Key Points
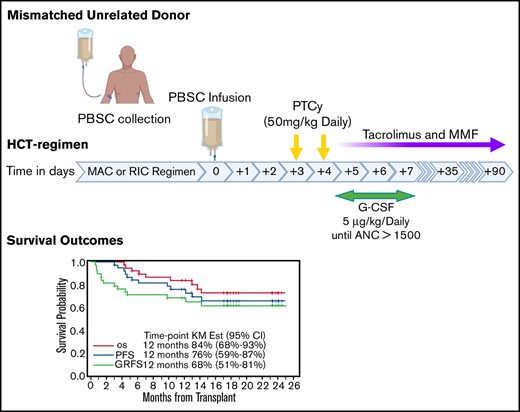
Highly promising OS and GRFS rates of 87% and 68%, respectively, were achieved after MMUD-HCT with PTCy.
Our data support further development of PTCy in the MMUD setting to improve outcomes in patients without a matched donor.
Efficacy of PTCy after mismatched unrelated donor (MMUD) HCT is unknown. In this pilot clinical trial, we enrolled 38 patients with hematologic malignancies scheduled to undergo MMUD-HCT (≥6/8 HLA-matched donors) onto 1 of 2 conditioning strata: myeloablative using fludarabine and fractionated total body irradiation (n = 19) or reduced intensity with fludarabine/melphalan (n = 19). Graft source was peripheral blood stem cells (PBSCs), and GVHD prophylaxis was PTCy, tacrolimus, and mycophenolate mofetil. Patients’ median age was 53 years (range, 21-72 years). Median number of HLA mismatches was 2 (range, 1-4) of 12 loci. Twenty-three patients (61%) were considered racial (n = 12) or ethnic (n = 11) minorities. Median time to neutrophil engraftment was 16 days (range, 13-35 days). With a median follow-up of 18.3 months (range, 4.3-25.0 months) for surviving patients, 1-year overall survival (OS) and GVHD-free/relapse-free survival (GRFS) were 87% (95% confidence interval [CI]: 71-94) and 68% (95% CI: 51-81), respectively. Cumulative incidence of nonrelapse mortality at 100 days and 1 year were 0% and 11% (95% CI: 4-27), respectively, whereas relapse/progression was 11% (95% CI: 4-27). Cumulative incidence of 100-day acute GVHD grades 2-4 and 3-4 and 1-year chronic GVHD were 50% (95% CI: 36-69), 18% (95% CI: 9-36), and 48% (95% CI: 34-68), respectively. The rate of moderate/severe chronic GVHD was 3% in the entire cohort. We showed highly promising OS/GRFS rates with an acceptable risk profile after PBSC-MMUD-HCT with PTCy. This trial was registered at www.clinicaltrials.gov as #NCT03128359.
Introduction
Donor-recipient HLA compatibility is a major prognostic factor for outcomes following allogeneic hematopoietic cell transplantation (HCT).1-6 HLA-identical sibling or fully matched unrelated donors (MUD) were historically the preferred donor choices, but only about 30% of patients have a suitable HLA-identical sibling.7 Despite the rapid growth of donor registries such as the National Marrow Donor Program,8 the likelihood of identifying a MUD varies among racial and ethnic groups, with the highest probability among Whites of European descent (75%) and the lowest probability among Hispanic (34%) and Blacks of South or Central American descent (16%).8
For patients without a matched donor, options include mismatched unrelated (MMUD), cord blood, or haploidentical donors. Compared with MUD-HCT, MMUD has been associated with higher rates of acute/chronic graft-versus-host disease (GVHD) and nonrelapse mortality (NRM), particularly when the graft source is peripheral blood stem cells (PBSCs).3,5,9,10 We and others have previously reported encouraging results with tacrolimus/sirolimus-based GVHD prophylaxis after MUD-HCT.6,11,12 Results from our earlier single institution analysis of tacrolimus/sirolimus-based GVHD prophylaxis after MUD and MMUD HCT showed that relative to MUD, MMUD-HCT is associated with significantly lower overall survival (OS), primarily because of increased NRM associated with GVHD and infections.6
High-dose posttransplant cyclophosphamide (PTCy) was initially developed to prevent GVHD and allow for engraftment across the HLA barrier in haploidentical HCT.13 Accumulating data are demonstrating that PTCy in combination with tacrolimus and mycophenolate mofetil (MMF) results in reliable engraftment, low incidence of GVHD, and lower NRM even in the matched14,15 and bone marrow (BM) mismatched donor setting.16 Initial studies were focused on BM as a graft source; yet, PTCy has been successfully administered after PBSC-HCT.14,17 Additionally, in a retrospective study, similar survival outcomes were reported after MUD and MMUD-HCT using PTCy.18 Two currently ongoing phase 3 trials are evaluating PTCy as an investigational arm (NCT02345850 and NCT03959241); however, there have been no prospective trials reported to date that evaluate PTCy’s efficacy in PBSC MMUD-HCT.
Herein, we conducted a 2-strata pilot clinical trial (NCT03128359) of PTCy-based GVHD prophylaxis in which patients undergoing MMUD-HCT were conditioned with either a myeloablative (MAC) or reduced intensity conditioning (RIC) regimen. This trial was designed to pursue the following objectives, overall and within each stratum: (1) estimate GVHD-free, relapse-free survival (GRFS), OS, and progression-free survival (PFS); (2) estimate the cumulative incidence of acute/chronic GVHD and relapse/progression; (3) evaluate engraftment, toxicities, infections, and transplant-related complications; and (4) longitudinal characterization of immune cell reconstitution and inflammatory cytokines.
Methods
Patients and clinical trial design
As part of this pilot clinical trial (NCT03128359), from May 2017 to July 2019, patients were enrolled into 1 of 2 predetermined strata, RIC or MAC, which accrued patients in a concurrent manner. Adult patients (≤75 years old) with a hematologic malignancy who did not have a fully matched related/unrelated donor were eligible. Unrelated donors were HLA matched at ≥6/8 (-A, -B, -C, and -DR) with absence of donor-specific antibodies to the mismatched HLA locus/loci. When multiple donors were available, younger donors with lower number of mismatches were favored.
The primary trial end point was GRFS.19 Because of a lack of comparable historical data/estimates of 1-year GRFS in this setting, a total of 38 patients (19 patients per stratum) was determined to be sufficient to estimate GRFS rate at 1 year with adequate precision (standard error = 0.08 overall, =0.12 per stratum). Secondary end points included toxicity, infections through day +100, acute and chronic GVHD, engraftment (neutrophil/platelet recovery and donor chimerism), OS and PFS, and relapse/disease progression. Within each stratum, an early stopping rule for toxicity was assessed for each patient at day +30 after HCT per the Bearman Toxicity Scale20 and Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. For more information on the toxicity monitoring stopping rules, see supplemental Materials. Safety data were independently monitored by the institutional Data Safety Monitoring Committee. This study was approved by the City of Hope Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Conditioning, GVHD prophylaxis, and supportive care
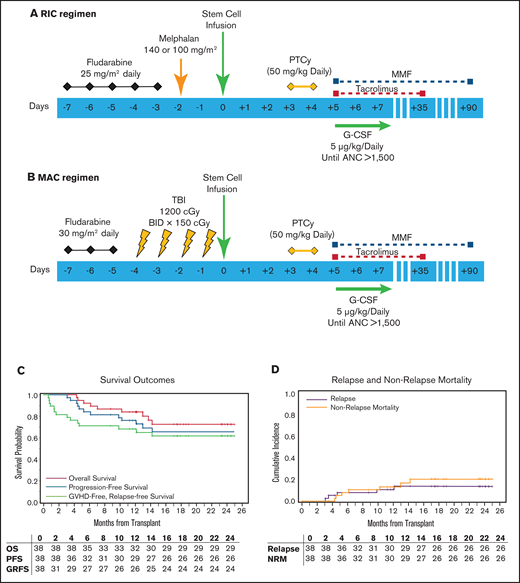
The RIC regimen was fludarabine (25 mg/m2 per day intravenously [IV] × 5 days) and melphalan (140 mg/m2) on day −2. For patients ≥ 60 of age, melphalan was at 100 mg/m2. The MAC regimen was fludarabine (30 mg/m2 IV) and total body irradiation at 150 cGy twice a day in 8 fractions (Figure 1). PBSC was the graft source. PTCy (50 mg/kg/day) was administered on days +3 and +4. Tacrolimus (1 mg continuous IV) was started on day +5 with dose adjustment to maintain a level of 5 to 15 ng/mL and then changed to the equivalent oral dose. A tacrolimus taper was started on day +90 in patients without active GVHD. MMF was administered at a 15-mg/kg dose 3 times per day beginning on day +5 (maximum dose, 1 g orally, 3 times per day) and stopped on day +35 if there was no severe GVHD. Granulocyte colony-stimulating factor (5 µg/kg per day) was given from day +5 until there was an absolute neutrophil count > 1500/mm3 for 3 consecutive days. Additional supportive care was provided according to institutional guidelines, including prophylactic antibiotics and sinusoidal obstruction syndrome prophylaxis. Letermovir for cytomegalovirus (CMV) prophylaxis was initiated as part of institutional guidelines for CMV-seropositive recipients on 1 March 2018.
Study schema and HCT outcomes. (A) RIC regimen. Fludarabine was administered at the daily dose of 25 mg/m2 from days −7 to −3 before HCT. Melphalan was given on day −2 at 140 mg/m2 for patients who were younger than 60 years old. Melphalan dose for patients ≥60 years old was 100 mg/m2. (B) MAC regimen consisted of daily fludarabine at 30 mg/m2, from days −7 to −5 before HCT. Total body irradiation was administered in 8 fractions of 150 cGy, 2 times a day, from days −4 to −1, for a total of 1200 cGy. Graft source was PBSCs for both strata, and GVHD prophylaxis was PTCy at 50-mg/kg daily dose on days +3 and +4. Granulocyte colony-stimulating factor administration (5 µg/kg per day) was started on day +5 and continued until absolute neutrophil count >1500/mm3 for 3 consecutive days. Tacrolimus (1 mg continuous IV) administration started on day +5 with dose adjustment to maintain a level of 5 to 15 ng/mL and then changed to equivalent oral dose once stable. Tacrolimus taper was started on day +90 if patient did not have active GVHD. MMF was administered at 15 mg/kg dose 3 times per day beginning on day +5 (maximum dose, 1 g orally, 3 times per day). MMF administration was stopped on day +35 if there was no severe GVHD. (C) Kalan-Meier curve showing survival outcomes and (D) relapse and nonrelapse mortality outcomes.
Study schema and HCT outcomes. (A) RIC regimen. Fludarabine was administered at the daily dose of 25 mg/m2 from days −7 to −3 before HCT. Melphalan was given on day −2 at 140 mg/m2 for patients who were younger than 60 years old. Melphalan dose for patients ≥60 years old was 100 mg/m2. (B) MAC regimen consisted of daily fludarabine at 30 mg/m2, from days −7 to −5 before HCT. Total body irradiation was administered in 8 fractions of 150 cGy, 2 times a day, from days −4 to −1, for a total of 1200 cGy. Graft source was PBSCs for both strata, and GVHD prophylaxis was PTCy at 50-mg/kg daily dose on days +3 and +4. Granulocyte colony-stimulating factor administration (5 µg/kg per day) was started on day +5 and continued until absolute neutrophil count >1500/mm3 for 3 consecutive days. Tacrolimus (1 mg continuous IV) administration started on day +5 with dose adjustment to maintain a level of 5 to 15 ng/mL and then changed to equivalent oral dose once stable. Tacrolimus taper was started on day +90 if patient did not have active GVHD. MMF was administered at 15 mg/kg dose 3 times per day beginning on day +5 (maximum dose, 1 g orally, 3 times per day). MMF administration was stopped on day +35 if there was no severe GVHD. (C) Kalan-Meier curve showing survival outcomes and (D) relapse and nonrelapse mortality outcomes.
Flow cytometry
Peripheral blood was collected on days 30, 100, and 180. Mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (GE Healthcare, Bath, United Kingdom). For T-regulatory cell (Treg) staining, PBMCs were surface stained for CD3, CD4, CD25, and CD127 (eBioscience, San Diego, CA) and intracellularly stained for Foxp3 using Foxp3/Transcription Factor Staining Buffer Set (eBioscience). For other lymphocyte subsets, anti-CD8, -CD56, -CD19, and -CD27 antibodies (eBioscience) were used. Flow cytometry was performed using a BD FACSCelesta (BD Biosciences, East Rutherford, NJ), and data were analyzed using Flowjo software (Tree Star, Ashland, OR).
Plasma cytokines and GVHD biomarkers
Serum samples were obtained each week for 4 weeks and analyzed for cytokines using the “Human Cytokine Thirty-Plex Antibody Magnetic Bead Kit” (Invitrogen, Camarillo, CA) per the manufacturer’s recommendations. The Flexmap 3D luminex system (Luminex Corp.) was used for analysis, and cytokine concentrations were calculated using Bio-Plex Manager 6.2 software with a 5-parameter curve-fitting algorithm applied for standard curve calculations for duplicate samples.
Statistical analysis
Survival estimates were calculated using the Kaplan-Meier method, the Greenwood formula was used to calculate standard error, and the log-log transformation method was used to construct 95% confidence intervals (CIs). The cumulative incidence of relapse/progression, NRM, and acute and chronic GVHD were calculated as competing risks according to Gooley et al.21 Descriptive statistics were used to summarize patient and disease characteristics, transplant features, and adverse event data. Fisher’s exact test was used to assess the contingency tables, and the Wilcoxon rank sum test was performed to examine the difference on medians. P values were not adjusted for multiple comparisons because of the exploratory nature of the study. All calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC). All tests were 2 sided at a significance level of .05.
Results
Patient and transplant characteristics
The median age at the time of HCT was 53 years (range, 21-72 years); half of the patients were male. For most patients (>80%), HCT indication was acute leukemia (n = 25) or myelodysplastic syndrome (n = 6). Disease Risk Index (DRI)22 was low (n = 3, 8%), intermediate (n = 22, 58%), and high/very high (n = 13, 34%). HCT-CI23 was >2 in 17 patients (45%). Donors (median age, 32 years; range, 19-53 years) were mismatched at HLA-A (n = 15), -B (n = 12), -C (n = 8), or -DR loci (n = 5). Of the 12 HLA loci, the median number of mismatches were 2 (range, 1-4), whereas 2 (5%) and 28 (74%) patients had HLA-DQ and/or DP mismatches, respectively. The median CD34+ cell dose was 5.45 × 106/kg (range, 2.39-9.35 × 106/kg). Notably, 23 patients (61%) represented racial/ethnic minorities (Asian: n = 8, African American: n = 4, Hispanic: n = 11; Table 1).
Demographics, disease, and transplant characteristics
| Variable . | N (%) or median (range) . | ||
|---|---|---|---|
| RIC (n = 19) . | MAC (n = 19) . | All (n = 38) . | |
| Sex | |||
| Female | 8 (42) | 11 (58) | 19 (50) |
| Male | 11 (58) | 8 (42) | 19 (50) |
| Race | |||
| White | 12 (63) | 12 (63) | 24 (63) |
| Asian | 4 (21) | 4 (21) | 8 (21) |
| Black | 2 (11) | 2 (11) | 4 (11) |
| Unknown | 1 (5) | 1 (5) | 2 (5) |
| Ethnicity | |||
| Hispanic | 3 (16) | 8 (42) | 11 (29) |
| Non-Hispanic | 15 (79) | 11 (58) | 26 (68) |
| Unknown | 1 (5) | 0 (0) | 1 (3) |
| Patient’s age at HCT (y) | 63 (33-72) | 44 (21-57) | 53 (21-72) |
| Primary disease | |||
| AML | 5 (26) | 12 (64) | 17 (45) |
| ALL | 3 (16) | 5 (26) | 8 (21) |
| MDS | 5 (26) | 1 (5) | 6 (16) |
| NHL | 3 (16) | 0 (0) | 3 (8) |
| CML | 2 (11) | 1 (5) | 3 (8) |
| CLL | 1 (5) | 0 (0) | 1 (2) |
| HCT comorbidity index | 3 (0-6) | 2 (0-6) | 2 (0-6) |
| 0 | 1 (5) | 5 (26) | 6 (16) |
| 1-2 | 8 (42) | 7 (37) | 15 (39) |
| >2 | 10 (53) | 7 (37) | 17 (45) |
| DRI | |||
| Low | 3 (16) | 0 (0) | 3 (8) |
| Intermediate | 8 (42) | 14 (74) | 22 (58) |
| High | 5 (26) | 5 (26) | 10 (26) |
| Very high | 3 (16) | 0 (0) | 3 (8) |
| Sex donor/recipient | |||
| Female/male | 4 (21) | 0 (0) | 4 (11) |
| Others | 15 (79) | 19 (100) | 34 (89) |
| Donor age at HCT (y) | 35 (24-53) | 29 (19-48) | 32 (19-53) |
| Patient CMV status | |||
| Negative | 3 (16) | 5 (26) | 8 (21) |
| Positive | 16 (84) | 14 (74) | 30 (79) |
| CD34+ dose (106 cells/kg) | 5.28 (2.39-9.35) | 6.28 (3.53-9.04) | 5.45 (2.39-9.35) |
| Number of mismatches | 3 (1-4) | 2 (1-4) | 2 (1-4) |
| HLA class I (A B C) | |||
| Matched | 3 (16) | 2 (11) | 5 (13) |
| Mismatched (≥1) | 16 (84) | 17 (89) | 33 (87) |
| HLA class II (DR DQ DP) | |||
| Matched | 3 (16) | 5 (26) | 8 (21) |
| Mismatched (≥1) | 16 (84) | 14 (74) | 30 (79) |
| HLA DQ | |||
| Matched | 18 (95) | 18 (95) | 36 (95) |
| Mismatched (≥1) | 1 (5) | 1 (5) | 2 (5) |
| HLA DP | |||
| Matched | 4 (21) | 6 (32) | 10 (26) |
| Mismatched (≥1) | 15 (79) | 13 (68) | 28 (74) |
| Variable . | N (%) or median (range) . | ||
|---|---|---|---|
| RIC (n = 19) . | MAC (n = 19) . | All (n = 38) . | |
| Sex | |||
| Female | 8 (42) | 11 (58) | 19 (50) |
| Male | 11 (58) | 8 (42) | 19 (50) |
| Race | |||
| White | 12 (63) | 12 (63) | 24 (63) |
| Asian | 4 (21) | 4 (21) | 8 (21) |
| Black | 2 (11) | 2 (11) | 4 (11) |
| Unknown | 1 (5) | 1 (5) | 2 (5) |
| Ethnicity | |||
| Hispanic | 3 (16) | 8 (42) | 11 (29) |
| Non-Hispanic | 15 (79) | 11 (58) | 26 (68) |
| Unknown | 1 (5) | 0 (0) | 1 (3) |
| Patient’s age at HCT (y) | 63 (33-72) | 44 (21-57) | 53 (21-72) |
| Primary disease | |||
| AML | 5 (26) | 12 (64) | 17 (45) |
| ALL | 3 (16) | 5 (26) | 8 (21) |
| MDS | 5 (26) | 1 (5) | 6 (16) |
| NHL | 3 (16) | 0 (0) | 3 (8) |
| CML | 2 (11) | 1 (5) | 3 (8) |
| CLL | 1 (5) | 0 (0) | 1 (2) |
| HCT comorbidity index | 3 (0-6) | 2 (0-6) | 2 (0-6) |
| 0 | 1 (5) | 5 (26) | 6 (16) |
| 1-2 | 8 (42) | 7 (37) | 15 (39) |
| >2 | 10 (53) | 7 (37) | 17 (45) |
| DRI | |||
| Low | 3 (16) | 0 (0) | 3 (8) |
| Intermediate | 8 (42) | 14 (74) | 22 (58) |
| High | 5 (26) | 5 (26) | 10 (26) |
| Very high | 3 (16) | 0 (0) | 3 (8) |
| Sex donor/recipient | |||
| Female/male | 4 (21) | 0 (0) | 4 (11) |
| Others | 15 (79) | 19 (100) | 34 (89) |
| Donor age at HCT (y) | 35 (24-53) | 29 (19-48) | 32 (19-53) |
| Patient CMV status | |||
| Negative | 3 (16) | 5 (26) | 8 (21) |
| Positive | 16 (84) | 14 (74) | 30 (79) |
| CD34+ dose (106 cells/kg) | 5.28 (2.39-9.35) | 6.28 (3.53-9.04) | 5.45 (2.39-9.35) |
| Number of mismatches | 3 (1-4) | 2 (1-4) | 2 (1-4) |
| HLA class I (A B C) | |||
| Matched | 3 (16) | 2 (11) | 5 (13) |
| Mismatched (≥1) | 16 (84) | 17 (89) | 33 (87) |
| HLA class II (DR DQ DP) | |||
| Matched | 3 (16) | 5 (26) | 8 (21) |
| Mismatched (≥1) | 16 (84) | 14 (74) | 30 (79) |
| HLA DQ | |||
| Matched | 18 (95) | 18 (95) | 36 (95) |
| Mismatched (≥1) | 1 (5) | 1 (5) | 2 (5) |
| HLA DP | |||
| Matched | 4 (21) | 6 (32) | 10 (26) |
| Mismatched (≥1) | 15 (79) | 13 (68) | 28 (74) |
Engraftment
All patients engrafted. Median time to neutrophil engraftment was 16 days (range, 13-35 days) for the entire cohort, 15 days (range, 13-19 days) for MAC, and 17 days (range, 14-35 days) for RIC. Median time to platelet engraftment was 28 days (range, 13-152 days) for the entire cohort, 20 days (range, 13-35 days) for MAC, and 32 days (range, 17-152 days) for RIC. Engraftment analysis showed complete donor chimerism (by STR or PCR) in 37 patients (97%) on day 30 ± 7 days. One patient with myelofibrosis had 90% donor chimerism by day 30 ± 7, which improved to full donor chimerism by day 100.
Survival outcomes
With the median follow-up of 18.3 months (range, 8.7-25 months) for surviving patients, the primary end point of 1-year GRFS was 68% (95% CI: 51-81) for the entire cohort (Figure 1C), 84% (95% CI: 59-95) for MAC, and 53% (95% CI: 29-72) for RIC. One-year OS was 84% (95% CI: 68-93) for the entire cohort (Figure 1C), 100% (95% CI: not applicable) for MAC, and 68% (95% CI: 42-84) for RIC. One-year PFS was 76% (95% CI: 59-87) for the entire cohort (Figure 1C), 95% (95% CI: 68-99) for MAC, and 57% (95% CI: 33-76) for RIC. NRM at 100 days and 1 year was 0% (95% CI: not applicable) and 13% (95% CI: 6-30) for the entire cohort (Figure 1D) and 0% (95% CI: not applicable) and 27% (95% CI: 13-57) at 1 year for the MAC and RIC arm. The cumulative incidence of relapse/progression at 1 year was 11% (95% CI: 4-27) for the entire cohort (Figure 1D), 5% (95% CI: 1-35) for MAC, and 16% (95% CI: 6-45) for RIC. Causes of death were relapse of underlying disease (n = 2), infections (n = 4), and GVHD (n = 3).
GVHD outcomes
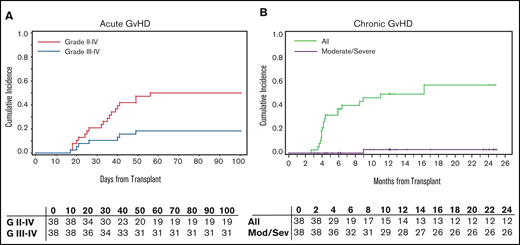
Cumulative incidence of 100-day acute GVHD (aGVHD) grades 2-4 and 3-4 were 50% (95% CI: 36-69) and 18% (95% CI: 9-36), respectively, for the entire cohort (Figure 2A). Grade 2-4 aGVHD was 53% (95% CI: 34-81) and 47% (95% CI: 29-76) for the MAC and RIC cohort, respectively. Grade 3-4 aGVHD was 11% (95% CI: 3-39) and 26% (95% CI: 12-56) for the MAC and RIC cohorts, respectively. One-year chronic GVHD (cGVHD) was 49% (95% CI: 35-69) in the entire cohort. Moderate/severe cGVHD by National Institutes of Health criteria was 3% (95% CI: 0.4-19) for the entire cohort (Figure 2B), 58% (95% CI: 39-85) for MAC, and 37% (95% CI: 20-66) for RIC.
GVHD outcomes. (A) Acute GVHD grade II-IV and III-IV in 100 days. (B) Chronic GVHD outcomes.
GVHD outcomes. (A) Acute GVHD grade II-IV and III-IV in 100 days. (B) Chronic GVHD outcomes.
Of the 31 patients who survived for at least 1 year after HCT, 21 (68%) were able to completely discontinue immunosuppressive medications at 1 year (8 patients in MAC and 13 patients in RIC). Among patients with grade 2-4 aGVHD (n = 19), 16 responded to systemic steroids, whereas 3 developed steroid refractory (SR)–GVHD and subsequently died because of GVHD at 130, 186, and 394 days after HCT.
Early toxicities, infections, and cytokine release syndrome
The trial progressed without excessive early toxicities; there was 1 protocol-defined unacceptable toxicity event (delayed engraftment, beyond day 30, in a patient with myelofibrosis who ultimately engrafted on day 35). For nonhematologic adverse events by the Bearman Toxicity Grading scale, the most common adverse events for both arms were grade 1 and 2 stomatitis: (n = 23, 61%) and (n = 5, 13%); and gastrointestinal toxicity grades 1 and 2 (n = 24, 63%) and (n = 4, 11%).
Infectious complications from days −9 to +100 are summarized in Table 2. CMV viremia was at 42% (n = 16; 4 patients, G2; 12 patients, G1), with only 40% of CMV-seropositive recipients receiving prophylaxis. Other viral infections included respiratory infections (n = 5; 4 with upper respiratory and 1 with lower respiratory infections), BK virus cystitis (n = 5 mostly G1), and human herpesvirus 6 (n = 1; G1). Bacterial infection was at 50% (n = 19; G3 was only in 2 patients); only 4 developed Clostridioides difficile colitis. Cytokine release syndrome (CRS) was observed in 27 patients (71%): grade 1 (n = 16, 42%), grade 2 (n = 10, 26%), and grade 3 (n = 1, 3%; Table 3) by the American Society for Transplantationand Cellular Therapy (ASTCT) consensus criteria.24 We observed CRS more commonly in the RIC arm (n = 17) than the MAC (n = 10; P = .03 by Fisher’s exact test).
Infectious complications with BMT CTN severity grades (version 3.0) from day −9 to day 100
| . | CTN grade . | . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | RIC . | MAC . | . | |||||
| G1 . | G2 . | G3 . | G1 . | G2 . | G3 . | Total . | ||
| Bacterial | ||||||||
| Clostridioides difficle | 3 | 0 | 0 | 1 | 0 | 0 | 4 | |
| Gram negative | 1 | 3 | 2 | 3 | 2 | 0 | 11 | |
| Gram positive | 4 | 1 | 0 | 1 | 2 | 0 | 8 | |
| Unknown* | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Viral | ||||||||
| Adenovirus | 2 | 0 | 0 | 1 | 0 | 0 | 3 | |
| BK | 2 | 0 | 0 | 3 | 0 | 0 | 5 | |
| CMV | 3 | 3 | 0 | 9 | 1 | 0 | 16 | |
| HHV6 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| HSV | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Respiratory | 0 | 0 | 1 | 0 | 4 | 0 | 5 | |
| Fungal | ||||||||
| Mold | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| . | CTN grade . | . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | RIC . | MAC . | . | |||||
| G1 . | G2 . | G3 . | G1 . | G2 . | G3 . | Total . | ||
| Bacterial | ||||||||
| Clostridioides difficle | 3 | 0 | 0 | 1 | 0 | 0 | 4 | |
| Gram negative | 1 | 3 | 2 | 3 | 2 | 0 | 11 | |
| Gram positive | 4 | 1 | 0 | 1 | 2 | 0 | 8 | |
| Unknown* | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Viral | ||||||||
| Adenovirus | 2 | 0 | 0 | 1 | 0 | 0 | 3 | |
| BK | 2 | 0 | 0 | 3 | 0 | 0 | 5 | |
| CMV | 3 | 3 | 0 | 9 | 1 | 0 | 16 | |
| HHV6 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| HSV | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Respiratory | 0 | 0 | 1 | 0 | 4 | 0 | 5 | |
| Fungal | ||||||||
| Mold | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
Culture-negative pneumonia.
Immune reconstitution
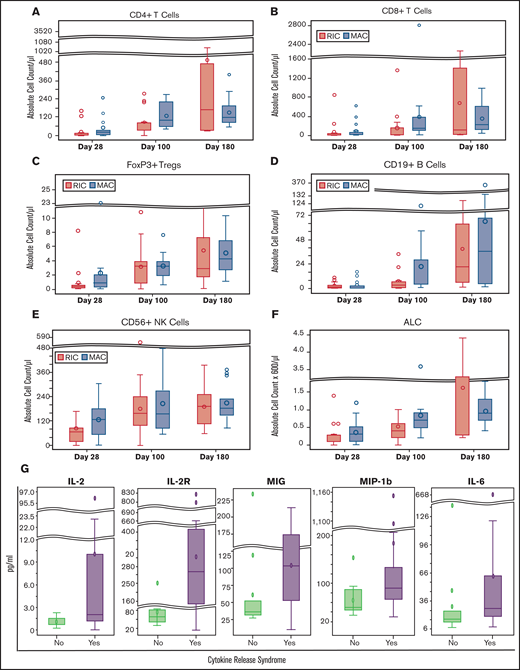
Cellular immune recovery was measured on days 28, 100, and 180 after HCT by flow cytometry (Figure 3A-F). The median lymphocyte, CD4 T-cell, CD8 T-cell, natural killer–cell, B-cell, and Treg counts (per microliter) on day 100 were 600 (range, 0-3600), 78.4 (range, 0-275.5), 107.7 (range, 0-2809.3), 155.7 (range, 0-564.4), 4.5 (range, 0-121.9), and 3.2 (range, 0-10.9), respectively. As part of planned exploratory analyses, we examined an association between day +30 absolute lymphocyte, Treg, and natural killer–cell counts with 1-year GRFS. Excluding 3 patients who developed a GRFS event before day 28 and 2 without available flow cytometry data (n = 33), among the 14 patients who had Treg ≥ 0.5 on day 28, 2 patients developed a GRFS event compared with 8 of 19 with Treg < 0.5 (P = .13 by Fisher's exact test). Using this cutoff, in a landmark analysis (day 28), 1-year GRFS was 86% vs 62% (P = .11, log-rank; supplemental Figure 1).
Immune reconstitution and plasma cytokine profile. (A-F) Immune reconstitution analysis on days +28, +60, and +100 after HCT in RIC and MAC arms by flow cytometry per cells in microliter of blood, calculated using the absolute lymphocyte count on the complete blood count with differential. (G) Comparison of plasma cytokine levels on day +7 after HCT in patients who did and did not experience CRS. Outliers have been removed from the cytokine graphs.
Immune reconstitution and plasma cytokine profile. (A-F) Immune reconstitution analysis on days +28, +60, and +100 after HCT in RIC and MAC arms by flow cytometry per cells in microliter of blood, calculated using the absolute lymphocyte count on the complete blood count with differential. (G) Comparison of plasma cytokine levels on day +7 after HCT in patients who did and did not experience CRS. Outliers have been removed from the cytokine graphs.
Inflammatory cytokines
We next explored the associations between plasma cytokine levels and CRS. Several cytokines (epidermal growth factor, fibroblast growth factor, interferon-α and -γ, interleukin 17 [IL-17], IL-4, and IL-7) were unmeasurable in most patients on day 7 and thus excluded from analyses (supplemental Table 1). We found that IL-12, sIL-2R, monokine induced by gamma (MIG), and macrophage inflammatory protein-1 b (MIP-1b) levels, but not IL-6 levels, on day 7 after HCT were elevated in patients with CRS compared with those without CRS (Figure 3G). A median IL-6 level on day 7 in patients with CRS was 28.1 pg/mL (range, 8.2-668.7 pg/mL) compared with 16.5 pg/mL (range, 7.5-138 pg/mL) in those without CRS (P = .075, unadjusted for multiple testing). These differences were no longer observed on samples from day 28.
Discussion
Given the increasing availability of alternative donors, finding a fully matched donor is not the key access barrier to HCT, even for patients among racial/ethnic minorities. Unfortunately, outcomes after MMUD-HCT remains inferior to other alternative donors, when calcineurin inhibitor–based GVHD prophylaxis is administered. Our study, which enrolled a majority racial/ethnic minorities (61%), demonstrated a highly promising 1-year GRFS (68%) in patients undergoing a PBSC MMUD-HCT, which compares favorably with studies using other alternative donors12,13,25 and a recent study by the National Marrow Donor Program (NMDP) using a BM allograft for MMUD-HCT.26 Although the composite end point of GRFS can be significantly affected by patient, disease, and transplant characteristics (ie, HCT-CI, underlying DRI), it provides a good approximation of current expectations related to successful HCT (efficacy) using standard methods.27 GRFS serves as a benchmark for studies with relatively short follow-up (often 1 year) and thus is well suited for pilot studies designed to obtain estimates of efficacy. In fact, GRFS was the primary end point of a multicenter phase 2 trial of 3 different GVHD prophylaxis (BMT CTN1203, PROGRESS-II: NCT02208037). The PROGRESS-II trial, which enrolled mostly 8/8 MUD, showed adjusted 1-year Kaplan-Meier estimates for GFRS of 34% (90% CI: 28%-40%) for the comparator arm of tacrolimus/methotrexate and 43% (90% CI: 34%-54%) for the PTCy arm.25
Although our study was not designed to compare RIC and MAC, reasons for the lower GRFS in RIC cohort are likely baseline patient/disease characteristics because the RIC cohort had older patients, higher DRI, and higher HCT-CI, with baseline difference in the Functional Assessment of Cancer Therapy–Bone Marrow Transplantation Functional Well-Being (FACT-BMT FWB) scores between MAC and RIC indicating the MAC patients were functionally doing better than RIC at enrollment. In addition, donor age and sex in patients undergoing RIC contributed to more severe GVHD and subsequently lower GRFS. Nonetheless, our GRFS data from the RIC cohort seem at least comparable to the abovementioned PROGRESS-II data of PTCy in RIC PBSCs from mostly 8/8 HLA-matched donors,25 suggesting that PTCy may overcome the historically poorer outcome of MMUD compared with MUD-HCT.
Consistent with the GRFS outcome, and in accordance with outcomes of the prospective phase 2 study by the NMDP that used BM as the graft source for MMUD-HCT with 1-year OS of 76% (90% CI: 67.3-83.3),26 1-year OS and PFS were highly promising in our trial, especially in the MAC arm, attributable to low rates of NRM/severe GVHD without an apparent increase in short-term relapse. Although it is possible that these favorable outcomes are because of patient selection in this relatively small trial, our cohort had a significant number of high-risk patients with high/very high DRI in one third and HCT-CI of >2 in almost half of the patients, which is historically associated with adverse outcomes.2,6,28,29
With the use of PBSCs, our data showed rate of grade 2I-4 aGVHD at 50% and grade 3-4 at 18%, which is relatively high compared with published data in PBSC haploidentical HCT (14%)17 and the BM MMUD (14%).26 With the sample size of 38 in our trial, the 95% CI was 9% to 36%, and a larger trial is needed to better define the rate of severe aGVHD in MMUD-PBSC using PTCy. It is important to note that we observed only 2 deaths attributable to aGVHD, and the GVHD incidence did not translate into poor OS or GRFS. cGVHD was also common (52%), but the National Institutes of Health moderate/severe cGVHD was very rare at 1 year, consistent with previous reports.17 Overall, our data suggest that the PTCy approach effectively prevented severe/debilitating acute and chronic GVHD in the PBSC MMUD-HCT.
We observed a significant number of infectious complications such as CMV and BK virus infections between days 0 and +100, consistent with experiences with PTCy in the matched donor and haploidentical HCT.30,31 CMV viremia was around 42% in this study and mostly was G1 by Blood and Marrow Transplant Clinical Trials Network (BMT CTN) grading with prophylaxis introduced midway through the study (40% of CMV-seropositive patients). Other viral infections including Epstein-Barr virus, adenovirus, and HHV6 were rare.
Cellular and humoral immune reconstitution data on HCTs using PTCy are scarce. To our knowledge, our study is the first to describe a pattern for immune reconstitution in the PBSC MMUD-HCT with PTCy. Overall, the recovery of CD4, CD8 T cells, and natural killer cells appeared consistent with the data from haploidentical HCT with PTCy32,33 and matched donor BMT using PTCy as single-agent GVHD prophylaxis34; whereas natural killer cells reconstituted early, most patients had persistent B- and T-lymphocytopenia (especially CD4 T cells). Kanakry et al35 reported that human CD4+ Tregs are more resistant to PTCy in mixed lymphocyte cultures because of increased expression of aldehyde dehydrogenase both in vivo and in vitro. They also reported in both xenogeneic and major histocompatibility complex–matched HCT models with PTCy, CD4+ Tregs are necessary for GVHD prevention at early time points after transplant.36 Our exploratory data preliminarily support the potential role of Tregs as a biomarker in PBSC MMUD-HCT with PTCy, although it remains to be confirmed in larger sample if Tregs are biologically modifying the clinical outcomes or simply a surrogate for overall immune reconstitution.
CRS is a complex systemic inflammatory condition involving multiple signaling molecules.37 Recent advances include development of clinical grading systems38 and CRS management by tocilizumab administration.39 However, these studies are mainly in the context of chimeric antigen receptor T-cell therapies38,40,41 and bispecific T-cell engagers.42 CRS is also recognized after haploidentical HCT, particularly when PBSCs are infused as the graft source,43-45 with prognostic implications.42 CRS in matched donor or MMUD-HCT has not been fully characterized. In our study, CRS was common and generally mild. Our pilot data suggest a few cytokines (IL-12, sIL-2R, monokine induced by gamm and macrophage inflammatory protein-1b) may be associated with CRS. IL-6 levels were not significantly associated with CRS, but it should be noted that the sampling time was on day +7, by which time lymphocytes activation and proliferation are often subsiding by PTCy. Furthermore, lower rates of CRS in MAC cohort might be caused by the fractionated total body irradiation (FTBI)–based myeloablative regime, which could better eliminate preexisting host alloreactive lymphocytes and/or antigen presenting cells. Another possible contributing factor may be the older age of patients, which should be confirmed in a larger study.
Our data should be interpreted with caution. Although our data, in combination with the NMDP’s recent analysis using BM as the graft source for MMUD-HCT,26 demonstrate feasibility and safety (low NRM and adverse events) of this approach and possibly its efficacy with promising early results, the sample size was small with wide 95% CIs for survival end points. Long-term follow-up is required to better estimate the relapse/OS outcomes. Regarding the transplant outcomes in the RIC arm, better assessment and interventional tools for older patients, regarding frailty and resiliency, would likely improve patient selection and treatment stratification (NCT03992352).46
In summary, we showed promising results in a prospective pilot trial designed to estimate GRFS in patients without a matched donor who received PBSC MMUD-HCT with PTCy. Our data support further development of PTCy in the MMUD setting, which will likely expand the access and improve the HCT results in many patients without a matched donor, particularly in racial/ethnic minorities.
Acknowledgments
The authors thank City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible, and Anna Keryan (CRC), Pamela Sunga, and Nikki Lim (CRN) for their hard work.
This trial was funded by the City of Hope Internal Funding Prioritization Committee and National Institutes of Health, National Cancer Institute grant P30 CA033572 (Biostatistics Core).
Authorship
Contribution: M.M.A.M. and R.N. contributed to study concept and design and data interpretation; W.T. performed the immune assays and developed SOPs for the flow analysis; N.-C.T. and J.P. were the study biostatisticians and did the statistical analysis; S.M. drafted the report; the remaining authors contributed to critical revision of the manuscript for intellectual content; and all authors read and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
ORCID profiles: M.M.A.M., 0000-0001-8226-471X; J.Z., 0000-0003-4308-629X; R.N., 0000-0002-9082-0680.
Correspondence: Monzr M. Al Malki, Department of Hematology and HCT, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: malmalki@coh.org.
References
Author notes
For original data, please contact malmalki@coh.org. Individual participant data will not be shared.
The full-text version of this article contains a data supplement.