Key Points
Vitamin D deficiency is a predictor for poor overall survival in patients with multiple myeloma, even after adjusting for age and stage.
This difference is only observed in white patients, not African Americans, even under a lower threshold for deficiency.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy affecting morbidity and mortality through damage to multiple organs, including bone lesions, renal dysfunction, anemia, and immunosuppression. Osteoclasts in the bone marrow microenvironment play a key role in the pathophysiology of MM and associated bone disease and also affect overall outcome.1 Vitamin D plays a crucial role in maintaining bone health and affects osteoclast activity.2 Moreover, it decreases proinflammatory cytokines and reins in the negative pathological activation of Th17 cells,3 playing a significant role in MM’s pathophysiology.4
Recent studies suggest an important role of vitamin D in improving outcomes of patients with cancer.5 In MM specifically, several studies report a high incidence of vitamin D deficiency in patients with MM ranging from 24% (≤20 ng/mL) to 87% (≤30 ng/mL).6,7 However, the role of vitamin D in MM is not fully understood. Moreover, despite the prevalence of vitamin D deficiency, screening for vitamin D levels is not part of the routine MM workup.8
In the general population, there is wide variability by race in reported incidence of vitamin D deficiency. However, overall, these reports suggest a higher rate of deficiency in African American (AA) patients compared with white patients. For example, in 1 study using a threshold of 25(OH)D of 20 ng/mL or less, AA patients had a 82.1% prevalence of deficiency compared with 30.9% for white patients.9 Vitamin D supplementation has been shown to be beneficial to both AA and white patients, but the level necessary to render a benefit appears to be higher among AA.10,11
Here we studied the differential effect of vitamin D deficiency on outcome in patients with symptomatic MM, in particular by race, in the Veterans Affairs (VA) system. The VA offers a unique platform to study vitamin D deficiency in MM in a large and racially heterogeneous patient population.
Methods
Patients diagnosed with MM in the VA’s nationwide database of electronic health records were identified using the VA Corporate Data Warehouse, as described in our previous work.12 As this study’s focus was on racial disparities between AA patients and white patients, patients not identified as AA or white were excluded. Except where noted, we assessed vitamin D deficiency using a threshold of 20 ng/mL. Differences in vitamin D levels across race, sex, International Staging System stage at diagnosis, initial myeloma therapy (immunomodulatory imide drugs, proteasome inhibitors, both, or other), and transplant status were evaluated using Student t tests/analysis of variance. Revised International Staging System was not used because of the lack of readily available fluorescent in situ hybridization data. We assessed survival impact of vitamin D deficiency, using Kaplan-Meier curves and logrank tests, and the magnitude was studied further, using univariate and multivariate Cox models. This study was approved by the VA Boston Healthcare System Institutional Review Board.
Results and discussion
We identified 1889 patients with MM (29% AA, 61% white, 10% other/unknown) who had serum 25-OH vitamin D measurements within ±2 months of MM diagnosis (Table 1). Median serum vitamin D level was 26.5 ng/mL (interquartile range, 18.0-36.9 ng/mL), with 46.3% AA and 23.6% white patients having vitamin D deficiency (<20 ng/mL). However, we did not observe any association between serum vitamin D levels and sex (P = .496), stage (P = .675), initial therapy (P = .096), or transplant (P = .472); these associations were also not observed in AA or white subgroups.
Cohort characteristics
| . | All . | White . | African American . |
|---|---|---|---|
| Number of patients | 1889 | 1158 | 551 |
| Age at multiple myeloma diagnosis, mean ± SD, y* | 68.9 ± 10.2 | 70.3 ± 10.0 | 66.2 ± 9.9 |
| Female sex, % | 3.2 | 2.8 | 4.1 |
| International Staging System stage† | I, II, III:18.3%, 30.2%, 51.5% | I, II, III:15.6%, 32.5%, 51.9% | I, II, III:24.0%, 25.3%, 50.6% |
| Vitamin D level, median/mean | 26.5/29.2 | 28.6/31.3 | 21.5/24.6 |
| Vitamin D level <20, %* | 30.9 | 23.6 | 46.3 |
| . | All . | White . | African American . |
|---|---|---|---|
| Number of patients | 1889 | 1158 | 551 |
| Age at multiple myeloma diagnosis, mean ± SD, y* | 68.9 ± 10.2 | 70.3 ± 10.0 | 66.2 ± 9.9 |
| Female sex, % | 3.2 | 2.8 | 4.1 |
| International Staging System stage† | I, II, III:18.3%, 30.2%, 51.5% | I, II, III:15.6%, 32.5%, 51.9% | I, II, III:24.0%, 25.3%, 50.6% |
| Vitamin D level, median/mean | 26.5/29.2 | 28.6/31.3 | 21.5/24.6 |
| Vitamin D level <20, %* | 30.9 | 23.6 | 46.3 |
Significant difference between race groups P < .001.
Significant difference between race groups P < .05.
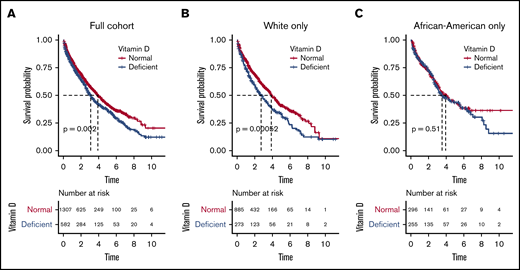
We next evaluated outcome in relationship to vitamin D levels and observed that patients with vitamin D deficiency had significantly worse OS (median, 3.10 years; 95% confidence interval [CI], 2.73-3.52) compared with patients with normal levels (median, 3.91 years; CI, 3.59-4.38; logrank P = .002; Figure 1A). The estimated mortality risk (hazard) increase resulting from vitamin D deficiency was 24% (hazard ratio [HR], 1.24; P = .002), and vitamin D deficiency remained an independent predictor of OS when adjusted for race, age, and stage at diagnosis (HR, 1.34; P = .008).
Overall survival probabilities in full cohort and in white and African American subgroups. Overall survival probabilities in full cohort (A), and in white (B) and African American (C) subgroups.
Overall survival probabilities in full cohort and in white and African American subgroups. Overall survival probabilities in full cohort (A), and in white (B) and African American (C) subgroups.
Given the significant difference in incidence of vitamin D deficiency between the AA and white populations, we evaluated outcome in these racial subgroups. For white patients, OS was significantly lower in patients with vitamin D deficiency compared with patients with normal levels (median, 2.71 years [CI, 2.18-3.47] vs 3.87 years [CI, 3.59-4.42], respectively; logrank P = .005; Figure 1B). The estimated mortality risk (hazard) increase was 38% (HR, 1.38; P = .001). On multivariate analysis, vitamin D deficiency was an even stronger independent predictor of OS (HR, 1.45; P = .005) after adjustment for age and stage at diagnosis.
In contrast, there was no detectable OS difference in AA patients with vitamin D deficiency compared with patients with normal serum vitamin D levels (median, 3.54 years [CI, 2.99-5.52] vs 3.95 years [CI, 3.25-5.35], respectively; logrank P = .510; Figure 1C). Vitamin D deficiency was also not an independent predictor of OS in AA after adjustment for age and stage at diagnosis (P = .692). Because serum vitamin D level was significantly lower in AA patients, we also analyzed the results using a 10 ng/mL cutoff for deficiency and did not observe a statistically significant difference in OS on either univariate (P = .078) or multivariate (P = .704) analysis.
Finally, we investigated the effect of vitamin D level as a continuous predictor of overall survival. A log transformation was applied to account for nonnormality. The log-transformed vitamin D level was a significant predictor of survival in white patients in univariate (HR, 0.77; P = .002) and multivariate (HR, 0.74; P = .009) analysis, but not in AA patients (P = .390 and 0.563, respectively).
It is important to acknowledge our study’s limitations. First, the data are from a primarily male patient population. A confirmation of these data in female population, although predicted, will be helpful to generalize the results. Second, it is possible that vitamin D supplementation before serum vitamin D measurement may affect findings. To investigate any effect of vitamin D supplementation, we identified 331 (17.5%) patients in the cohort who received at least 1 vitamin D supplement before their serum vitamin D measurement (and within ±2 months of MM diagnosis) and reanalyzed the data after excluding these patients. However, even after excluding these patients, the results described here and conclusions remained unchanged.
Our study shows the importance of screening for vitamin D deficiency at diagnosis in MM and highlights the differential effect of vitamin D across race, where white patients with deficiency had much worse outcomes. When studying the effect of vitamin D deficiency on other comorbidities across race, deficiency has been associated with both increased overall mortality and increased cardiovascular-related mortality in AA and non-AA.13 The Health ABC study demonstrated increased overall and cardiovascular mortality among AA and white participants, but only showed increased risk for cancer mortality among white participants.14 Hence, we hypothesize our findings of disparate effect of vitamin D deficiency relate to increased cancer (myeloma)-related mortality in white vs AA patients rather than other comorbidities.
Of note, a 2019 study of non-MM cancers concluded that vitamin D supplementation did not lower the incidence of invasive cancer, but excluding the first year of follow-up, the rate of death from cancer was significantly lower in patients receiving vitamin D supplementation compared with placebo (HR, 0.79; CI, 0.63-0.99).15
Although identifying this important association between vitamin D deficiency and differences in racial outcome, our study does not clarify any specific reason for the decreased survival in white population with deficiency. Although it is possible that vitamin D deficiency may affect mortality because of unrelated causes such as skeletal-related events, the differential effect between racial groups remains an intriguing observation. With the known association between bone marrow microenvironment, especially the bone elements, and MM cell growth and survival,1 vitamin D may play a unique role in MM compared with other cancers. A study investigating vitamin D deficiency’s prevalence observed that serum vitamin D levels lower than 10 ng/mL were associated with increased plasma cells in the bone marrow (44.8% vs 13.3%), and supplementation with vitamin D resulted in an increase in hemoglobin, leukocyte, and erythrocyte levels.7 More studies of vitamin D deficiency in MM are warranted to further explore the interplay between vitamin D and racial differences in its physiological impact, including potential roles in preventing tumorigenesis and promoting tumor suppression.
Our results, in addition suggesting a need to screen patients with MM for vitamin D deficiency and consider replacement if deficient, also highlight racial differences in disease biology that require further in-depth evaluation.
Veterans Affairs policy does not permit public sharing of identifiable health record data as used in this study.
Acknowledgments
This work was supported by the VA Office of Research and Development, Cooperative Studies Program (N.R.F., N.V.D., and M.T.B.), the VA Merit Review Award 1I01BX001584 (N.C.M.), and National Institutes of Health, National Cancer Institute grants P01-155258-07 and P50-100707 (N.C.M.).
The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Authorship
Contribution: S.V.Y., N.R.F., and N.C.M. conceived and designed the study; N.R.F. and A.F. prepared and analyzed the data; Y.Z., P.D., H.Y., C.I., N.V.D., and M.T.B. provided scientific input; S.V.Y., N.R.F., and N.C.M. wrote the manuscript; and all authors edited and critically reviewed the final version of the manuscript.
Conflict-of-interest disclosure: S.V.Y. receives research funding from Takeda and Celgene. M.T.B. receives research funding from Novartis. N.C.M. is a consultant for Celgene, Janssen, AbbVie, and Takeda and is on the board of directors for OncoPep. The remaining authors declare no competing financial interests.
Correspondence: Nikhil Munshi, Jerome Lipper Myeloma Center, Dana-Farber Cancer Institute, 440 Brookline Ave, M230, Boston, MA 02115; e-mail: nikhil_munshi@dfci.harvard.edu.
References
Author notes
S.V.Y. and N.R.F. contributed equally to this study.