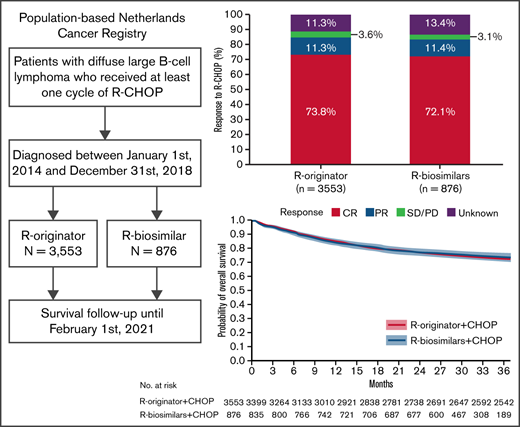
Key Points
Three-year overall survival did not differ between DLBCL patients treated with rituximab biosimilars and those receiving rituximab originator.
By the end of 2018, 91% of purchased rituximab in the Netherlands were biosimilars, accounting for a 43% reduction in annual costs.
Abstract
In 2017, the European Medicines Agency approved rituximab biosimilars (R-biosimilars) for treatment of diffuse large B-cell lymphoma (DLBCL). Thereafter, the Netherlands was one of the first countries to implement R-biosimilars, given lower costs compared with rituximab originator (R-originator). This study’s objective was to investigate whether overall survival (OS) of patients with DLBCL receiving R-biosimilars is similar to patients treated with R-originator. DLBCL patients ≥18 years, diagnosed between 2014 and 2018, who received at least 1 cycle of rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) were identified in the Netherlands Cancer Registry. Patients were categorized into R-originator or R-biosimilars groups based on data from a central repository of the Dutch medicinal drug market. The primary end point was 3-year OS, defined as the time between diagnosis and all-cause death. By the end of 2018, 91% of purchased rituximab were biosimilars. In total, 4429 patients were identified with 876 in the R-biosimilars group and 3553 in the R-originator group. Patients in the R-biosimilars group less frequently received >6 cycles of R-CHOP compared with patients treated with R-originator (24% vs 30%, P = .003). The 3-year OS did not differ between patients treated with R-originator or R-biosimilars (73% vs 73%, P = .855). This was confirmed with a multivariable Cox regression analysis accounting for sex, age, International Prognostic Index score, and number of R-CHOP cycles. In conclusion, the 3-year OS is similar for patients treated with CHOP in combination with R-originator or R-biosimilars and, therefore, favors the use of R-biosimilars in DLBCL treatment management.
Introduction
The standard treatment of patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) consists of the anti-CD20 monoclonal antibody rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP).1 In 2017, the rituximab biosimilars (R-biosimilars) CT-P10 and GP2013 were approved by the European Medicines Agency (EMA) for the treatment of DLBCL.2,3 This approval of R-biosimilars was based on preclinical data and prospective studies in patients with rheumatoid arthritis and naïve follicular lymphoma. Whereas efficacy data in naïve follicular lymphoma showed equal overall response rates (ORR) for R-biosimilars and R-originator, data on ORR and overall survival (OS) for patients with newly diagnosed DLBCL is limited to small post marketing studies.4,5
In 2017, the Dutch Association of Medical Specialists stated to adopt biosimilars into standard of care. Hence, because of considerably lower purchase costs of R-biosimilars compared with R-originator (up to 50%), the Netherlands was one of the first countries to implement R-biosimilars in its health care system. However, it is still uncertain if the effect of R-biosimilars on OS of patients with newly diagnosed DLBCL is truly equivalent to that of R-originator. Complementary to prospective intervention studies, population-based studies can provide valuable information to assess treatment strategies and outcome in patients at the population level. Therefore, we evaluated the 3-year OS of newly diagnosed patients with DLBCL either treated with R-biosimilars or R-originator, using a contemporary, nationwide, population-based cancer registry in the Netherlands.
Methods
Patients
Patients with newly diagnosed DLBCL who were ≥18 years of age and diagnosed between 1 January 2014 and 31 December 2018, who received at least 1 cycle of R-CHOP, were identified in the Netherlands Cancer Registry (NCR). This population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organization and has, since 1989, nationwide coverage of at least 95% of all malignancies. All newly diagnosed malignancies are reported to the NCR via the Nationwide Network of Histopathology and Cytopathology, and the National Registry of Hospital Discharges (eg, inpatient and outpatient discharges). Information on patient characteristics and treatment (y/n) is routinely recorded in the NCR by trained registrars of the NCR through retrospective medical records review. Unique to the NCR, since 1 January 2014, detailed information on diagnostic and treatment characteristics for all hematological malignancies diagnosed in the Netherlands are also recorded in the NCR. For DLBCL, the available prognostic factors include Ann Arbor stage, serum lactate dehydrogenase (LDH) levels, World Health Organization (WHO) performance status, International Prognostic Index (IPI) score, type of therapeutic regimen, number of R-CHOP cycles, initiation date of first-line therapy, and best response to first-line treatment.
Topography and morphology of hematological malignancies are coded according to the International Classification of Diseases for Oncology. Patients with newly diagnosed DLBCL were defined using morphology code 9680, thereby excluding patients with primary central nervous system lymphomas, primary mediastinal B-cell lymphoma, and primary testicular lymphoma. Information on the last known vital status for all patients (eg, alive, death, emigration) was obtained through annual linkage with the Nationwide Population Registries Network that holds vital statistics on all residents in the Netherlands.
Patients were categorized into R-originator or R-biosimilars groups using purchase information of the Dutch hospitals for every month, derived from FarmInform. This information was validated by the confirmation of transition date from R-originator to R-biosimilars, which was provided by the respective clinical pharmacists from the Dutch hospitals. In more detail, FarmInform maintains a central data pooling system of the medicinal drug market of pharmaceutical companies, importers, and wholesalers in the Netherlands with coverage of >98% of the total market. FarmInform performs internal and external audits to guarantee a high quality of the collected data and is monitored according to Dutch and European competition law. In the Netherlands, central pharmacies coordinate the distribution of medicines among and within hospitals. Therefore, we validated the categorization of patients into R-biosimilars or R-originator groups in a 2-step approach. First, the registrars of the NCR performed an additional medical records review of a random sample of 30% of the patients categorized in the R-biosimilars group, to validate the use of R-biosimilars according to purchase information from FarmInform. Detailed pharmacists' information about drug prescription was not accessible for all patients in the random sample via these medical records. Second, we therefore directly approached the responsible pharmacists of hospitals for which detailed information was not available in medical records with an inquiry about the exact transition date to R-biosimilars. Patients for whom the use of R-originator or R-biosimilars could not be conclusively deducted were excluded, including patients who either switched from R-originator to R-biosimilars or vice versa during first-line treatment.
The primary end point was 3-year OS, defined as the time between DLBCL diagnosis and all-cause death. The secondary end point was best response to first-line treatment. Best response (ie, complete response, partial response, or stable/progressive disease) was determined by physician assessment.
According to the Central Committee on Research involving Human Subjects, this type of observational study does not require approval from an ethics committee in the Netherlands. The Privacy Review Board of the NCR approved the use of anonymous data for this study.
Statistical analysis
Age, sex, Ann Arbor stage, serum LDH level, WHO performance score, number of extranodal sites, IPI score, time until treatment, and the number of R-CHOP cycles were compared between R-originator- and R-biosimilar-treated patients. The Pearson χ2 test was applied to compare age (≤60 vs >60 years), sex, Ann Arbor stage, serum LDH level (elevated vs normal), WHO performance score, number of extranodal sites, IPI score, and number of cycles. The Kruskal-Wallis test was used for median age and time until treatment between R-originator- and R-biosimilar-treated patients. The Kaplan-Meier method served to estimate OS, and the log-rank test to examine differences in survival distributions between R-originator- and R-biosimilar-treated patients. Cox proportional hazard regression analyses were performed to investigate the effect of age, sex, Ann Arbor stage, WHO performance score, IPI score, and the number of R-CHOP cycles on OS. The results from the Cox regression analyses produce hazard ratios with associated 95% confidence intervals (CIs). The proportional hazard assumption was tested based on the Schoenfeld residuals.6 Covariates were introduced in the regression models with a forward selection method, after adjusting for influence of those already selected. The final model was accomplished when the P value for entering an additional covariate was above .05. Patients alive were censored on 1 February 2021.
To account for differences in duration of follow-up between patients treated with R-originator and R-biosimilars, we additionally performed a sensitivity analysis. First, we selected patients with DLBCL diagnosed between 1 April 2017 and 31 December 2018 (the period following EMA approval of R-biosimilars) and compared patients in the R-originator group with patients in the R-biosimilars group on OS. Second, we selected patients with DLBCL in the R-originator group and compared patients diagnosed in the period before EMA approval (pre-EMA) of R-biosimilars to patients diagnosed in the period after EMA approval (post-EMA) of R-biosimilars.
P values < .05 indicated statistical significance. All statistical analyses were performed with STATA version 16.1 (StataCorp, College Station, TX).
Results
Patients
In total, we identified 4968 patients with DLBCL treated with R-CHOP. For 539 patients with newly diagnosed DLBCL, information on the use of R-biosimilars was unknown and were excluded from analysis, leaving 4429 patients in our study population.
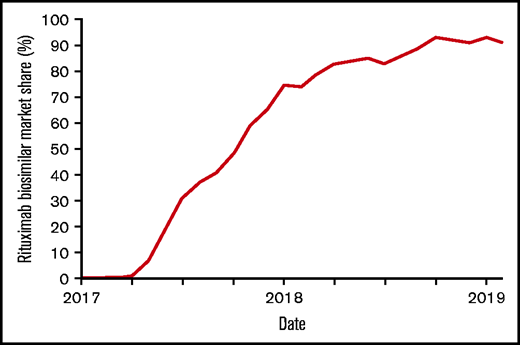
Pre-EMA (ie, between 1 January 2014 and 31 March 2017), 3183 patients received R-originator. Post-EMA (from 1 April 2017 onward), the purchase percentage to R-biosimilars, compared with R-originator, had risen to 91% by the end of 2018, resulting in 370 patients receiving R-originator and 876 R-biosimilars (CT-P10 86%, GP2013 14%) (Figure 1). The baseline characteristics of all 4429 patients, including age, sex, IPI score, and the number of R-CHOP cycles, are presented in Table 1, according to patients treated with R-originator (n = 3553) and R-biosimilars (n = 876). In the R-biosimilars group, a higher proportion of patients had a WHO performance status of 0-2 (57% vs 53%; P = .044), and a lower proportion of patients received >6 cycles of R-CHOP (24% vs 30%; P = .004) compared with the R-originator group.
Pace of conversion of rituximab originator (R-originator) to rituximab biosimilars (R-biosimilars) in the Netherlands. Line graph depicting the market share of rituximab biosimilars from their approval by the European Medicines Agency in 2017 until January 2019.
Pace of conversion of rituximab originator (R-originator) to rituximab biosimilars (R-biosimilars) in the Netherlands. Line graph depicting the market share of rituximab biosimilars from their approval by the European Medicines Agency in 2017 until January 2019.
Characteristics of DLBCL patients receiving rituximab originator or rituximab biosimilars as part of R-CHOP treatment regimen
| . | Rituximab originator . | Rituximab biosimilars . | . | ||
|---|---|---|---|---|---|
| Characteristic . | (N = 3553) . | (N = 876) . | P value . | ||
| Female, no. (%) | 1466 | (41.3) | 344 | (39.3) | .283 |
| Age, y, median (range) | 68 | (18-95) | 68 | (21-95) | .054 |
| Age, y, no. (%) | |||||
| ≤60 | 1092 | (30.7) | 232 | (26.5) | .014 |
| Ann Arbor stage (%) | |||||
| I-II | 1282 | (36.1) | 305 | (34.8) | .333 |
| III-IV | 2257 | (63.5) | 570 | (65.1) | |
| Unknown | 14 | (0.4) | 1 | (0.1) | |
| Lactate dehydrogenase, no. (%) | |||||
| Normal | 1668 | (46.9) | 374 | (42.7) | .066 |
| Elevated | 1819 | (51.2) | 487 | (55.6) | |
| Unknown | 66 | (1.9) | 15 | (1.7) | |
| WHO performance status, no. (%) | |||||
| 0-2 | 1880 | (52.9) | 503 | (57.4) | .044 |
| 3-4 | 75 | (2.1) | 20 | (2.3) | |
| Unknown | 1598 | (45.0) | 353 | (40.3) | |
| Number of extranodal sites, no. (%) | |||||
| 0–1 | 2550 | (71.8) | 653 | (74.5) | .210 |
| >1 | 1001 | (28.2) | 223 | (25.5) | |
| Unknown | 2 | (0.06) | 0 | (0) | |
| IPI score, no. (%) | |||||
| Low (0-1) | 682 | (1929) | 150 | (17.1) | .133 |
| Low-intermediate (2) | 464 | (13.1) | 129 | (14.7) | |
| High-intermediate (3) | 463 | (13.0) | 129 | (14.7) | |
| High (4-5) | 546 | (15.4) | 148 | (16.9) | |
| Unknown | 1398 | (39.4) | 320 | (36.5) | |
| Time until treatment, days, median | 24 | (15-36) | 24 | (14-38) | .314 |
| Number of cycles, no. (%) | |||||
| <6 | 922 | (25.9) | 241 | (27.5) | .004 |
| 6 | 1566 | (44.1) | 426 | (48.6) | |
| >6 | 1062 | (29.9) | 209 | (23.9) | |
| Unknown | 3 | (0.1) | 0 | (0) | |
| . | Rituximab originator . | Rituximab biosimilars . | . | ||
|---|---|---|---|---|---|
| Characteristic . | (N = 3553) . | (N = 876) . | P value . | ||
| Female, no. (%) | 1466 | (41.3) | 344 | (39.3) | .283 |
| Age, y, median (range) | 68 | (18-95) | 68 | (21-95) | .054 |
| Age, y, no. (%) | |||||
| ≤60 | 1092 | (30.7) | 232 | (26.5) | .014 |
| Ann Arbor stage (%) | |||||
| I-II | 1282 | (36.1) | 305 | (34.8) | .333 |
| III-IV | 2257 | (63.5) | 570 | (65.1) | |
| Unknown | 14 | (0.4) | 1 | (0.1) | |
| Lactate dehydrogenase, no. (%) | |||||
| Normal | 1668 | (46.9) | 374 | (42.7) | .066 |
| Elevated | 1819 | (51.2) | 487 | (55.6) | |
| Unknown | 66 | (1.9) | 15 | (1.7) | |
| WHO performance status, no. (%) | |||||
| 0-2 | 1880 | (52.9) | 503 | (57.4) | .044 |
| 3-4 | 75 | (2.1) | 20 | (2.3) | |
| Unknown | 1598 | (45.0) | 353 | (40.3) | |
| Number of extranodal sites, no. (%) | |||||
| 0–1 | 2550 | (71.8) | 653 | (74.5) | .210 |
| >1 | 1001 | (28.2) | 223 | (25.5) | |
| Unknown | 2 | (0.06) | 0 | (0) | |
| IPI score, no. (%) | |||||
| Low (0-1) | 682 | (1929) | 150 | (17.1) | .133 |
| Low-intermediate (2) | 464 | (13.1) | 129 | (14.7) | |
| High-intermediate (3) | 463 | (13.0) | 129 | (14.7) | |
| High (4-5) | 546 | (15.4) | 148 | (16.9) | |
| Unknown | 1398 | (39.4) | 320 | (36.5) | |
| Time until treatment, days, median | 24 | (15-36) | 24 | (14-38) | .314 |
| Number of cycles, no. (%) | |||||
| <6 | 922 | (25.9) | 241 | (27.5) | .004 |
| 6 | 1566 | (44.1) | 426 | (48.6) | |
| >6 | 1062 | (29.9) | 209 | (23.9) | |
| Unknown | 3 | (0.1) | 0 | (0) | |
Statistically significant P values (P < .05) are presented in bold.
Response rate
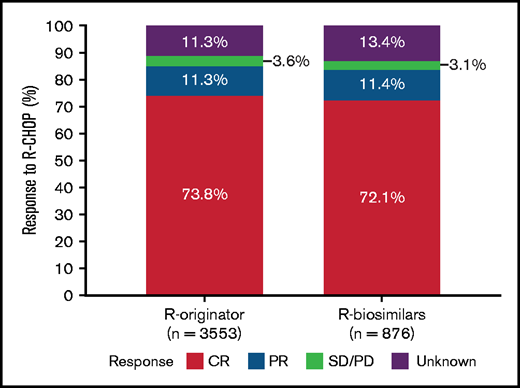
The ORR between R-originator and R-biosimilars was not significantly different (85% vs 84%, P = .326) (Figure 2). In detail, 74% of the patients in the R-originator group had a CR compared with 72% in the R-biosimilars group. In addition, the observed proportion of patients with a partial response was similar in both groups (11% and 11%).
Best response after first-line therapy with rituximab originator (R-originator) or rituximab biosimilars (R-biosimilars). Stacked bar graph depicting tumor response in patients with diffuse large B-cell lymphoma treated with rituximab originator (R-originator) or rituximab biosimilars (R-biosimilars) combined with standard CHOP chemotherapy regimens.
Best response after first-line therapy with rituximab originator (R-originator) or rituximab biosimilars (R-biosimilars). Stacked bar graph depicting tumor response in patients with diffuse large B-cell lymphoma treated with rituximab originator (R-originator) or rituximab biosimilars (R-biosimilars) combined with standard CHOP chemotherapy regimens.
OS
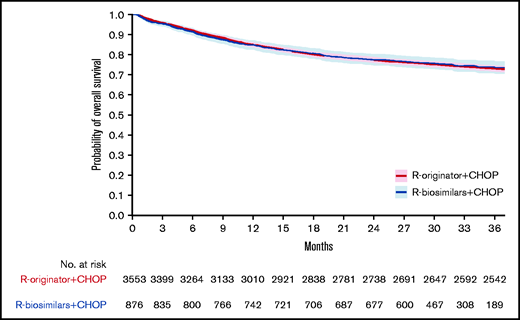
The median follow-up for patients with DLBCL alive treated with R-originator and those who received R-biosimilars were 61.7 months (range, 8.1-85.0 months) and 32.3 months (range, 23.6-45.2 months), respectively. The 3-year OS of patients treated with R-originator or R-biosimilars were 73% (95% CI, 72-74) and 73% (95% CI, 70-76; P = .855), respectively, and is presented in Figure 3. When simultaneously adjusted for age at diagnosis, sex, IPI score, and the number of R-CHOP cycles, the risk of mortality was similar for patients treated with R-biosimilars (hazard ratio, 0.89; 95% CI, 0.76-1.05; P = .182) and those treated with R-originator (supplemental Table 1).
Overall survival of patients treated with rituximab originator or rituximab biosimilars. Kaplan-Meier curves depicting 3-year overall survival in patients with DLBCL treated with rituximab originator (R-originator, in red) or rituximab biosimilars (R-biosimilars, in blue) combined with standard CHOP chemotherapy regimens. The 95% confidence intervals are depicted as blue/red shaded areas.
Overall survival of patients treated with rituximab originator or rituximab biosimilars. Kaplan-Meier curves depicting 3-year overall survival in patients with DLBCL treated with rituximab originator (R-originator, in red) or rituximab biosimilars (R-biosimilars, in blue) combined with standard CHOP chemotherapy regimens. The 95% confidence intervals are depicted as blue/red shaded areas.
Sensitivity analysis
To account for differences in duration of follow-up between patients receiving R-originator and R-biosimilars, we performed a sensitivity analysis using a 2-way approach. First, all patients in the R-originator group diagnosed pre-EMA were excluded, leaving 370 patients who received R-originator and 876 patients who received R-biosimilars between 1 April 2017 and 31 December 2018. No differences in baseline characteristics were observed between both groups of patients, as well as in response rates (data not shown). The median follow-up was 42.7 months (range, 15.8-46.0) for alive patients treated with R-originator and 32.3 months (range, 23.6-45.2) for alive patients treated with R-biosimilars. The shorter median follow-up for the R-biosimilar-treated patients was due to the timing R-biosimilars were introduced in routine clinical practice. The 3-year OS was 74% (95% CI, 70-79) for the R-originator group and 73% (95% CI, 70-76) for the R-biosimilar group (P = .798).
Second, all patients who received R-biosimilars were excluded, leaving 3183 patients who received R-originator between 1 January 2014 and 31 March 2017, and 370 patients who received R-originator between 1 April 2017 and 31 December 2018. Here, patients who were diagnosed pre-EMA more often received >6 cycles of R-CHOP compared with patients diagnosed in the post-EMA (31% vs 22%; P < .001). Otherwise, no differences in baseline characteristics and response rates were observed between patients diagnosed in the pre-EMA and patients post-EMA (data not shown). The median follow-up was 64.2 months (range, 8.1–85.0) for alive patients treated with R-originator pre-EMA compared with 42.7 months (range, 15.8–46.0) for alive patients treated with R-originator post-EMA. The 3-year OS was 74% for patients diagnosed post-EMA (95% CI, 70-79) and 73% for patients diagnosed in the R-originator group pre-EMA (95% CI, 71-74; P = .619). Overall, the results of the sensitivity analyses were similar to those of the primary analyses.
Discussion
We evaluated the 3-year OS of R-biosimilars compared with R-originator in patients with newly diagnosed DLBCL using a contemporary, nationwide, population-based cancer registry in the Netherlands. The OS and the best response of patients with DLBCL did not differ between the R-biosimilars and R-originator groups.
To our knowledge, this is the first population-based study post-EMA of R-biosimilars to link treatment with R-biosimilars to survival among patients with newly diagnosed DLBCL. The 3-year OS in the current study was similar to the survival of the R-originator CHOP reported 15 years ago.7 Collectively, our data show that patients with DLBCL benefit equally from R-originator or R-biosimilars combined with CHOP treatment.
The R-originator was approved by the US Food and Drug Administration (FDA) on 26 November 1997 for treatment of relapsed/refractory CD20+ follicular lymphoma and, subsequently, on 2 June 1998 by the EMA. It has since been the backbone of every treatment regimen in B-cell non-Hodgkin lymphomas. Although the glycoengineered anti-CD20 antibody obinutuzumab showed enhanced efficacy for follicular lymphoma and chronic lymphocytic leukemia, as compared with R-originator, this was not substantiated in DLBCL.8-10 The approval of the subcutaneous R-originator for the treatment of DLBCL has not been translated into widespread use. Therefore, R-originator administered intravenously remained the standard of care for most patients with DLBCL.11
Since the expiration of the patent of R-originator, several R-biosimilars have been approved by the EMA and subsequently by the FDA.2,3,12-14 Despite the preclinical similarity between R-biosimilars and R-originator, the latter having a considerable share of the global market, the implementation of R-biosimilars into routine clinical practice fluctuates greatly between countries. In the current study, we objectivized that the transition to R-biosimilars in the Netherlands occurred within 12 months following EMA approval without an adverse impact on 3-year OS, thus supporting the use of R-biosimilars in DLBCL.
Global spending on cancer medicines has risen from $96 billion in 2013 to $150 billion in 2018. It might increase to $200 billion by 2023.15 Biosimilars hold the promise of constraining health care spending with 55 products already approved by the EMA and 26 by the FDA in May 2020.16 Estimated cost reduction until 2026 is $24 to $150 billion for oncology products in the United States alone.17 With global sales of $7.6 billion of R-originator in 2017, R-biosimilars may have major health economic consequences. With a worldwide R-originator sales increase of 1% in 2017, purchase percentages of R-originator decreased by 8% and 4% in 2018 and 2019, respectively.18 The highest conversion rate occurred in Europe with a 33% purchase reduction of R-originator in 2019.18 Despite approval in 2018, the first R-biosimilar was only marketed in November 2019 in the United States. In that same year, the relative expenditure on R-originator in the Netherlands was 43% lower than in 2015.19
The main strength of this study is the use of a nationwide population-based cancer registry over clinical trial data because clinical trial data are based on selections of patients. Second, with the availability of comprehensive data on first-line treatment of a substantial number of patients, treatment practices could be assessed and linked to outcome. Moreover, by combining clinical data from the NCR and validated purchase information on rituximab from FarmInform, we were able to distinguish patients treated with R-originator and R-biosimilar. Limitations of this study mainly pertain to the lack of detailed information on dosage per cycle at the patient level and treatment-related toxicities, including infusion-related reactions. Third, we observed a change in standard-of-care treatment strategy for patients with DLBCL over time, with a reduction of the number of cycles R-CHOP used. However, 6 cycles of R-CHOP did not negatively affect survival compared with 8 cycles of R-CHOP, regardless of whether patients with DLBCL received R-originator or R-biosimilars in combination with CHOP.20,21 Fourth, although 2-year event-free-survival (EFS) is considered a robust end point in DLBCL,22,23 because the relapse rate after this time point is low, we acknowledge that 2-year EFS is lacking as an outcome parameter in our study. Because OS is affected by the efficacy of salvage treatment and a 2-year OS does not guarantee a similar 2-year EFS between R-originator and R-biosimilars, we evaluated a longer term OS (ie, 3 years). Despite the previously mentioned limitations, this cancer registry represents an important tool to gain insight into the clinical data and outcome of large numbers of patients pre- and post-EMA approval because all newly diagnosed patients are captured.
In conclusion, in this nationwide, population-based study, we observed no difference in OS between patients with DLBCL treated with R-biosimilars and patients with DLBCL treated with R-originator. These results favor the use of R-biosimilars in the first-line treatment of DLBCL because this helps to constrain worldwide health care spending without negatively affecting OS.
Acknowledgments
The authors thank the registration clerks of the Netherlands Cancer Registry for their dedicated data collection, Robert Beenes of FarmInform for his logistical support, and the pharmacists of the Dutch hospitals for their contribution to the validation check.
Authorship
Contribution: M.N. designed the study; M.B. collected the data; M.B. and X.U.K. analyzed the data; M.N. and M.B. interpreted the data and drafted the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.G.E.d.V. reports institutional financial support for advisory board services from Daiichi Sankyo, NSABP, Sanofi, Pfizer, and Synthon and institutional financial support for clinical trials or contracted research from Amgen, AstraZeneca, Bayer, Chugai Pharma, CytomX Therapeutics, Genentech, G1 Therapeutics, Nordic Nanovector, Radius Health, Roche, Servier, and Synthon, outside the submitted work. P.J.L. reports grants and personal fees from Roche, grants and personal fees from Takeda, and personal fees from Genmab, Sandoz, Regeneron, and Celgene, outside the submitted work. M.J.K. reports personal fees and nonfinancial support from Roche, Celgene/BMS, Kite/Gilead, Novartis, Miltenyi Biotech, and Takeda, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Marcel Nijland, University Medical Center Groningen, University of Groningen, Groningen, Department of Hematology, Hanzeplein 1, DA21, 9713 GZ Groningen, The Netherlands; e-mail: m.nijland@umcg.nl.
References
Author notes
Send data sharing requests via e-mail to the corresponding author at m.nijland@umcg.nl.
The full-text version of this article contains a data supplement.