication bias . | Pretest probability of 3%* . | Pretest probability of 20%† . | Pretest probability of 50%‡ . | Test accuracy CoE . |
| True positives (patients with suspected patients) | 7 studies, 112 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Not serious | Not serious | None | 23 (20-25) | 150 (132-165) | 376 (331-413) | ⨁⨁⨁⨁ HIGH |
| False negatives (patients incorrectly classified as not having suspected patients) | 7 (5-10) | 50 (35-68) | 124 (87-169) | ||||||||
| True negatives (patients without suspected patients) | 7 studies, 863 patients | Cross-sectional (cohort type accuracy study) | Not serious | Not serious | Serious§ | Not serious | None | 523 (284-744) | 431 (234-614) | 270 (147-384) | ⨁⨁⨁◯ MODERATE |
| False positives (patients incorrectly classified as having suspected patients) | 447 (226-686) | 369 (186-566) | 230 (116-353) | ||||||||
CoE, certainty of evidence.
Typically seen in patients investigated for VWD because of a personal history of abnormal laboratory test (eg, increased APTT).
Typically seen in patients investigated for VWD because of a personal history of bleeding symptoms (eg, mucocutaneous bleeding).
Typically seen in in patients investigated for VWD as a first-degree relative for a patient with VWD.
The point estimates of specificity are not homogenous, which was not explained by a priori determined analysis (eg, based on difference in risk of bias of the studies), and can be due to differences in the setting and disease prevalence. The majority of included studies were judged to be low risk of bias for test and reference standard interpretation. Although there was unclear reporting regarding flow and timing in some studies, the certainty of evidence was generally not downgraded for risk of bias. The patient selection risk of bias was low in 7 cohort studies and high in 6 case control studies that were not included to calculate the pooled estimate.
Discussion
This review presents pooled estimates of test accuracy for commonly available BATs used as a screening test for VWD. Importantly, the certainty of evidence was moderate to high. BATs had a sensitivity and specificity of 75% (95% confidence interval, 66-83) and 54% (29-77), respectively. These are overall results that include men and children; if adult women are evaluated separately, the sensitivity is much higher (100% in some studies). The benefit of using BATs is to identify patients suspected of having VWD who may otherwise be missed without this tool in clinic. Additionally, using a BAT will allows for the quantification of bleeding symptoms in patients. However, recommendations on whether to use BATs as a screening tool in clinical practice will depend on multiple factors, including the prevalence of VWD in the clinical setting/patient population and the resources required to complete the BAT such as provider training and time needed in the clinical setting. Additionally, the clarity and precision of the structured questions within a BAT may help patients voice their concerns compared with unstructured symptoms questionnaires; therefore, patients may benefit from the standardized and objective way of obtaining bleeding data. This is consistent with patients’ values and preferences aimed at ensuring their symptoms are validated and addressed. Limitations of BATs include lack of availability in languages other than English, although the ISTH-BAT has been translated into German, Italian, Norwegian, Spanish, and Japanese, and the time necessary to complete the BAT (often 10-20 minutes, but up to 30 minutes depending on the version and operator experience). That being said, the amount of time needed to administer a BAT may be a barrier to feasibility, including the requirement of appropriate training and education for the individual administering the tool. The Self-BAT addresses this issue because patients can fill in the questionnaire at home before visiting their doctor, and can be done using mobile devices to collect data; however, drawing comparative conclusions about Self-BAT was limited because of the limited number of individuals assessed. Self-BAT is currently available in English and French only.
When we looked at the trend of diagnostic test accuracy based on the date the different studies were conducted, we did not observe a clear trend of the sensitivity and specificity that reflect a relation with publication time. This might be due to the use of different BATs with no standardization of the questions asked. The changes that were made after the first few Vicenza-based BATs were about how long it takes to administer and who administers it, rather than to improve accuracy.
This review has several strengths. First, this is the first systematic review to comprehensively examine diagnostic test accuracy of bleeding assessment tools in both adults and children suspected of having VWD. Second, the comprehensive and systematic approach for identifying studies makes it unlikely that relevant studies were missed. Finally, we assessed the certainty of evidence in this area and identified sources of bias.
We note a few limitations in this comprehensive systematic review. The pooled sensitivity and specificity estimates of the tests from this review apply only when the test is performed alone; however, BATs can be used as part of different diagnostic strategies to inform clinical decision-making.
Conclusion
This comprehensive systematic review is the first to synthesize and evaluate the accuracy of BATs as a screening tool for the diagnosis of VWD in adults and children. Estimates of sensitivity and specificity from this review were used to inform evidence-based recommendations for a clinical practice guideline. Prevalence or pretest probability of VWD in a population is essential to consider when making clinical decisions about relying on the BAT results to rule in or rule out VWD diagnosis.
Acknowledgments
The systematic review team acknowledges Jenny Castano, members of the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, the World Federation of Hemophilia, and the von Willebrand disease (VWD) diagnosis guidelines panel members for their assistance and administrative support.
This systematic review was conducted to support the development of the ASH 2020 guidelines for diagnosis and management of VWD. The entire guideline development process was funded by ASH. Through the Outcomes and Implementation Research Unit at the University of Kansas Medical Center, some researchers received salary or grant support and others participated to fulfill requirements of an academic degree or program or volunteered their time.
Authorship
Contribution: R.A.M., M.A.K., and N.H. contributed to study design, study selection, data extraction, statistical analysis, and interpretation of results; M.A.K., N.H., O.A., O.D., A.E.A, S.T., B.M., A.B.D., and A.Q. contributed to study selection and data extraction; M.A.K. and R.A.M. contributed to drafting the report; B.A., J.D.P., J.C.J.E., V.J.-P., C.M., R.M., J.S.O., R.S., P.D.J., N.T.C., and V.F. contributed to the interpretation of results, and critical revision of the report; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: P.D.J. and J.C.J.E. receive research funding from CSL Behring, Bayer, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Reem A. Mustafa, Division of Nephrology and Hypertension, Department of Medicine, 3901 Rainbow Blvd, University of Kansas Medical Center, Kansas City, KS 66160; e-mail: rmustafa@kumc.edu.
References
Author notes
Vicky Jacobs-Pratt is retired from Hemophilia Alliance of Maine, Augusta, Maine
Requests for data sharing may be submitted to Reem Mustafa (rmustafa@kumc.edu).
The full-text version of this article contains a data supplement.