Key Points
This is the first prospective cohort study to report ATL incidence rate among HTLV-1 carriers in endemic areas of South America.
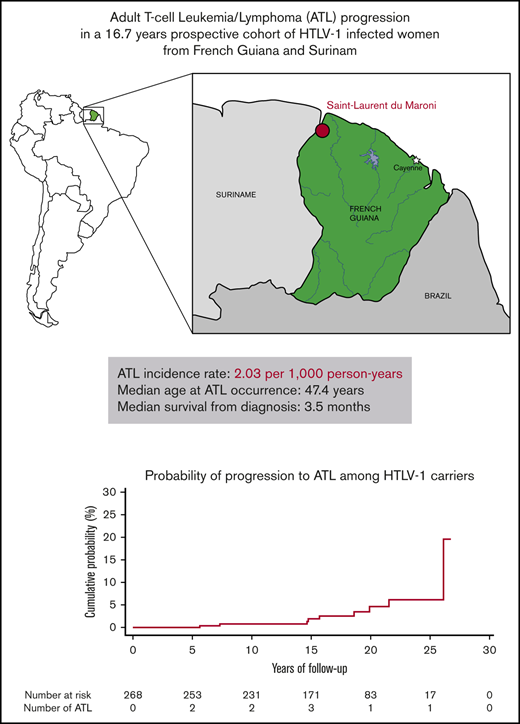
ATL incidence rate in women in French Guiana was 2.03 per 1000 person-years with median survival of 3.5 months.
Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy caused by the human T-cell leukemia virus type 1 (HTLV-1). The incidence of ATL among HTLV-1 carriers remains largely unknown in endemic countries other than Japan as very few prospective studies have been performed. We assessed the ATL incidence rate among HTLV-1 infected women in a prospective cohort in French Guiana. This is the first prospective study to assess the ATL incidence rate in an area of South America where HTLV-1 prevalence is high. Patients were enrolled between 1991 and 2005, and follow-up continued until April 2018. In the general hospital in Saint-Laurent-du-Maroni, 307 pregnant women were diagnosed with HTLV-1 infection, and 268 of them were observed for a median of 16.7 years. During follow-up, 9 ATL incident cases occurred resulting in an ATL incidence rate of 2.03 per 1000 HTLV-1 carrier-years (95% confidence interval, 0.93-3.85 per 1000 HTLV-1 carrier-years). The median age at diagnosis was 47.4 years, and median survival from diagnosis was low at 3.5 months. The ATL incidence rate was elevated for a study population consisting mostly of young people, which could either be a general feature in South America or could be specific to the Noir Marron population that constituted most of the cohort.
Introduction
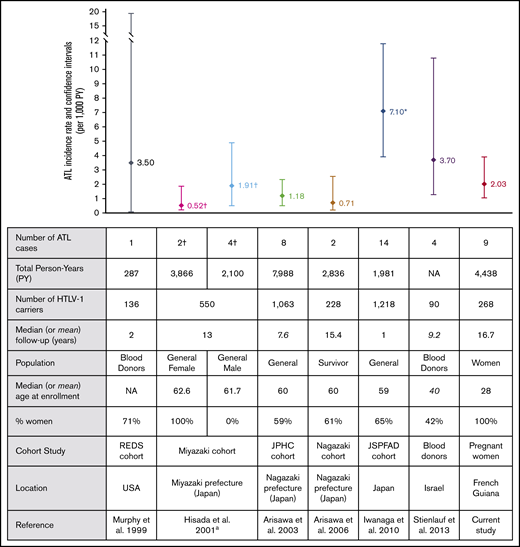
Adult T-cell leukemia/lymphoma (ATL) is a very aggressive malignancy caused by the human T-cell leukemia virus type 1 (HTLV-1).1,2 In Japan and Jamaica, it is estimated that HTLV-1 carriers have a risk of developing ATL during their lifetime of 2% to 7%, but the risk of ATL occurrence remains largely unknown in other countries.3,4 ATL cases have mainly been reported in areas where the prevalence of HTLV-1 is high, such as South America, the Caribbean area, sub-Saharan Africa, and the southwestern part of Japan.5 Most of the epidemiologic data on ATL come from cross-sectional studies that estimate the annual cumulative incidence of ATL, based on the estimations of either the number of incident ATL cases or the expected number of HTLV-1 carriers in a given population.3 Such estimations could bias the result, and the reported incidences should therefore be interpreted with care. Cohort studies are the only accurate method for determining incidence rate, but there have been very few of them to assess the incidence rate of ATL (Figure 1). In French Guiana, a French territory located between Brazil and Surinam, we observed a clustering of ATL cases in the Noir Marron ethnic group, a population with West African ancestry,6,7 suggesting a higher incidence of ATL in this group.8 Several epidemiologic studies showed that HTLV-1 infection was highly prevalent in this population, with a seroprevalence of 8.0% in the villages9 and about 4% among pregnant women.10-12
ATL incidence rates and cohort characteristics. ªATL mortality rates are represented separately for males and females from the same study. †ATL mortality rate from this study. However, given that most patients died within a year, this mortality rate can be considered an approximation of ATL incidence rate. *Confidence intervals were not available in the article and were estimated based on Poisson distribution. NA, not available.
ATL incidence rates and cohort characteristics. ªATL mortality rates are represented separately for males and females from the same study. †ATL mortality rate from this study. However, given that most patients died within a year, this mortality rate can be considered an approximation of ATL incidence rate. *Confidence intervals were not available in the article and were estimated based on Poisson distribution. NA, not available.
We conducted a prospective cohort study among women infected with HTLV-1 to assess the incidence rate of ATL in French Guiana. This study is one of the few prospective cohort studies of HTLV-1 carriers, and it provides the first report of incidence rate in South America, where HTLV-1 is endemic.
Study design
This prospective cohort study was conducted in Saint-Laurent-du-Maroni, the second most populous city in French Guiana, which is located on the Maroni River on the northwest border shared with Surinam. Its regional hospital, Centre Hospitalier de l’Ouest Guyanais (CHOG) provides the only gynecologic and obstetric unit in the western region, so its patients are derived from people living along both the French and the Surinamese sides of the Maroni River.
Upon recommendation from the French Ministry of Health, there is now systematic screening for HIV and HTLV-1 infections in pregnant women in French Guiana. All women who delivered their babies in the hospital from July 1991 to January 2005 were enrolled in the study, and 8546 pregnant women were tested for HTLV-1. Several previous studies described this baseline testing up to 2001,10-12 and our work extended the inclusion period to January 2005. Informed consent was obtained for all participants. This study was authorized by the Commission Nationale de l’Informatique et des Libertés and the Comité de Protection des Personnes dans la Recherche Biomedicale (Necker Hospital and Sud-Ouest et Outremer III).
Our cohort included all women infected with HTLV-1, which was confirmed by a positive western blot assay. The follow-up period began at the first delivery date on which HTLV-1 infection was detected, and the end point for each woman enrolled in the study was the date of either ATL diagnosis, last follow-up, or death. Follow-up was carried out by combining regular home surveys and frequent analysis of medical records from the CHOG up to May 1, 2018. Incidence rates were defined as the number of new cases per 1000 person-years (PY), and exact 95% confidence intervals (CIs) were obtained by assuming a Poisson distribution. Median survival time from ATL diagnosis was calculated by the Kaplan-Meier method. All analyses were performed using Stata 15.0 (Stata Corporation, College Station, TX).
Results
The study population consisted of 307 women infected with HTLV-1 with a median age of 28 years (range, 13-46 years) at inclusion. Most of the women were born in Surinam (64%) and French Guiana (29%), and the majority belonged to the Noir Marron ethnic group (89%).
One women diagnosed with chronic ATL before entry to the study cohort and 38 women without any follow-up were excluded from analysis. The remaining 268 women (88%) were observed for a median of 16.7 years (range, 3.3 months to 26.7 years) for a total of 4438 PY. During follow-up, 9 women developed ATL giving a crude incidence rate of 2.03 cases of ATL per 1000 HTLV-1 carrier-years (95% CI, 0.93-3.85 carrier-years). Our data showed no association with age, although the incidence rate seemed to increase after age 50 years (Table 1). Based on Shimoyama classification,13 there were 4 lymphoma subtypes, 4 acute and 1 undetermined subtype (Table 2). The median age at ATL diagnosis was 47.4 years (range, 26-59 years). Prognosis was extremely poor. Seven of the 9 ATL patients died during follow-up, with a median survival from diagnosis of 3.5 months (range, 0 days to 10 years) (Table 2). Of the 21 deaths that occurred during follow-up, ATL was the cause for 33% of them. Of the other causes of death, 2 resulted from cervical neoplasms, 2 from AIDS, 2 from stroke, 1 from cardiac arrest, 1 from diabetes, and 6 from unknown causes. No cases of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) were reported during the study period. Three incident cases of cervical carcinoma and 1 case of breast cancer occurred, resulting in incidence rates of 0.68 per 1000 PY (95% CI, 0.14-1.98 per 1000 PY) and 0.23 per 1000 PY (95% CI, 0.005-1.25 per 1000 PY), respectively. In our cohort, the incidence rate for ATL was higher than that for breast cancer (P = .01) and tended to be higher than that for cervical cancer (P = .09), the most prevalent cancers in women.
Age-specific incidence rate
| Age group, y . | Total PY . | No. of ATL incident cases . | Incidence rate per 1000 PY . | 95% CI per 1000 PY . |
|---|---|---|---|---|
| 13-19 | 136 | 0 | 0 | 0-27.1* |
| 20-29 | 969 | 2 | 2.06 | 0.25-7.46 |
| 30-39 | 1594 | 1 | 0.63 | 0.02-3.50 |
| 40-49 | 1259 | 2 | 1.59 | 0.19-5.74 |
| 50-59 | 445 | 4 | 8.99 | 2.45-23.0 |
| 60-67 | 35 | 0 | 0 | 0-105.4* |
| All | 4438 | 9 | 2.03 | 0.93-3.85 |
| Age group, y . | Total PY . | No. of ATL incident cases . | Incidence rate per 1000 PY . | 95% CI per 1000 PY . |
|---|---|---|---|---|
| 13-19 | 136 | 0 | 0 | 0-27.1* |
| 20-29 | 969 | 2 | 2.06 | 0.25-7.46 |
| 30-39 | 1594 | 1 | 0.63 | 0.02-3.50 |
| 40-49 | 1259 | 2 | 1.59 | 0.19-5.74 |
| 50-59 | 445 | 4 | 8.99 | 2.45-23.0 |
| 60-67 | 35 | 0 | 0 | 0-105.4* |
| All | 4438 | 9 | 2.03 | 0.93-3.85 |
One-sided 97.5% CI.
Description of ATL cases
| Case . | Age at ATL diagnosis, y . | Time in cohort, y . | Place of birth . | Ethnic group . | HIV serology . | Observed HTLV-1 seroconversion . | ATL subtype . | Status (April 2018) . | Survival time . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 5.6 | Surinam | NM | Negative | No | Lymphoma | Dead | 0 d |
| 2 | 45 | 7.3 | French Guiana | NM | Negative | No | Lymphoma | Unknown | Unknown |
| 3 | 47 | 8.6 | Surinam | NM | Negative | No | Lymphoma | Alive | >10 y |
| 4 | 29 | 14.7 | Surinam | NM | Negative | No | Acute | Dead | 3.5 mo |
| 5 | 54 | 15.7 | Surinam | NM | Negative | No | Acute | Dead | 4.6 mo |
| 6 | 51 | 18.6 | Surinam | NM | Negative | No | ND | Dead | 6.6 mo |
| 7 | 52 | 19.9 | Surinam | NM | Negative | No | Acute | Dead | 3.9 mo |
| 8 | 39 | 21.4 | Surinam | ND | Negative | No | Lymphoma | Dead | 1.0 mo |
| 9 | 59 | 25.8 | Surinam | NM | Negative | No | Acute | Dead | 0.1 mo |
| Case . | Age at ATL diagnosis, y . | Time in cohort, y . | Place of birth . | Ethnic group . | HIV serology . | Observed HTLV-1 seroconversion . | ATL subtype . | Status (April 2018) . | Survival time . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 5.6 | Surinam | NM | Negative | No | Lymphoma | Dead | 0 d |
| 2 | 45 | 7.3 | French Guiana | NM | Negative | No | Lymphoma | Unknown | Unknown |
| 3 | 47 | 8.6 | Surinam | NM | Negative | No | Lymphoma | Alive | >10 y |
| 4 | 29 | 14.7 | Surinam | NM | Negative | No | Acute | Dead | 3.5 mo |
| 5 | 54 | 15.7 | Surinam | NM | Negative | No | Acute | Dead | 4.6 mo |
| 6 | 51 | 18.6 | Surinam | NM | Negative | No | ND | Dead | 6.6 mo |
| 7 | 52 | 19.9 | Surinam | NM | Negative | No | Acute | Dead | 3.9 mo |
| 8 | 39 | 21.4 | Surinam | ND | Negative | No | Lymphoma | Dead | 1.0 mo |
| 9 | 59 | 25.8 | Surinam | NM | Negative | No | Acute | Dead | 0.1 mo |
ND, not determined; NM, Noir Marron.
Discussion
Measuring the incidence only from the time of HTLV-1 detection at pregnancy results in an overestimation of the incidence rate by reducing the period of infection. However, it is impractical to determine whether the HTLV-1 infection was acquired during childhood or from sexual transmission for each individual, and most of the studies measured ATL incidence from the time of HTLV-1 detection. In Japan, prospective cohort studies of HTLV-1 carriers reported ATL incidence rates ranging from 0.71 to 7.10 per 1000 HTLV-1 infected PY.14-17 Among blood donors, ATL incidence rate was 3.7 per 1000 PY in Israel18 and 3.5 per 1000 PY in the United States19 (Figure 1). There is considerable variation in ATL incidence rates among all published cohort studies. The wide range of results can be explained by differences in population size and patients’ characteristics, such as the age of the participants, the sex ratio, and the length of follow-up (Figure 1). Indeed, ATL occurs predominantly among the elderly, especially in Japan, where median age at onset is about 68 years.20 This contrasts with reports from Central and South America, where median age at onset is 49 years.21 In our cohort, ATL occurred early with a median age of 47.4 years, similar to the age observed in Jamaica.22 Conversely, women have a lower risk than men of developing ATL, although this sex-related difference was reported only in Japan and not in South America or in the Caribbean area.17,21 Considering only women infected with HTLV-1, the ATL incidence rate was 0.5 per 1000 in Japan,14 4 times lower than the rate in French Guiana, whereas in Jamaica, estimations of annual ATL incidence using a modeling approach ranged from 0.08 to 0.3 per 1000 HTLV-1–infected women.4 Under these assumptions, given that our cohort had the youngest participants and was composed of women only, the incidence rate was relatively high compared with these studies. Moreover, given the median age at ATL onset of 49 years in South America21 and the mean age at the end of follow-up in our study (42 years; range, 14-67 years), a longer follow-up would probably have resulted in a higher incidence rate.
Nevertheless, these estimated incidence rates were probably underestimated. Indeed, among those lost to follow-up and the 6 deaths from unknown causes, some participants could have developed a tumor, including ATL. Furthermore, given the high proportion of women from Surinam in our study, we cannot exclude that ATL cases might have been missed, with patients developing ATL after they moved back to Surinam. Little is known about HTLV-1 prevalence and the associated diseases in Surinam. A previous study reported that 0.4% of blood donors in Surinam were positive for HTLV-1.23 However, this number likely underestimates the actual prevalence of the virus in the general population. Indeed, some cases of TSP/HAM were previously reported in Surinam,24 and many cases of ATL have been reported in the Netherlands and in French Guiana among migrants from Surinam.25 These data strongly suggest that a large proportion of the Surinamese population is infected with HTLV-1, especially among the Noir Marron population.
Further studies are required to determine the incidence rates of ATL among men infected with HTLV-1 in French Guiana and to investigate any predisposition in the Noir Marron population. Given geographical differences in ATL features, such as the incidence rate, the age at diagnosis, the type of ATL, and probable exposure to different risk factors, there is a real need to implement studies on ATL in the areas of the Americas where HTLV-1 is endemic.
A high ATL incidence rate associated with a high fatality rate would impose a health burden in French Guiana, where HTLV-1 prevalence is high. HTLV-1 infection can also lead to HAM/TSP, a progressive neurological debilitating condition. No cases of HAM/TSP were reported during our cohort study. In Japan, the lifetime risk of HAM/TSP is expected to be considerably lower than ATL in HTLV-1 carriers and in Trinidad and Tobago, the annual incidence of HAM/TSP was estimated to be between 17.3 and 24.7 cases per 100 000 infected individuals.26,27
Besides ATL and HAM/TSP, HTLV-1 infected individuals are also at risk of developing a broad spectrum of diseases, and recent studies show that HTLV-1 infection was associated with an increase in overall mortality rate that remains unexplained.28 Studies are thus needed to better understand the consequences of HTLV-1 infection on health and to investigate overall mortality among HTLV-1 carriers compared with non-infected population.
Individual participant data will not be shared.
Acknowledgments
The authors thank the staff of the Service de Protection Maternelle et Infantile de la Direction Départementale des Affaires Sanitaires et Sociales de Guyane and the Gynecology-Obstetrics Department of the Centre Hospitalier de l’Ouest Guyanais for their help during this study.
This study was supported by the Centre National de la Recherche Scientifique (UMR 3569) (A.G.), the Institut Pasteur, Paris, France (A.G.), and through the Investissement d’Avenir as part of a French Laboratoire d’Excellence research program (Integrative Biology of Emerging Infectious Diseases [ANR10-LBX-62 IBEID]) (A.G.). J.-L.R. received financial support from the European Union (FOOD/2016/379-660).
Authorship
Contribution: J.-L.R. collected and analyzed data and helped write the article; P.T. collected the data; B.N., B.S., G.C., and D.G. helped collect the data; Y.M. and A.F. contributed to statistical analysis and helped write the article; and A.G. designed research, helped collect data, and helped write the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jill-Léa Ramassamy, Institut Pasteur, 28 Rue du Docteur Roux, 75015 Paris, France; e-mail: jill.lea.ramassamy@pasteur.fr; and Antoine Gessain, Institut Pasteur, 28 Rue du Docteur Roux, 75015 Paris, France; e-mail: antoine.gessain@pasteur.fr.