Key Points
Red blood cell exchange should be considered in sickle cell trait with refractory stuttering priapism.
Abstract
Sickle cell trait (SCT) is typically an asymptomatic carrier state, but sickling complications can occur under extreme conditions. Priapism is known to be associated with sickle cell disease (SCD); The link with SCT is less well established. We report the case of a 19-year-old man with SCT presenting with prolonged priapism and a refractory, stuttering course requiring multiple invasive procedures over a 5-day period with no clear alternative triggers. In light of ongoing, stuttering priapism, he underwent red blood cell (RBC) exchange transfusion with decrease of hemoglobin S from 45.8% to 11.7%. This was followed by immediate and sustained cessation of stuttering priapism, with no further episodes at 5 months. Multiple cases of priapism associated with SCT have been reported in the literature. In most cases invasive interventions were required but RBC exchange was not attempted. RBC exchange has been reported in 2 people with exertional rhabdomyolysis in the context of SCT, with improvement in 1 case. In patients with SCT and priapism, conservative measures are used to treat brief episodes, but invasive management is required for persistent or prolonged episodes. RBC exchange transfusion may be considered for treatment of refractory, stuttering priapism in individuals with SCT.
Introduction
The inheritance of 1 sickle β globin gene, βS, and a normal β globin gene defines sickle cell trait (SCT).1 Although SCT is typically an asymptomatic carrier trait, sickling can occur under extreme conditions, such as severe tissue hypoxia, acidosis, or dehydration. SCT is associated with increased risk of complications, including exertional rhabdomyolysis or exercise-related sudden death.1
Priapism is a sustained erection caused by increased arterial inflow (high flow) or reduced venous outflow (low flow) to the corpora cavernosa. The risk of ischemic priapism is higher in some hematologic conditions, including sickle cell disease (SCD),2 in which stuttering priapism with recurrent brief episodes occurs.3 In SCD, low-flow priapism is theorized to be caused by red blood cell (RBC) sickling, hemolysis, and nitric oxide depletion, leading to vasoconstriction.4 Low-flow priapism leads to local acidosis and deoxygenation, thus further exacerbating sickling.
Initial management of brief episodes of priapism in SCD includes hydration, oxygenation, analgesics, trial of voiding, masturbation, ice packs, and/or warm bath.5 Advanced management for prolonged episodes (>4 hours) includes corporeal aspiration and irrigation with saline and/or sympathomimetics, with distal shunting from the cavernosa to the glans being a last resort because of invasiveness and risk of impotence.5
Although evidence of its efficacy is limited, RBC exchange transfusion may be used in priapism with SCD. Potential adverse effects can include transfusion reactions and ASPEN (association of sickle cell disease, priapism, exchange transfusion, and neurologic events) syndrome.6 There are a few case reports of RBC exchange transfusion for acute complications in SCT; however, none report its use for priapism.
Case description
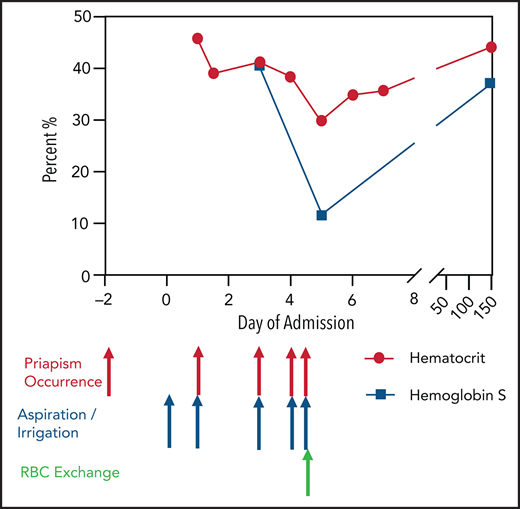
A 19-year-old man presented to the emergency department with priapism lasting 48 hours, without preceding penile trauma or sexual activity. He was not taking medications and occasionally smoked marijuana, with no recent use. He had SCT with no history of blood transfusions or hospitalizations. Family history included a maternal aunt with SCD.
Physical examination was notable for priapism. Initial investigations revealed hemoglobin of 16.3 mg/dL, with hematocrit of 45.8%. Urology aspirated and irrigated with phenylephrine until detumescence. Penile cavernosa blood gas showed acidosis with hypoxia and hypercarbia, consistent with ischemic priapism. Additional investigations found no evidence of hemolysis and revealed positive sickle solubility screen. High-performance liquid chromatography showed hemoglobin S (HbS) of 40.5%, consistent with SCT (HbAS). Conservative measures were initiated early and continued until discharge, including IV hydration, cold avoidance, and maintenance of adequate oxygenation.
Priapism recurred after 3 hours. Repeat aspiration followed by saline and phenylephrine irrigation was initially unsuccessful, so plans for a semiurgent corpora cavernosa–glans distal shunt were made, with priapism lasting >4 hours. Saline and phenylephrine irrigation was repeated intraoperatively, eventually resulting in detumescence. Urology decided against proceeding with distal shunting.
On the third day of admission, priapism recurred. After 3 hours, aspiration with saline and phenylephrine irrigation was performed, with detumescence lasting several hours followed by brief recurrence, necessitating repeat irrigation on the same day. He had additional brief recurrences on the fourth and fifth days, requiring aspiration and irrigation.
In light of ongoing stuttering priapism with no clear trigger, and in recognition of his SCT diagnosis, he underwent RBC exchange transfusion to prevent additional episodes. High-performance liquid chromatography after the procedure showed HbS of 11.7% and hematocrit of 34.9%. Priapism did not recur after 5 months of follow-up.
Methods
Preexchange hematocrit was 41%. The desired postprocedure hematocrit was 38%, and the desired fraction of cells remaining was 40%. The Spectra Optia Pheresis (Terumo BCT) system was used, with 9 units (2562 mL) of packed RBCs.
Results and discussion
We detail the first published case of SCT-associated stuttering priapism that resolved after RBC exchange transfusion. Several cases have been reported linking priapism with SCT. The first was published 5 decades ago, involving a man with SCT and priapism triggered by trauma, which resolved after Hemovac suction.7 There are 2 case reports of priapism in patients with SCT and marijuana use; the first patient used marijuana and alcohol and required corpora cavernosa–glans shunting,8 and the other used cocaine, alcohol, quetiapine, and marijuana and required intracorporeal phenylephrine injection.9 Other case reports detail priapism in SCT precipitated by different factors, including 2 patients with priapism precipitated by sexual activity (1 resolved with corpora cavernosa evacuation,10 and the other required a saphenous vein corpus cavernosum shunt11 ) and another patient with protein S deficiency who required cavernosal glanular shunting.12
There are 2 case reports of phosphodiesterase-5 inhibitor use as a possible precipitant for priapism with SCT; the first was an infant taking sildenafil for pulmonary hypertension with priapism post–cardiac transplantation surgery,13 and the second was an adult who used sildenafil.14 Both cases had resolution of priapism, with no recurrence after stopping sildenafil. Interestingly, researchers are investigating whether phosphodiesterase-5 inhibitors may be used to treat priapism in SCD by enhancing nitric oxide production.4 Another case report describes a patient with SCT and stuttering priapism managed with self-injecting metaraminol, a vasoconstricting amine, with no hospital visits in >10 years.15
A case series of 46 patients with priapism included 7 with SCT.16 Two patients with SCT were included in 2 different case series.17,18 Another case series included a 14-year-old boy with SCT and priapism requiring a sapheno-crural shunt, which was complicated by skin loss and required split-thickness skin grafting.19
Evidence supporting the efficacy of RBC exchange is anecdotal. In a case series of 10 patients with SCD and priapism not responding to initial measures, patients underwent RBC exchange, with resolution in 8, whereas 2 required surgical interventions.20 There were no neurologic events, but ASPEN syndrome has been described in a pediatric case report21 and a case series22 ; in the series, however, postexchange hemoglobin was high, and imaging showed vascular abnormalities in 5 of 6 patients. Another pediatric case series documented 8 patients with SCD and priapism, 5 of whom underwent RBC exchange transfusion. Only 2 had resolution, whereas the others required distal shunts. One patient had a cerebrovascular accident.23
Target HbS in RBC exchange transfusion for SCD is typically <30%, with hematocrit of ≥30% to suppress HbS production, reduce sickle cells, and improve oxygen-carrying capacity.6 Targets in SCT are unknown. Two case reports detail the use of RBC exchange transfusion in SCT, both for rhabdomyolysis. The first was a patient with worsening exertional rhabdomyolysis despite IV fluids and RBC exchange transfusion with no resolution, eventually requiring fasciotomies for compartment syndrome.24 The second was a hypoxic asthmatic male patient with rhabdomyolysis that was nonresponsive to IV fluids and plasma exchange but resolved with RBC exchange transfusion.25
Priapism in patients with SCT should be managed with conservative measures, with invasive interventions reserved for persistent priapism. In our case, RBC exchange transfusion was attempted for persistent stuttering priapism with underlying SCT. Automated RBC exchange was followed by an immediate decrease in HbS and sustained absence of recurrent priapism. Manual partial RBC exchange may be considered in centers without access to automated RBC exchange. Although stuttering priapism ceased immediately after RBC exchange transfusion, coincidental resolution may have been part of the natural history, and a causal link cannot be definitively concluded.
Authorship
Contribution: M.S.E. conducted the literature review and prepared the visual abstract; M.V. reviewed and approved the manuscript; both authors wrote the manuscript and participated in the care of the patient.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madeleine Verhovsek, Hematology, Department of Medicine, McMaster University, Room F-304, St Joseph’s Hospital, 50 Charlton Ave East, Hamilton, ON L8N 4A6, Canada; e-mail: verhovm@mcmaster.ca.
References
Author notes
Please contact verhovm@mcmaster.ca for more data.