Key Points
Pola is an effective treatment in heavily pretreated patients with r/r LBCL, but long-term remissions are rare.
Pola serves as a valuable bridging treatment to CAR T-cell therapy and allogeneic hematopoietic cell transplantation.
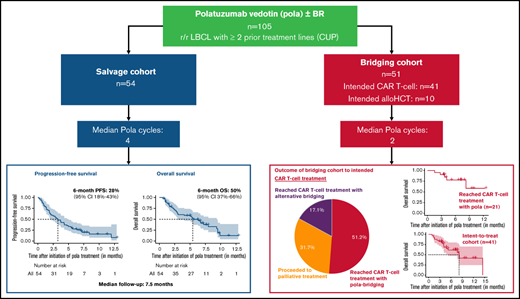
The antibody-drug conjugate polatuzumab vedotin (pola) has recently been approved in combination with bendamustine and rituximab (pola-BR) for patients with refractory or relapsed (r/r) large B-cell lymphoma (LBCL). To investigate the efficacy of pola-BR in a real-world setting, we retrospectively analyzed 105 patients with LBCL who were treated in 26 German centers under the national compassionate use program. Fifty-four patients received pola as a salvage treatment and 51 patients were treated with pola with the intention to bridge to chimeric antigen receptor (CAR) T-cell therapy (n = 41) or allogeneic hematopoietic cell transplantation (n = 10). Notably, patients in the salvage and bridging cohort had received a median of 3 prior treatment lines. In the salvage cohort, the best overall response rate was 48.1%. The 6-month progression-free survival and overall survival (OS) was 27.7% and 49.6%, respectively. In the bridging cohort, 51.2% of patients could be successfully bridged with pola to the intended CAR T-cell therapy. The combination of pola bridging and successful CAR T-cell therapy resulted in a 6-month OS of 77.9% calculated from pola initiation. Pola vedotin-rituximab without a chemotherapy backbone demonstrated encouraging overall response rates up to 40%, highlighting both an appropriate alternative for patients unsuitable for chemotherapy and a new treatment option for bridging before leukapheresis in patients intended for CAR T-cell therapy. Furthermore, 7 of 12 patients with previous failure of CAR T-cell therapy responded to a pola-containing regimen. These findings suggest that pola may serve as effective salvage and bridging treatment of r/r LBCL patients.
Introduction
Large B-cell lymphomas (LBCL) encompass a group of aggressive B-cell lymphomas including diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma, and high-grade B-cell lymphoma (HGBCL) including both HGBCL NOS and HGBCL with MYC and BCL2 and/or BCL6 rearrangements. Patients with LBCL who have failed autologous stem cell transplantation (autoHCT) or are ineligible for transplant have a dismal outcome.1-6 With the recent approval of the antibody-drug conjugate polatuzumab vedotin (pola) in combination with bendamustine and rituximab (pola-BR), a novel treatment option is available for this challenging cohort of LBCL patients. Pola consists of a CD79b-binding monoclonal antibody conjugated to monomethyl auristatin E, a potent anti-mitotic agent. CD79b functions as a signaling component of the B-cell receptor and is highly expressed on most types of B-cell malignancies including DLBCL.7-10 Hence, pola selectively targets B cells into which monomethyl auristatin E is internalized and cleaved from its linker by lysosomal proteases before binding to microtubules to inhibit cell division and induce apoptosis.11 In the approval phase 1b/2 study (GO29365), pola-BR was compared with a BR control arm with 40 patients with relapsed and refractory (r/r) DLBCL in each arm.12 The overall response (OR) rate and complete response (CR) rate in the pola-BR arm was 45% and 40%, respectively, and significantly higher than in the BR control arm (OR and CR rate: 18%). The median progression-free survival (PFS) and overall survival (OS) of patients treated with pola-BR was 9.5 months and 12.4 months, respectively, and was significantly longer than in the BR control cohort. The efficacy of pola-BR was recently confirmed in an extended cohort of 106 DLBCL patients.13
Chimeric antigen receptor (CAR) T-cell therapy has become a promising and potentially curative treatment option for r/r LBCL patients.14,15 However, a substantial proportion of patients considered eligible for CAR T-cell therapy fails to proceed to dosing because of rapid LBCL progression.16,17 Moreover, evidence is emerging that high tumor load and active tumor proliferation at the time of lymphodepletion for CAR T-cell therapy are associated with an unfavorable outcome.17-19 Thus, effective bridging treatments to CAR T-cell therapy appear to be highly desirable. The promising efficacy and the safety profile suggest that pola could be suitable for this purpose. To this end, we analyzed 105 r/r LBCL patients who received pola under the German compassionate use program (CUP) either as salvage treatment (n = 54) or as bridging treatment (n = 51) to CAR T-cell therapy or allogeneic hematopoietic cell transplantation (alloHCT).
Methods
Study design and patient eligibility
All patients eligible for this retrospective multicenter study were adults with r/r LBCL treated with pola under the German CUP since January 2019. The CUP offered the possibility to treat r/r DLBCL patients with pola-BR in Germany before pola was approved by the European Medicine Agency in January 2020. Patients were eligible for treatment under the CUP when they had failed at least 2 lines of therapy including rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone or similar first-line treatment regimens.
Baseline characteristics, treatment details, and outcome data were extracted by chart review. The study was performed in accordance with the Declaration of Helsinki. Patients signed informed consent before starting the treatment under the CUP. The central institutional review board and the local institutional review boards of the participating centers approved the study protocol. The study is a project of the German Lymphoma Alliance.
Statistical analysis
The primary end point of this study was OS from the initiation of pola treatment. Secondary end points included best OR and CR rate, PFS, and prognostic factor analysis. In the cohort of patients who received pola as bridging treatment, the proportion of patients proceeding to intended cellular immunotherapy was a further cohort-specific secondary end point.
Response to therapy was determined by the investigators using computed tomography (CT) scans. In case of absence of CT scans, response assessment was based on physician’s clinical judgment including results of physical examination and laboratory assessment. Both clinical responses and objective responses including CR and partial response (PR) were considered as OR.
Probabilities of OS and PFS were calculated using the Kaplan-Meier estimate. Events for OS were defined as death from any cause, and events for PFS as disease relapse/progression or death from any cause. Surviving patients were censored at the date of last follow‐up. In the bridging cohort, no censoring of patients was performed when patients reached the intended cellular immunotherapy. Survival curves were compared using the log-rank test. For univariate and multivariate analysis of survival times, Cox proportional hazard modeling was performed. The median observation time was calculated by the reverse Kaplan-Meier estimate.20 Differences in patient characteristics and response rates between treatment groups were estimated using Fisher’s exact test for categorical variables and the Mann-Whitney test for quantitative variables. Significance levels were set at P = .05. Calculations were done by R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Data were analyzed as of October 20, 2020.
Results
Patient characteristics
Altogether, 105 patients were included in this study. Fifty-four patients received salvage treatment with a pola-containing regimen (salvage cohort; supplemental Figure 1) and 51 patients were treated with a pola-containing regimen with the intention of bridging to a cellular immunotherapy (bridging cohort: CAR T-cell therapy, n = 41; alloHCT, n = 10; supplemental Figure 1). Patient characteristics for both cohorts are summarized in Table 1. Patients in the bridging cohort were significantly younger than patients in the salvage cohort, had more often undergone a prior autoHCT, and tended to have a shorter interval between diagnosis and study entry. Patients of both cohorts were heavily pretreated with a median of 3 prior treatment lines (range, 2-8). In both cohorts, the majority of patients were refractory to their last treatment line (bridging cohort: 84.3%; salvage cohort: 87%).
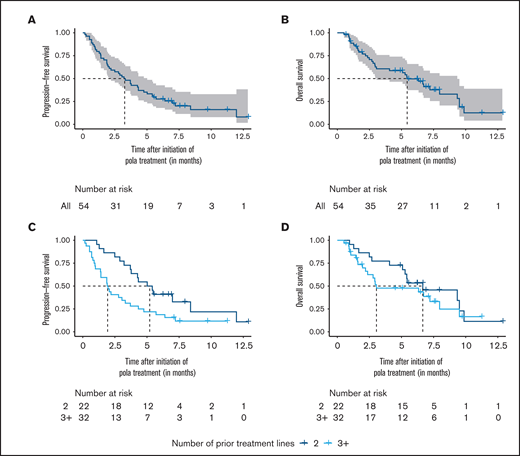
Outcome of patients in the salvage cohort. (A-B) Progression-free survival and overall survival of the salvage cohort. (C-D) Progression-free survival and overall survival stratified by the number of prior systemic treatment lines. Survival times were calculated from the initiation of polatuzumab vedotin (pola) treatment.
Outcome of patients in the salvage cohort. (A-B) Progression-free survival and overall survival of the salvage cohort. (C-D) Progression-free survival and overall survival stratified by the number of prior systemic treatment lines. Survival times were calculated from the initiation of polatuzumab vedotin (pola) treatment.
Patient baseline characteristics
| Characteristic . | Salvage cohort (n = 54) . | Bridging cohort (n = 51) . | P . |
|---|---|---|---|
| Median age, y (range) | 73.5 (37-87) | 61 (22-82) | <.001 |
| Male (%) | 37 (68.5) | 39 (76.5) | .4 |
| Diagnosis (%) | |||
| DLBCL | 49 (90.7) | 48 (94.1) | .7 (DLBCL vs other) |
| GCB | 16 (29.6) | 19 (37.3) | |
| ABC | 13 (24.1) | 7 (13.7) | |
| COO unknown | 20 (37.0) | 22 (43.1) | |
| PMBCL | 0 | 1 (2) | |
| HGBCL | 5 (9.3) | 2 (3.9) | |
| NOS | 3 (5.6) | 0 | |
| With MYC and BCL2 and/or BCL6 rearrangements | 2 (3.7) | 2 (3.9) | |
| Proportion of transformed lymphoma (%) | 15 (27.8) | 9 (17.6) | .3 |
| Time from diagnosis, mo (range) | 18.6 (4.6-143) | 13.4 (2.4-120) | .096 |
| Prior systemic treatment lines (range) | 3 (2-8) | 3 (2-6) | .49 |
| Failed autoHCT (%) | 5 (9.3) | 16 (31.4) | .007 |
| Failed alloHCT (%) | 2 (3.7) | 5 (9.8) | .3 |
| Failed CAR T-cell therapy (%) | 5 (9.3) | 7 (13.7) | .5 |
| Refractoriness to last pretreatment (%) | 47 (87) | 43 (84.3) | .8 |
| Characteristic . | Salvage cohort (n = 54) . | Bridging cohort (n = 51) . | P . |
|---|---|---|---|
| Median age, y (range) | 73.5 (37-87) | 61 (22-82) | <.001 |
| Male (%) | 37 (68.5) | 39 (76.5) | .4 |
| Diagnosis (%) | |||
| DLBCL | 49 (90.7) | 48 (94.1) | .7 (DLBCL vs other) |
| GCB | 16 (29.6) | 19 (37.3) | |
| ABC | 13 (24.1) | 7 (13.7) | |
| COO unknown | 20 (37.0) | 22 (43.1) | |
| PMBCL | 0 | 1 (2) | |
| HGBCL | 5 (9.3) | 2 (3.9) | |
| NOS | 3 (5.6) | 0 | |
| With MYC and BCL2 and/or BCL6 rearrangements | 2 (3.7) | 2 (3.9) | |
| Proportion of transformed lymphoma (%) | 15 (27.8) | 9 (17.6) | .3 |
| Time from diagnosis, mo (range) | 18.6 (4.6-143) | 13.4 (2.4-120) | .096 |
| Prior systemic treatment lines (range) | 3 (2-8) | 3 (2-6) | .49 |
| Failed autoHCT (%) | 5 (9.3) | 16 (31.4) | .007 |
| Failed alloHCT (%) | 2 (3.7) | 5 (9.8) | .3 |
| Failed CAR T-cell therapy (%) | 5 (9.3) | 7 (13.7) | .5 |
| Refractoriness to last pretreatment (%) | 47 (87) | 43 (84.3) | .8 |
Salvage chemoimmunotherapy followed by high-dose chemotherapy and autologous hematopoietic cell transplantation was counted as 1 treatment line. Refractoriness to last pretreatment was defined as no response or progression within 6 mo of last treatment.
ABC, activated B cell; COO, cell of origin; GCB, germinal-center B cell; PMBCL, primary mediastinal B-cell lymphoma.
Treatment and outcome of the salvage cohort
The majority of patients in the salvage cohort were treated with pola-BR (59.3%) or alternative chemotherapy combinations including pola-bendamustine (1.85%) and pola-R-gemcitabine (1.85%). The remaining 37% of patients received pola in combination with rituximab without a chemotherapy backbone (Table 2). The median number of administered pola cycles was 4 (range, 1-9). Twenty-two of 54 patients (40.7%) completed 6 cycles of pola treatment, whereas treatment of 28 patients (51.8%) was prematurely stopped because of lymphoma progression (Table 2).
Treatment characteristics
| Characteristic . | Salvage cohort (n = 54) . | Bridging cohort (n = 51) . | P value . |
|---|---|---|---|
| Pola treatment | |||
| Chemotherapy backbone | .6 (chemotherapy backbone vs no chemotherapy backbone) | ||
| pola-BR | 32 (59.3%) | 27 (52.9%) | |
| pola-B | 1 (1.85%) | 1 (1.96%) | |
| pola-R-CHP | 0 | 1 (1.96%) | |
| pola-R-gemcitabine | 1 (1.85%) | 0 | |
| No chemotherapy backbone | |||
| pola-R | 20 (37.0%) | 19 (37.3%) | |
| pola-monotherapy | 0 | 3 (5.9%) | |
| Median number of pola cycles (range) | 4 (1-9) | 2 (1-6) | .001 |
| Reason for treatment cessation | |||
| Treatment completed with 6 cycles | 22 (40.7%) | 4 (7.8%) | |
| Availability of intended IT | n/a | 23 (45.1%) | |
| Progressive disease | 28 (51.8%) | 21 (41.2%) | |
| AEs | 2 (3.7%) | 0 | |
| Withdrawal of patients | 1 (1.9%) | 0 | |
| Unknown | 1 (1.9%) | 3 (5.9%) |
| Characteristic . | Salvage cohort (n = 54) . | Bridging cohort (n = 51) . | P value . |
|---|---|---|---|
| Pola treatment | |||
| Chemotherapy backbone | .6 (chemotherapy backbone vs no chemotherapy backbone) | ||
| pola-BR | 32 (59.3%) | 27 (52.9%) | |
| pola-B | 1 (1.85%) | 1 (1.96%) | |
| pola-R-CHP | 0 | 1 (1.96%) | |
| pola-R-gemcitabine | 1 (1.85%) | 0 | |
| No chemotherapy backbone | |||
| pola-R | 20 (37.0%) | 19 (37.3%) | |
| pola-monotherapy | 0 | 3 (5.9%) | |
| Median number of pola cycles (range) | 4 (1-9) | 2 (1-6) | .001 |
| Reason for treatment cessation | |||
| Treatment completed with 6 cycles | 22 (40.7%) | 4 (7.8%) | |
| Availability of intended IT | n/a | 23 (45.1%) | |
| Progressive disease | 28 (51.8%) | 21 (41.2%) | |
| AEs | 2 (3.7%) | 0 | |
| Withdrawal of patients | 1 (1.9%) | 0 | |
| Unknown | 1 (1.9%) | 3 (5.9%) |
AEs, adverse events; IT, immunotherapy.
Best responses achieved with pola were a CR in 8 patients (14.8%) and a PR or clinical response in 18 patients (33.3%), resulting in an OR rate of 48.1% (Table 3). Next, we separately analyzed response rates for patients who either received a chemotherapy-containing or a chemotherapy-free pola-based treatment. The OR rate of patients who received pola in combination with chemotherapy was 52.9%, whereas the OR rate of patients treated with pola vedotin-rituximab (pola-R) was 40% (P = .4). To evaluate if pola should be used earlier or later during the LBCL disease, we compared the OR rate for patients with 2 vs ≥3 prior lines of therapy and found no significant difference between both subgroups (2 vs ≥3 lines: 54.5% vs 43.8%; P = .6).
Responses to polatuzumab vedotin in the salvage cohort
| Best response . | Salvage cohort (n = 54) . |
|---|---|
| OR rate* | 26 (48.1) |
| CR | 8 (14.81) |
| PR | 15 (27.78) |
| Clinical response | 3 (5.56) |
| Nonresponse rate | 28 (51.9) |
| SD | 4 (7.41) |
| MR | 3 (5.56) |
| PD | 11 (20.37) |
| Clinical progression | 10 (18.52) |
| Best response . | Salvage cohort (n = 54) . |
|---|---|
| OR rate* | 26 (48.1) |
| CR | 8 (14.81) |
| PR | 15 (27.78) |
| Clinical response | 3 (5.56) |
| Nonresponse rate | 28 (51.9) |
| SD | 4 (7.41) |
| MR | 3 (5.56) |
| PD | 11 (20.37) |
| Clinical progression | 10 (18.52) |
Objective responses specified as CR, PR, SD, MR, and PD were assessed by CT. In absence of CT scans, response assessment was based on physician’s clinical judgment including results of physical examination and laboratory assessment and documented as clinical response, clinical stable disease, or clinical progression. Response assessment with CT scans was available in 75.9% (41/54) patients. The median time of first CT response assessment was 50 d (range, 12-193 d). The median time to best CT response was 68 d (range, 12-217 d).
MR, mixed response; PD, progressive disease; SD, stable disease.
*Objective responses (including CR and PR) and clinical responses were considered as OR.
The 6- and 12-month PFS from initiation of pola were 27.7% (95% confidence interval [CI], 17.9-42.6) and 8.0% (95% CI, 1.7-38.3), respectively (median follow-up [FU]: 7.5 months; 95% CI, 6.6-not reached) (Figure 1A). The 6- and 12-month OS were 49.6% (95% CI, 37.4-65.9) and 12.6% (95% CI, 4.1-38.9), respectively (Figure 1B). Similar to the response rates, we did not observe significant differences of PFS and OS between patients who either received a chemotherapy-containing or a chemotherapy-free pola-based treatment (PFS: P = .68; OS: P = .84; supplemental Figure 2A-B). The pretreatment status impacted the treatment outcome; patients with a maximum of 2 prior lines of treatment had a significantly longer median PFS than patients with ≥3 treatment lines (median PFS 5.2 vs 1.9 months; HR 2.2; 95% CI, 1.2-4.1; P = .016; Figure 1C). Accordingly, the median OS tended to be higher in the group of patients with 2 prior treatment lines compared with patients with ≥3 prior treatment lines, although the difference was not significant (median OS 6.7 vs 3.1 months; HR 1.4; 95% CI, 0.7-2.8; P = .3; Figure 1D).
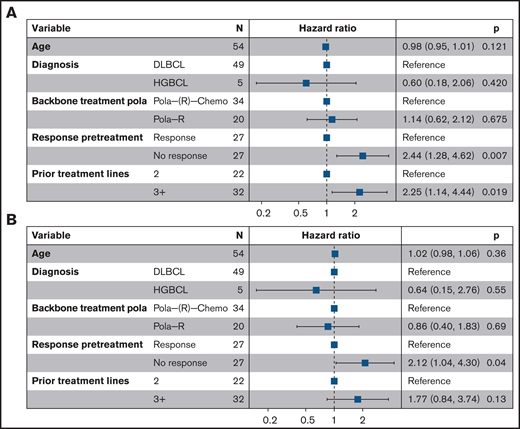
Multivariate analysis of risk factors for inferior outcome. Forest plots for (A) progression-free survival and (B) overall survival. Pola(-R)-chemo, polatuzumab vedotin and chemotherapy ± rituximab.
Multivariate analysis of risk factors for inferior outcome. Forest plots for (A) progression-free survival and (B) overall survival. Pola(-R)-chemo, polatuzumab vedotin and chemotherapy ± rituximab.
We further conducted a multivariate analysis for PFS and OS in the salvage cohort considering age, diagnosis, concurrent chemotherapy, prior lines of treatment, and response to the last prior treatment as covariates. The adverse impact of ≥3 prior treatment lines on the PFS was confirmed (≥3 vs 2 lines: HR 2.3; 95% CI, 1.1-4.4; P = .02; Figure 2). Response to the last pretreatment was an independent predictor of both PFS and OS (PFS, nonresponse vs response: HR 2.4; 95% CI. 1.3-4.6; P = .007; OS, nonresponse vs response: HR 2.1; 95% CI, 1.04-4.3; P = .04). As expected, patients with response to pola had better outcomes, but the median OS was still limited with 5.7 months calculated from the time of best response to pola (95% CI, 4.4-not reached; supplemental Figure 3). However, some individual patients experienced an ongoing remission of up to 9 months at the time of last follow-up.
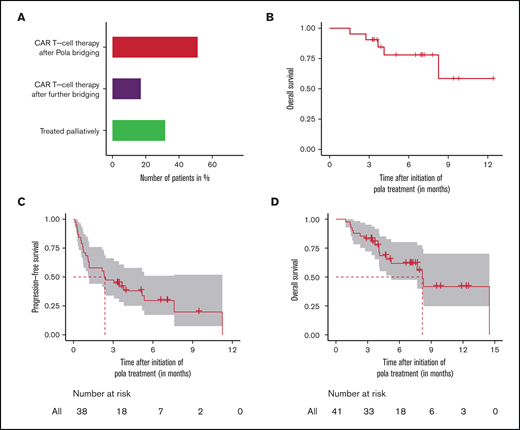
Outcome of patients in the bridging cohort. (A) Actual treatment of patients intended for CAR T-cell therapy and (B) overall survival of patients who received the intended CAR T-cell therapy after polatuzumab vedotin (pola) bridging. (C-D) Intent-to-treat-analysis with progression-free survival and overall survival of complete bridging cohort intended for CAR T-cell therapy. Survival times were calculated from the initiation of pola treatment. For the estimation of progression-free survival, data were only assessed in the efficacy-evaluable population. Three patients had no response evaluation and were excluded from the progression-free survival estimation.
Outcome of patients in the bridging cohort. (A) Actual treatment of patients intended for CAR T-cell therapy and (B) overall survival of patients who received the intended CAR T-cell therapy after polatuzumab vedotin (pola) bridging. (C-D) Intent-to-treat-analysis with progression-free survival and overall survival of complete bridging cohort intended for CAR T-cell therapy. Survival times were calculated from the initiation of pola treatment. For the estimation of progression-free survival, data were only assessed in the efficacy-evaluable population. Three patients had no response evaluation and were excluded from the progression-free survival estimation.
Treatment and outcome of the bridging cohort
In the bridging cohort, 56.9% of patients received pola combined with a chemotherapy-backbone (pola-BR: n = 27; pola-bendamustine: n = 1, pola-rituximab, cyclophosphamide, doxorubicin hydrochloride, and prednisone [R-CHP]: n = 1), whereas 43.1% were treated either with pola-R (n = 19) or pola-monotherapy (n = 3) (Table 2). Patients in the bridging cohort received a median of only 2 cycles (range, 1-6) of a pola-containing regimen, whereas 4 cycles (range, 1-9) were administered in the salvage group (P = .001) (Table 2).
The CAR T-cell therapy was successfully reached by 68.3% (28/41) of the patients who were initially intended to do so, with pola being the most recent regimen before lymphodepletion in 51.2% of the patients, whereas 17.1% underwent alternative bridging attempts following pola failure (Figure 3A). Responses to pola before CAR T-cell therapy were achieved in 7 patients, and stable disease and mixed response were observed in further 7 and 2 patients, respectively. Five patients progressed on pola but still received the CAR T-cell treatment (Table 4). Notably, 3 of these 5 patients with progression during pola achieved a remission after CAR T-cell therapy (CR: n = 1; PR: n = 2; PD: n = 1, not available: n = 1). The 6-month OS of patients who received the intended CAR T-cell therapy after pola was 77.9% (95% CI, 60.7-100) and the 12 month OS was 58.5% (95% CI, 31.5-100) (Figure 3B).
Responses to polatuzumab vedotin in the bridging cohort
| Best responses polatuzumab vedotin . | n . |
|---|---|
| Intended CAR T-cell therapy, n = 41 | |
| Proceeded to CAR T-cell therapy after pola bridging, n = 21 (51.2%) | |
| CR | 0 |
| PR | 5 |
| Clinical response | 2 |
| SD/clinical stable disease | 7 |
| MR | 2 |
| PD/clinical progression | 5 |
| Proceeded to CAR T-cell therapy after alternative bridging, n = 7 (17.1%) | |
| PD/clinical progression | 7 |
| Treated palliatively, n = 13 (31.7%) | |
| CR* | 1 |
| Clinical response† | 2 |
| MR | 1 |
| PD/clinical progression | 6 |
| Not evaluable | 3 |
| Intended alloHCT, n = 10 | |
| Proceeded to alloHCT after pola bridging, n = 5 | |
| CR | 1 |
| PR | 3 |
| SD | 1 |
| Proceeded to alloHCT after alternative bridging, n = 2 | |
| SD | 1 |
| PD | 1 |
| Treated palliatively, n = 3 | |
| PR† | 1 |
| Clinical response† | 1 |
| MR | 1 |
| Best responses polatuzumab vedotin . | n . |
|---|---|
| Intended CAR T-cell therapy, n = 41 | |
| Proceeded to CAR T-cell therapy after pola bridging, n = 21 (51.2%) | |
| CR | 0 |
| PR | 5 |
| Clinical response | 2 |
| SD/clinical stable disease | 7 |
| MR | 2 |
| PD/clinical progression | 5 |
| Proceeded to CAR T-cell therapy after alternative bridging, n = 7 (17.1%) | |
| PD/clinical progression | 7 |
| Treated palliatively, n = 13 (31.7%) | |
| CR* | 1 |
| Clinical response† | 2 |
| MR | 1 |
| PD/clinical progression | 6 |
| Not evaluable | 3 |
| Intended alloHCT, n = 10 | |
| Proceeded to alloHCT after pola bridging, n = 5 | |
| CR | 1 |
| PR | 3 |
| SD | 1 |
| Proceeded to alloHCT after alternative bridging, n = 2 | |
| SD | 1 |
| PD | 1 |
| Treated palliatively, n = 3 | |
| PR† | 1 |
| Clinical response† | 1 |
| MR | 1 |
Objective responses specified as CR, PR, SD, MR, and PD were assessed by CT. In absence of CT scans, response assessment was based on physician’s clinical judgment including results of physical examination and laboratory assessment and documented as clinical response, clinical stable disease, or clinical progression. Response assessment with CT scans was available in 68.6% (35/51) patients. The median time of first CT response assessment was 39 d (range, 9-124 d). The median time to best CT response was 42 d (range, 9-200 d).
MR, mixed response; PD, progressive disease; SD, stable disease.
Patient was followed up in palliative intention because of a failed T-cell apheresis.
Patients developed progressive disease during further treatment.
A total of 31.7% of the patients who were initially intended to receive a CAR T-cell therapy did not reach the CAR T-cell therapy and were treated palliatively primarily because of insufficient tumor control with the pola bridging treatment. The 6-month OS of these patients after pola initiation was 22% (95% CI, 6.8-70.7).
When calculating survival from study entry, including the pre-CAR T-cell treatment failures, 6-month PFS and OS from initiation of pola were 29.6% (95% CI, 17.2-51.1) and 61.8% (95% CI, 47.6-80.2), respectively (median FU: 7.2 months; 95% CI, 6.5-11.7) (Figure 3C-D).
Thirty-one patients of the intended CAR T-cell cohort underwent leukapheresis, of whom 10 patients were treated with pola-BR before leukapheresis. A second leukapheresis was performed in 2 patients because the first T-cell apheresis had failed after bridging treatment with pola-BR. One of these patients was then able to proceed to CAR T-cell therapy, whereas the other patient was treated palliatively because of a repeatedly failed leukapheresis. The manufacturing process of efficient CAR T cells failed in an additional patient who underwent the first leukapheresis after 1 course of pola-R and the second leukapheresis after 1 course of pola-BR.
In the intended alloHCT cohort of 10 patients, 5 could be successfully bridged after achieving a response (CR, n = 1; PR, n = 3) or stable disease (n = 1). Two patients achieved no response and pola bridging was replaced with an alternative bridging treatment enabling them to undergo alloHCT. The remaining 3 patients progressed despite initial response to pola and proceeded to a palliative treatment concept.
Outcome of pola treatment after failed CAR T-cell therapy
Altogether, 12 patients had failed CAR T-cell therapy before receiving pola as bridging treatment to alloHCT or as palliative treatment. Seven of those 12 patients responded to pola. Interestingly, 1 patient in the bridging cohort was successfully retreated with pola after CAR T-cell therapy failure. Initially, he was bridged with 3 cycles of pola-BR and was able to proceed to CAR T-cell therapy in PR, but relapsed within 2 months after CAR T-cell therapy. He was retreated with 5 cycles of pola-BR and achieved a PR again, enabling him to undergo consolidative alloHCT.
Tolerability and adverse events
The most frequently recorded adverse events of pola treatment are listed in Table 5. Cytopenias were the most common both for all-grade and grade 3-4 adverse events. Febrile neutropenia grade 3-4 occurred in 15.4% of patients in the salvage cohort and 6.1% of patients in the bridging cohort. Polyneuropathy was limited to grade 1-2 and recorded in 21.1% and 14.3% of patients in the salvage and bridging cohort, respectively. Tumor lysis occurred in 6 patients without severe long-term consequences.
Most frequently recorded adverse events during polatuzumab vedotin treatment
| . | Salvage cohort (n = 52)* . | Bridging cohort (n = 49)* . | ||
|---|---|---|---|---|
| Adverse event . | All grades (%) . | Grades 3-4 (%) . | All grades (%) . | Grades 3-4 (%) . |
| Blood disorders | ||||
| Anemia | 41 (78.8) | 14 (26.9) | 35 (71.4) | 14 (28.6) |
| Thrombocytopenia | 33 (63.5) | 17 (32.7) | 25 (51.0) | 10 (20.4) |
| Neutropenia | 31 (59.6) | 20 (38.5) | 17 (34.7) | 12 (24.5) |
| Febrile neutropenia | 12 (23.1) | 8 (15.4) | 3 (6.1) | 3 (6.1) |
| Infections† | 20 (38.5) | 10 (19.2) | 14 (28.6) | 11 (22.4) |
| Polyneuropathy | 11 (21.2) | 0 | 7 (14.3) | 0 |
| Tumor lysis | 2 (3.8) | 2 (3.8) | 4 (8.2) | 4 (8.2) |
| . | Salvage cohort (n = 52)* . | Bridging cohort (n = 49)* . | ||
|---|---|---|---|---|
| Adverse event . | All grades (%) . | Grades 3-4 (%) . | All grades (%) . | Grades 3-4 (%) . |
| Blood disorders | ||||
| Anemia | 41 (78.8) | 14 (26.9) | 35 (71.4) | 14 (28.6) |
| Thrombocytopenia | 33 (63.5) | 17 (32.7) | 25 (51.0) | 10 (20.4) |
| Neutropenia | 31 (59.6) | 20 (38.5) | 17 (34.7) | 12 (24.5) |
| Febrile neutropenia | 12 (23.1) | 8 (15.4) | 3 (6.1) | 3 (6.1) |
| Infections† | 20 (38.5) | 10 (19.2) | 14 (28.6) | 11 (22.4) |
| Polyneuropathy | 11 (21.2) | 0 | 7 (14.3) | 0 |
| Tumor lysis | 2 (3.8) | 2 (3.8) | 4 (8.2) | 4 (8.2) |
For 2 patients per cohort, no adverse events were reported and patients were excluded from analysis of adverse events.
Febrile neutropenia included.
In total, 57 patients died during follow-up (salvage cohort: 34; bridging cohort: 23). The majority of patients (n = 47) died from disease progression (salvage cohort: 28 of 34 patients; bridging cohort: 19 of 23 patients). Four patients died of infection, of which 2 infections were related to pola treatment. One patient with 3 prior treatment lines developed a secondary acute myeloid leukemia after treatment with pola and died. Five further patients died of an underlying disease other than lymphoma, CAR T-cell therapy-related complications, or from unknown reasons.
Discussion
Patients who relapse after standard therapy often develop chemorefractory disease and have a very dismal outcome.1-6,21 In this retrospective multicenter study, we reported on a large group of r/r LBCL patients who received pola as a salvage therapy or as a bridging treatment to cellular immunotherapies. Despite several lines of treatment having previously failed, an OR rate of 48.1% was achieved in the salvage cohort. However, the outcome of the salvage cohort was unsatisfying, with a median OS of 5.4 months. Although a comparison with the pivotal study (median OS of 12.4 months)12 is only partially possible because of the heterogeneity of treatments in our cohort, the differences in outcomes might also reflect differences of patients’ baseline characteristics. Patients in our study had a predominance of 3 or more prior treatment lines, whereas patients in the approval study had received a median of only 2 prior treatment lines. Moreover, our study included r/r LBCL patients who had relapsed after CAR T-cell therapy or alloHCT. These patients were excluded from the approval study. A similar trend of a worse outcome in a real-world study of pola was reported by Segman et al.22 Although outcomes in real-world settings may not be as promising as demonstrated in the approval study, pola still may have a valuable role to play in LBCL salvage therapy, even in patients following several failed treatment lines.
Despite the adverse baseline characteristics, the moderate toxicity profile of pola was confirmed in this study. This suggests that pola can be a reasonable treatment option even in heavily pretreated and also elderly patients. A noteworthy proportion of patients in the salvage cohort received pola-R (37%) without a chemotherapy backbone, resulting in an OR rate of 40%. This was comparable to the OR rate reported in both phase 1/2 studies that investigated pola as a monotherapy or in combination with rituximab (NCT01290549 and ROMULUS).23,24 These observations suggest that pola-R administered without chemotherapy might represent a valuable option for comorbid patients who cannot tolerate chemotherapy. Moreover, this is also important when pola-containing regimens are used as bridging to CAR T-cell therapy because bendamustine has been shown to compromise T-cell function25 and affect CAR T-cell apheresis success.26
The advent of the CAR T-cells offers a new treatment approach with curative potential for patients with r/r LBCL who have exhausted first- and second-line treatment options. To control the lymphoma growth during the time needed to manufacture the CAR T-cells, bridging treatments are required for the majority of patients with r/r LBCL. However, effective treatments for r/r LBCL patients are scarce. Pola has become a promising new and effective treatment opportunity, but response duration after pola treatment is limited in the majority of LBCL patients. The sequential combination of an effective pola-based bridging treatment followed by a cellular therapy that offers the potential for long-term disease control appears an attractive opportunity. Our study reports for the first time the outcome of a significant number of patients who were bridged with pola to the intended CAR T-cell therapy. Pola bridging enabled 51.2% of the patients who were planned for CAR T-cell therapy to successfully proceed to the intended CAR T-cell therapy infusion.
Patients who reached the CAR T-cell therapy with pola had a reasonable outcome with a 6-month OS of 77.9%. However, the survival of the entire bridging cohort calculated from study entry was only 61.8.% which was mainly attributed to the outcome of patients who could not successfully bridged to CAR T-cell therapy. This is in line with the observation that patients who need bridging have a poorer outcome.19,27 ,28
The outcome after CAR T-cell therapy failure is poor29 and effective treatment options are sparse. The efficacy of pola for relapse after CAR T-cell therapy is widely unknown. In this study, we reported on 12 patients who have received pola for relapse after CAR T-cell therapy, of whom 7 (58.3%) responded to pola. Although the duration of these responses was limited, this observation is noteworthy in the light of outcomes achieved with alternative immunotherapies such as mosunetuzumab (OR rate, 39%)30 and odronextamab (OR, 33%)31 after previous CAR T-cell therapy. This indicates that pola can be effective after CAR T-cell therapy failure in individual patients and might serve as a valuable bridging treatment to a second cellular therapy such as alloHCT.
Because of the retrospective character of this study, several limitations have to be taken into account, including the lack of a predefined image-based response assessment, the lack of a positron emission tomography CT scan-based response assessment, the heterogeneity of treatment regimens, and the limited granularity of toxicity assessment extracted by chart review. The relatively short FU could be a further limitation of the study. However, because of the high number of events during the first 6 months of observation, the PFS and OS are not likely to be underestimated despite the lack of a predefined image-based response assessment and the limited FU.
In summary, although further validation is needed, this study suggests that pola is a valuable treatment either as bridging to CAR T-cell therapy and alloHCT or as a salvage treatment concept for patients who are unfit for further intensive consolidative treatment approaches. Further studies are required to improve the identification of patients who will likely benefit from pola treatment. Additional treatment partners that might replace bendamustine are currently investigated in several different clinical trials including R-Gemcitabine-Oxaliplatin (POLARGO),32 R-CHP (POLARIX),33 mosunetuzumab (GO40516),34 obinutuzumab-lenalidomide (NCT02600897),34 and obinutuzumab-venetoclax (GO29833; NCT02611323)35 and may further improve the efficacy of pola.
Acknowledgments
N.L. was supported by the Heidelberg School of Oncology (HSO2) fellowship from the National Center for Tumor Diseases Heidelberg. S.D. was supported by a grant from the Hairy Cell Leukemia Foundation, Heidelberg Research Centre for Molecular Medicine, an e:med BMBF junior group grant, and Deutsche Forschungsgemeinschaft (DFG) through the SFB873 (project B7).
This study was not financially supported by F. Hoffmann-La Roche or Genentech.
Authorship
Contribution: N.L., J.D., and S.D. designed the study; N.L., J.D., A.K., D.N., E.K., F.A., S.F., C.L., M.W., J.C., J.-M.M., T.W., U.H., R.T., R.P., R.L., C.S., S.F., N.G., L.C., T.G., C.K., U.K., R.C., D.M., S.M., A.H., C.P., A.T., G.W., U.B., L.B., G.H., B.G., and G.L. collected data; N.L. and S.D. analyzed data and performed statistical analysis; N.L., D.F., B.G., G.L., P.D., and S.D. interpreted data; N.L., D.F., P.D., and S.D. wrote the paper; and J.D., S.F., J.C., J.-M.M., U.H., T.G., U.K., R.C., D.M., G.H., C.M.T., B.G., and G.L. edited the manuscript.
Conflict-of-interest disclosure: N.L. reports honoraria from Takeda and Roche. J.D. reports research funding from Morphosys and Regeneron. D.K. reports honoraria from BMS. S.F. reports consultancies for Kite-Gilead, Roche, BMS, and Celgene. C.L. reports lecture fees from Roche and traveling expenses and congress attendance fee from Celgene. J.C. reports honoraria from Amgen, Novartis, and Roche; travel grants and honoraria from Abbvie; and travel grants from Celgene. J.-M.-M. reports consultancies for Janssen, Roche, Gilead, Sanofi, Abbvie, Jazz, Pfizer, and Astellas; honoraria from Novartis, Roche, Janssen, Abbvie, Pfizer, Sanofi, and Astellas; and research funding from Sanofi and Janssen. U.H. reports honoraria from Novartis, Pfizer, Gilead Sciences, Janssen, BMS/Celgene, and Miltenyi Biotec B.V. & Co. KG. C.K. reports advisory fees from Roche. U.K. reports consultancies for AbbVie, Roche, BMS, Hexal, Novartis, MSD, Celgene, Janssen-Cilag, Takeda, Pfizer, Astra-Zeneca, and Pentixapharm. R.C. reports consultancy and speakers bureau for Abbvie; speakers bureau for AstraZeneca; consultancy for Celgene and Gilead; consultancy, speakers bureau, and travel grants from; consultancy and speakers Bureau for Roche; and research funding and speakers bureau for Novartis. D.M. reports consultancies for AbbVie, Roche, BMS, Hexal, Novartis, MSD, Celgene, Janssen-Cilag, Pfizer, Astra-Zeneca, and Jazz Pharma. S.M. reports honoraria and travel grants from Amgen, travel grants from Abbvie, and honoraria from Novartis and Roche. A.H. reports honoraria, travel, accommodations, and expenses from Celgene; honoraria from Gilead; honoraria, membership on an entity's Board of Directors or advisory committees, travel, accommodations, and expenses from Takeda; travel expenses from Roche; research funding from Seattle Genetics; and consultancy for Lead Discovery Center GmbH. U.B. reports membership on an entity's Board of Directors or advisory committees for Gilead, Hexal, and MSD; membership on an entity's Board of Directors or advisory committees and travel grants from Janssen and Roche; and travel grants from Amgen. L.R. reports membership on an entity's Board of Directors or advisory committees from Menarini, Gilead, Amgen, Pfizer, Daiichi Sankyo, Novartis, Hexal, Janssen, Jazz Pharmaceuticals, Abbvie, Seattle Genetics, Sanofi, Astellas, Celgene, and Bristol-Myers Squibb. G.H. reports research funding and honoraria from Roche and Celgene; research funding from Janssen; honoraria from Hono Genmab, M Gilead, and Astra; and research funding and honoraria from Abbvie; EUSA, and Morphosys. C.M.-T. reports membership on an entity's Board of Directors or advisory committees and research funding from Pfizer; membership on an entity's Board of Directors or advisory committees for Janssen-Cilag Gmbh; and research funding from Deutsche Krebshilfe, BMBF, Deutsche Forschungsgemeinschaft, Wilhelm-Sander-Stiftung, Jose-Carreras-Siftung, Bayer AG, Daiichi Sankyo, and BiolineRx. B.G. reports consultancy for Roche, AstraZeneca, BMS, Celgene, Gilead, AbbVie, Janssen, and Novartis and research funding from Roche. G.L. reports research grants from AQUINOX, Acerta, AGIOS, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Morphosys, Novartis, Roche, and Verastem and honoraria from Abbvie, AstraZeneca, Bayer, BMS, Celgene, Gilead, Incyte, Janssen, Karyopharm, Morphosys, Novartis, Nanostring, Roche, and Takeda. P.D. reports consultancy and speakers bureau for Roche, AbbVie, and Novartis; research funding from Neovii; consultancy for AstraZeneca and Janssen; consultancy and speakers bureau for Gilead; and consultancy, research funding, and speakers bureau for Riemser. S.D. reports membership on an entity's Board of Directors or advisory committees for Roche, Celgene, and KITE and membership on an entity's Board of Directors or advisory committees and research funding from Janssen.
Correspondence: Sascha Dietrich, Heidelberg University Hospital, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: sascha.dietrich@med.uni-heidelberg.de.
References
Author notes
Presented at the 62nd annual meeting of the American Society of Hematology, 5-8 December 2020.
Deidentified participant data that underlie the reported results can be requested by contacting the corresponding author (sascha.dietrich@med.uni-heidelberg.de). Individual participant data cannot be shared.
The full-text version of this article contains a data supplement.