Key Points
Incidental pulmonary embolisms are associated with poor short- and long-term outcomes in cancer patients.
Concurrent incidental venous thromboembolisms are common and lead to poorer outcomes.
Abstract
Incidental pulmonary embolisms (IPEs) are common in cancer patients. Examining the characteristics and outcomes of IPEs in cancer patients can help to ensure proper management, promoting better outcomes. To determine the clinical characteristics, management, and outcomes of IPEs for cancer patients, we conducted a 1:2 ratio case-control study and identified all consecutive patients with IPE who visited the emergency department at The University of Texas MD Anderson Cancer Center between 1 January 2006 and 1 January 2016. Each IPE case was matched with 2 controls using a propensity score obtained using logistic regression for IPE status with other factors affecting overall survival. A total of 904 confirmed cases were included in the analysis. IPE frequently occurred during the first year after cancer diagnosis (odds ratio [OR], 2.79; 95% confidence interval [95% CI], 2.37-3.29; P < .001). Patients receiving cytotoxic chemotherapy had a nearly threefold greater risk of developing IPE (OR, 2.87; 95% CI, 2.42-3.40; P < .001). In-hospital mortality was 1.9%. The 7- and 30-day mortality rates among the cases were 1.8% and 9.9%, respectively, which was significantly higher than in the control groups: 0.2% and 3.1%, respectively (P < .001). IPE was associated with reduced overall survival (hazard ratio [HR], 1.93; 95% CI, 1.74-2.14; P < .001). Concurrent incidental venous thromboembolism was identified in 189 of the patients (20.9%) and was also associated with reduced overall survival (HR, 1.65; 95% CI, 1.21-2.25; P = .001). Our results show that IPE events are associated with poor outcomes in cancer patients. Proper management plans similar to those of symptomatic pulmonary embolisms are essential.
Introduction
The incidence of venous thromboembolism (VTE), including pulmonary embolisms (PEs), in cancer patients is usually underestimated.1 In addition to the usual symptomatic presentation, PE can be found incidentally during routine imaging studies, including staging computed tomography (CT) scans of the chest or abdomen.2-4 With the introduction of multidetector CT scanners that are capable of providing good visualization of the pulmonary arteries up to the subsegmental level, the incidence of these asymptomatic incidental pulmonary embolisms (IPEs) increased dramatically.5,6 For symptomatic PEs, physicians stratify patients into different risk groups using clinical information, including the presenting symptoms, to assign pretest probability scores prior to testing.7,8 This process allows physicians to choose the optimal next step in determining which imaging or laboratory test needs to be done, if any.9 For confirmed IPEs, the management plan skips the PE diagnostic step and starts with clinical assessment of the patient, ordering secondary tests when necessary, including Doppler ultrasound, echocardiography, and respiratory reserve. The treatment plan is then devised, including medications, admission or discharge, and any interventional procedures needed, such as inferior vena cava (IVC) filter or thrombolysis.
The complexity of IPE is due to the multiple management possibilities and is often further complicated by multiple high-risk comorbidities.10-14 Similar rates of recurrent VTE, major bleeding, and mortality have been observed in patients with IPE compared with symptomatic PE,15,16 and most fatal PEs tend to be clinically unsuspected.11 Furthermore, the recommended use of anticoagulants varies among studies or guidelines. For example, the American College of Chest Physicians recommends observation for low-risk patients with subsegmental PE (with higher confidence when the PE is incidental and isolated) and normal bilateral ultrasonography of the legs, whereas the American Society of Clinical Oncology recommends treating incidental subsegmental PE on a case-by-case basis.12,14
In the current case-control study, we sought to identify important clinical characteristics and describe the management and outcomes of IPE in cancer patients.
Methods
Identification of final cases
First, we identified all cancer patients who visited The University of Texas MD Anderson Cancer Center emergency department (ED) between 1 January 2006 and 1 January 2016 and whose records included International Classification of Diseases, Ninth Revision codes for PE in the institutional billing databases. The list was then cross-referenced with the billing and radiology database to identify those who had CT studies of the chest with IV contrast performed within 24 hours prior to the ED visit. Patients who had CT pulmonary angiography were excluded as representative of symptomatic patients. The eligible cases were then reviewed to identify cancer patients with IPE who were sent to the ED for clinical evaluation and initiation of treatment upon discovery of the IPE. The exclusion criteria were (1) noncancer patient, (2) no acute PE (ie, chronic PE, no PE found on the chest CT study, tumor thrombus, or thrombus within pulmonary veins), (3) nonincidental PE (PE was suspected as a differential diagnosis by the treating physician or the indication on the CT was “suspected PE”), (4) incomplete medical records, and (5) deep venous thrombosis found within 72 hours prior to the chest CT study. The final cases were then matched with corresponding controls.
Matching and identification of controls
After identifying the final cases, we generated a pooled list of patients from which 2 controls could be matched to each of the final cases. This pool consisted of patients who had a CT study of the chest with IV contrast (excluding CT pulmonary angiography) at MD Anderson Cancer Center between 1 January 2006 and 1 January 2016, without a record of a previous International Classification of Diseases, Ninth Revision code related to PE or anticoagulation. From this pool, each final case was matched with 2 controls (1:2 ratio) using a propensity score. The propensity score was obtained using logistic regression for IPE status with other patient characteristics affecting overall survival, including CT study date, age at CT study, sex, race/ethnicity, type of cancer, stage of cancer, Charlson comorbidity index, renal function (serum creatinine), and payer/insurance type. Following the recommendation of matched sampling for casual effects, the 9 matching factors were selected a priori based on domain knowledge, and all of them were included in the propensity model.17 Propensity score matching was used to obtain matched 1:2 samples of patients who had IPE or not. After matching, an ad hoc check confirmed the balance of patient characteristics between the 2 groups using a 2-sample Student t test for continuous variables, Fisher’s exact test for binary variables, and the Cochran-Mantel-Haenszel test for ordinal variables. The exclusion criteria for the controls were noncancer patient, VTE within 1 year prior to the CT study, patient was receiving active anticoagulant therapy, and incomplete records. Each excluded matched control was replaced with the next-best matched control.
Sample size justification
We calculated the sample size using the following assumption. The median overall survival for the control group was estimated to be 12 months. Assuming that PE in cancer patients can increase the risk of death by 25% (based on prior studies done in the general population18,19 showing increased 1-year mortality after PE, which may be as high as 25%, as well as our own best guess among cancer patients), then assuming a hazard ratio (HR; PE vs non-PE) of 1.25, which translates into a median overall survival of 9.6 months for the PE group (cases), a total sample size of 3000 patients (1:2 ratio; 1000 cases and 2000 controls) achieves a power of 97.9% to detect a 2.4-month decrease in median overall survival from 12 months to 9.6 months, with a 2-sided significance level of 0.05 using the log-rank test. If the total number of IPEs between 1 January 2006 and 1 January 2016 was <1000, with a minimum of 562 cases and 1124 matched controls, a power of 85% could still be achieved.
Retrospective chart review, data collection, and interrater agreement
The institutional electronic medical record systems were used to extract the needed data. Following chart review guidelines,20,21 charts were reviewed by trained physicians who collected the clinical data. Questionable radiology reports were verified by a thoracic radiologist. First abstractors were trained by the primary investigator using a training set of charts that was reevaluated by the primary investigator. Abstractors joining at a later stage of the project were trained by the primary investigator or the trained first abstractors in the same manner. Biweekly meetings were held to monitor chart abstractor performance and address any abstraction questions, discrepancies, and disagreements. Variables from random sample charts (n = 150) were reviewed by a second reviewer to assess interrater agreement, calculating the κ statistic. The interobserver agreement was very good (κ = 0.84). Prior to data collection, a data dictionary was created to unify data and element collection, which included variable definitions, acceptable categories for each variable, interpretation of negative and missing values, and exclusion criteria. A collection form that included drop-down lists and checkboxes was used to record the data collected and the variables. The presence of IPE as the outcome of interest was defined as an incidence of acute PE incidentally found on a chest CT study with contrast. Advanced cancer status was defined as a solid tumor with growing primary or metastatic lesions and/or an increase in cancer biomarker levels or hematologic malignancies in relapsed or refractory phases. Time from cancer diagnosis was calculated from the time of confirmed pathologic diagnosis to the time of CT.
Data analysis
Baseline patient characteristics were analyzed using standard descriptive statistics. The Student t test, Wilcoxon Mann-Whitney U test, or χ2 test was used to compare variables, where appropriate. Univariate analysis was performed to determine the association between each variable and IPE. Statistically significant clinical variables from the univariate analyses were analyzed further using a multiple logistic regression model calculating the odds ratio (OR) with 95% confidence interval (CI). Kaplan-Meier survival analysis, followed by the log-rank test, was used to estimate the difference in overall survival (defined as time from CT scan date to death, with censoring for being alive or lost to follow-up) between the 2 groups. Univariate analysis of survival data for all patients was based on Cox proportional-hazards modeling; the HR was calculated with 95% CI. Furthermore, multivariable Cox proportional-hazards modeling was used to investigate the association between IPE characteristics and survival after controlling for common clinical and cancer-associated factors. For all analyses, P < .05 was considered statistically significant.
All statistical analyses were performed using R software (version 3.5.3; http://www.r-project.org). The study was approved by the institutional review board of MD Anderson Cancer Center, which granted waivers of informed consent. Anonymized patient-level data that are compliant with Health Insurance Portability and Accountability Act regulations will be shared upon acquiring MD Anderson Cancer Center Institutional Review Board approval.
Results
Patient characteristics
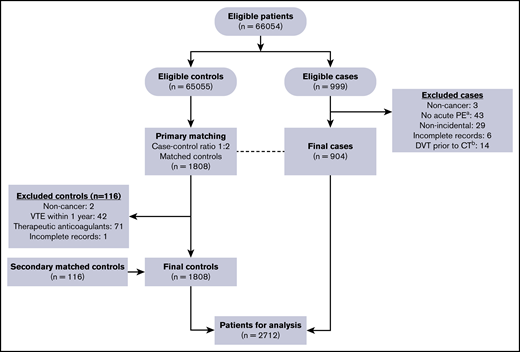
Of the 999 eligible cases initially identified, 904 final cases were confirmed as IPE once the exclusion criteria were applied (Figure 1). After matching, 1808 primary controls (patients who had routine CT study of the chest with IV contrast without evidence of PE) were reviewed. Of these, 116 were excluded and replaced with secondary matched controls. Table 1 shows the general characteristics of the patients in each group. The median age was 63 years in both groups, and the population was predominantly white (77% vs 23% nonwhite). More cases were receiving active cancer therapy compared with controls (55.4% vs 34.0%).
Flow chart of the case-control matching and exclusion criteria to determine study eligibility.a“No acute PE” included chronic PE, no PE found on the chest CT study, tumor thrombus, or thrombus within pulmonary veins. bDeep venous thrombosis (DVT) diagnosed within 72 hours prior to the chest CT study.
Flow chart of the case-control matching and exclusion criteria to determine study eligibility.a“No acute PE” included chronic PE, no PE found on the chest CT study, tumor thrombus, or thrombus within pulmonary veins. bDeep venous thrombosis (DVT) diagnosed within 72 hours prior to the chest CT study.
Baseline characteristics of the cases (patients with IPE) and controls (no IPE)
| Characteristic . | Cases . | Controls . | P . |
|---|---|---|---|
| Patients, n | 904 | 1808 | |
| Age, median (IQR), y | 63 (54-70) | 63 (54-71) | .639 |
| Sex | .673 | ||
| Female | 412 (45.6) | 841 (46.5) | |
| Male | 492 (54.4) | 967 (53.5) | |
| Race/ethnicity | .910 | ||
| Nonwhite | 204 (22.6) | 413 (22.8) | |
| White | 700 (77.4) | 1395 (77.2) | |
| CCI, median (IQR) | 6 (5-7) | 6 (4-7) | .123 |
| Serum creatinine, median (IQR), mg/dL | 0.84 (0.70-1.04) | 0.87 (0.71-1.01) | .334 |
| Cancer type | .387 | ||
| Breast | 69 (7.6) | 143 (7.9) | |
| Female genital | 63 (7.0) | 130 (7.2) | |
| Gastrointestinal | 266 (29.4) | 498 (27.5) | |
| Head and neck | 16 (1.8) | 61 (3.4) | |
| Leukemia | 20 (2.2) | 50 (2.8) | |
| Lung | 139 (15.4) | 294 (16.3) | |
| Lymphoma | 70 (7.7) | 143 (7.9) | |
| Male genital | 25 (2.8) | 64 (3.5) | |
| Melanoma | 55 (6.1) | 94 (5.2) | |
| Metastatic, unknown primary | 17 (1.9) | 22 (1.2) | |
| Others | 40 (4.4) | 71 (3.9) | |
| Sarcoma | 56 (6.2) | 94 (5.2) | |
| Urinary | 68 (7.5) | 144 (8.0) | |
| Cancer stage | .203 | ||
| Advanced | 730 (80.8) | 1419 (78.5) | |
| Local | 70 (7.7) | 177 (9.8) | |
| Hematologic | 104 (11.5) | 212 (11.7) | |
| Active cancer treatment | <.001 | ||
| Cytotoxic chemotherapy | 323 (35.7) | 347 (19.2) | |
| Targeted chemotherapy | 35 (3.9) | 99 (5.5) | |
| Radiotherapy | 29 (3.2) | 15 (0.8) | |
| Hormonal therapy | 20 (2.2) | 75 (4.1) | |
| Immunotherapy | 2 (0.2) | 9 (0.5) | |
| Multiple treatment regimens | 92 (10.2) | 69 (3.8) | |
| None | 403 (44.6) | 1194 (66.0) | |
| Surgery within 30 d | .002 | ||
| No | 887 (98.1) | 1797 (99.4) | |
| Yes | 17 (1.9) | 11 (0.6) |
| Characteristic . | Cases . | Controls . | P . |
|---|---|---|---|
| Patients, n | 904 | 1808 | |
| Age, median (IQR), y | 63 (54-70) | 63 (54-71) | .639 |
| Sex | .673 | ||
| Female | 412 (45.6) | 841 (46.5) | |
| Male | 492 (54.4) | 967 (53.5) | |
| Race/ethnicity | .910 | ||
| Nonwhite | 204 (22.6) | 413 (22.8) | |
| White | 700 (77.4) | 1395 (77.2) | |
| CCI, median (IQR) | 6 (5-7) | 6 (4-7) | .123 |
| Serum creatinine, median (IQR), mg/dL | 0.84 (0.70-1.04) | 0.87 (0.71-1.01) | .334 |
| Cancer type | .387 | ||
| Breast | 69 (7.6) | 143 (7.9) | |
| Female genital | 63 (7.0) | 130 (7.2) | |
| Gastrointestinal | 266 (29.4) | 498 (27.5) | |
| Head and neck | 16 (1.8) | 61 (3.4) | |
| Leukemia | 20 (2.2) | 50 (2.8) | |
| Lung | 139 (15.4) | 294 (16.3) | |
| Lymphoma | 70 (7.7) | 143 (7.9) | |
| Male genital | 25 (2.8) | 64 (3.5) | |
| Melanoma | 55 (6.1) | 94 (5.2) | |
| Metastatic, unknown primary | 17 (1.9) | 22 (1.2) | |
| Others | 40 (4.4) | 71 (3.9) | |
| Sarcoma | 56 (6.2) | 94 (5.2) | |
| Urinary | 68 (7.5) | 144 (8.0) | |
| Cancer stage | .203 | ||
| Advanced | 730 (80.8) | 1419 (78.5) | |
| Local | 70 (7.7) | 177 (9.8) | |
| Hematologic | 104 (11.5) | 212 (11.7) | |
| Active cancer treatment | <.001 | ||
| Cytotoxic chemotherapy | 323 (35.7) | 347 (19.2) | |
| Targeted chemotherapy | 35 (3.9) | 99 (5.5) | |
| Radiotherapy | 29 (3.2) | 15 (0.8) | |
| Hormonal therapy | 20 (2.2) | 75 (4.1) | |
| Immunotherapy | 2 (0.2) | 9 (0.5) | |
| Multiple treatment regimens | 92 (10.2) | 69 (3.8) | |
| None | 403 (44.6) | 1194 (66.0) | |
| Surgery within 30 d | .002 | ||
| No | 887 (98.1) | 1797 (99.4) | |
| Yes | 17 (1.9) | 11 (0.6) |
Unless otherwise noted, data are n (%).
CCI, Charlson comorbidity index; IQR, interquartile range.
IPE characteristics and management
Most IPEs were identified within the lobar (37.4%) and the segmental (26.2%) pulmonary arteries. Central IPEs were also common; 252 patients (27.9%) had saddle, main, or interlobar pulmonary artery embolisms. Only 77 patients (8.5%) had subsegmental IPE as the most proximal PE location identified. About one third (36.4%) of the patients had multiple levels of IPE at the same time (supplemental Table 1). Upon ED presentation, PE-related symptoms were identified in 181 (20.0%) patients (supplemental Table 2).
A total of 574 of the 904 IPE cases (63.5%) was discharged home. Of the remaining 330 patients (36.5%) who were admitted to the hospital, only 7 (2.1%) were admitted directly to the intensive care unit. The median length of ED stay was 5 hours (IQR, 4-8 hours). For the patients who were admitted, the median in-hospital length of stay was 4 days (IQR, 2-7 days), and in-hospital mortality was 1.9% (17 patients) for the entire cohort of cases.
Other incidental VTEs were also discovered at or within 72 hours after the chest CT study, with most (55.0%) detected on a CT study of the abdomen or pelvis. Concurrent incidental VTE was identified in 189 (20.9%) patients. Of these, femoral (45.0%), popliteal (23.3%), and iliac (22.8) veins were the most commonly affected sites (supplemental Table 3). Sixty patients (31.7%) had incidental central VTE (thrombosis in the iliac veins, inferior or superior vena cava, or the right atrium).
Most of the cases (84.2%) were initially treated with low molecular weight heparin (LMWH), reflecting the standard treatment of cancer-associated VTE during the period studied. Sixty-three patients (7.0%) were treated with IV unfractionated heparin, and 12 (1.3%) were treated with other types of anticoagulants (Table 2). Sixty-eight patients (7.5%) had no initial treatment. Similarly, most of the patients (88.1%) were discharged with LMWH, and 29 patients (3.2%) had other different types of anticoagulants as medications at discharge. In the remaining 79 patients (8.7%), no further discharge medication was prescribed. An IVC filter was implanted in 81 (9.0%) patients (Table 2). The most common reasons for no initial treatment (supplemental Table 4) were active bleeding (36.8%), thrombocytopenia (25%), and brain metastasis (13.2%).
Initial and discharged treatment regimens for cancer patients with IPE (n = 904)
| Treatment . | No. of patients (%) . |
|---|---|
| Initial treatment | |
| LMWH | 761 (84.2) |
| Enoxaparin | 578 (63.9) |
| Dalteparin | 183 (20.2) |
| Unfractionated heparin | 63 (7.0) |
| Direct oral anticoagulant | 6 (0.7) |
| Factor Xa inhibitor | 5 (0.6) |
| Direct thrombin inhibitor | 1 (0.1) |
| No initial treatment | 68 (7.5) |
| Discharged treatment | |
| LMWH | 796 (88.1) |
| Enoxaparin | 604 (66.8) |
| Dalteparin | 192 (21.2) |
| Direct oral anticoagulant | 14 (1.5) |
| Factor Xa inhibitor | 8 (0.9) |
| Warfarin | 6 (0.7) |
| Direct thrombin inhibitor | 1 (0.1) |
| No discharged treatment | 79 (8.7) |
| No medications or IVC filter | 27 (3.0) |
| IVC filter placement only | 52 (5.8) |
| In-hospital IVC filter placement | |
| No | 823 (91.0) |
| Yes | 81 (9.0) |
| Treatment . | No. of patients (%) . |
|---|---|
| Initial treatment | |
| LMWH | 761 (84.2) |
| Enoxaparin | 578 (63.9) |
| Dalteparin | 183 (20.2) |
| Unfractionated heparin | 63 (7.0) |
| Direct oral anticoagulant | 6 (0.7) |
| Factor Xa inhibitor | 5 (0.6) |
| Direct thrombin inhibitor | 1 (0.1) |
| No initial treatment | 68 (7.5) |
| Discharged treatment | |
| LMWH | 796 (88.1) |
| Enoxaparin | 604 (66.8) |
| Dalteparin | 192 (21.2) |
| Direct oral anticoagulant | 14 (1.5) |
| Factor Xa inhibitor | 8 (0.9) |
| Warfarin | 6 (0.7) |
| Direct thrombin inhibitor | 1 (0.1) |
| No discharged treatment | 79 (8.7) |
| No medications or IVC filter | 27 (3.0) |
| IVC filter placement only | 52 (5.8) |
| In-hospital IVC filter placement | |
| No | 823 (91.0) |
| Yes | 81 (9.0) |
Risk factors
Univariate analysis showed that patients receiving cytotoxic chemotherapy at the time of the CT had a nearly threefold greater risk for developing IPE compared with patients not receiving cytotoxic chemotherapy (OR, 2.87; 95% CI, 2.42-3.40; P < .001). An increased risk was also observed among patients receiving radiotherapy at the time of CT (OR, 5.41; 95% CI, 3.30-9.20; P < .001). In contrast, patients receiving hormonal therapy had a reduced risk for developing IPE (OR, 0.56; 95% CI, 0.35-0.87; P = .013). IPE frequently occurred during the first year after cancer diagnosis (OR, 2.79; 95% CI, 2.37-3.29; P < .001) and also among patients with active cancer status (OR, 6.17; 95% CI, 5.03-7.62; P < .001). Similar results were observed in the multivariable analysis, except that active hormonal therapy did not turn out to be significant (P = .726) after controlling for the other factors (Table 3).
Univariate and multivariable analysis for the association of cancer-related factors with IPE
| Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Time from cancer diagnosis | ||||
| >1 y | Reference | |||
| ≤1 y | 2.79 (2.37-3.29) | <.001 | 1.99 (1.66-2.38) | <.001 |
| Cancer status | ||||
| Stable | Reference | |||
| Active | 6.17 (5.03-7.62) | <.001 | 4.50 (3.63-5.61) | <.001 |
| Active cytotoxic chemotherapy | ||||
| No | Reference | |||
| Yes | 2.87 (2.42-3.40) | <.001 | 2.21 (1.84-2.66) | <.001 |
| Active radiotherapy | ||||
| No | Reference | |||
| Yes | 5.41 (3.30-9.20) | <.001 | 4.28 (2.51-7.54) | <.001 |
| Active hormonal therapy | ||||
| No | Reference | |||
| Yes | 0.56 (0.35-0.87) | .013 | 1.09 (0.66-1.76) | .726 |
| Surgery within 30 d | ||||
| No | Reference | |||
| Yes | 3.13 (1.48-6.92) | .003 | 3.03 (1.33-7.19) | .009 |
| Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Time from cancer diagnosis | ||||
| >1 y | Reference | |||
| ≤1 y | 2.79 (2.37-3.29) | <.001 | 1.99 (1.66-2.38) | <.001 |
| Cancer status | ||||
| Stable | Reference | |||
| Active | 6.17 (5.03-7.62) | <.001 | 4.50 (3.63-5.61) | <.001 |
| Active cytotoxic chemotherapy | ||||
| No | Reference | |||
| Yes | 2.87 (2.42-3.40) | <.001 | 2.21 (1.84-2.66) | <.001 |
| Active radiotherapy | ||||
| No | Reference | |||
| Yes | 5.41 (3.30-9.20) | <.001 | 4.28 (2.51-7.54) | <.001 |
| Active hormonal therapy | ||||
| No | Reference | |||
| Yes | 0.56 (0.35-0.87) | .013 | 1.09 (0.66-1.76) | .726 |
| Surgery within 30 d | ||||
| No | Reference | |||
| Yes | 3.13 (1.48-6.92) | .003 | 3.03 (1.33-7.19) | .009 |
Short-term mortality and overall survival
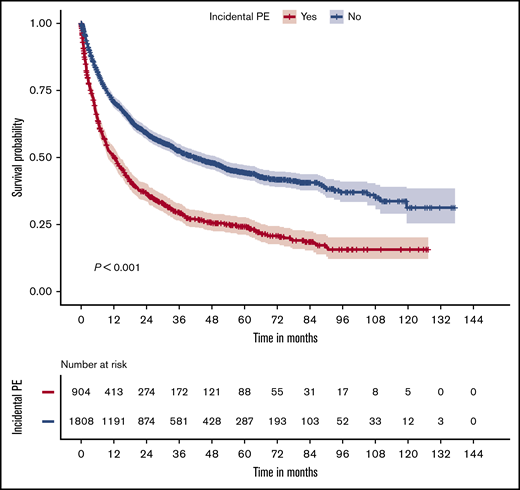
Significant short- and long-term survival differences were observed between patients with or without IPE. The 7-, 30-, and 90-day mortality among the cases were 1.8% (n = 16), 9.9% (n = 90), and 22.1% (n = 201), respectively, which were significantly higher than those in the control group (0.2%, n = 3; 3.1%, n = 56; and 9.8%, n = 179, respectively; all P < .001; Table 4). Kaplan-Meier analysis showed a significant difference (P < .001) in overall survival between patients with and without IPE (Figure 2), and the poorest overall survival was observed in patients with a central IPE (supplemental Figure 1). IPE was associated with reduced overall survival (HR, 1.93; 95% CI, 1.74-2.14; P < .001). For the patients with IPE, a separate analysis (Table 5) revealed that embolism in the central pulmonary arteries was associated with a worse prognosis than distal PE (HR, 1.35; 95% CI, 1.14-1.60; P < .001). Also, patients with concurrent VTE, especially central VTE, had worse overall survival than did those without a concurrent VTE (HR, 1.47; 95% CI, 1.07-2.01; P = .017).
Short-term mortality for cancer patients with or without IPE after an ED department visit
| Mortality, d . | IPE (n = 904) . | No IPE (n = 1808) . | P . |
|---|---|---|---|
| 7 | 16 (1.8) | 3 (0.2) | <.001 |
| 14 | 39 (4.3) | 20 (1.1) | <.001 |
| 30 | 90 (10) | 56 (3.1) | <.001 |
| 90 | 201 (22.2) | 179 (9.8) | <.001 |
| Mortality, d . | IPE (n = 904) . | No IPE (n = 1808) . | P . |
|---|---|---|---|
| 7 | 16 (1.8) | 3 (0.2) | <.001 |
| 14 | 39 (4.3) | 20 (1.1) | <.001 |
| 30 | 90 (10) | 56 (3.1) | <.001 |
| 90 | 201 (22.2) | 179 (9.8) | <.001 |
Data are n (%).
Association of IPE with poor overall survival. Kaplan-Meier curves for overall survival are shown for the control (blue) and case (red) groups (upper panel). The number of patients at various time points is shown in the table (lower panel).
Association of IPE with poor overall survival. Kaplan-Meier curves for overall survival are shown for the control (blue) and case (red) groups (upper panel). The number of patients at various time points is shown in the table (lower panel).
Univariate and multivariable Cox proportional-hazards model analysis of overall survival in patients with IPE
| Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR* (95% CI) . | P . | |
| IPE location | ||||
| Distal PE | Reference | |||
| Central PE | 1.35 (1.14-1.60) | <.001 | 1.28 (1.08-1.53) | .006 |
| Associated VTE | ||||
| None | Reference | |||
| Peripheral VTE | 1.38 (1.11-1.72) | .003 | 1.30 (1.04-1.63) | .019 |
| Central VTE | 1.65 (1.21-2.25) | .001 | 1.47 (1.07-2.01) | .017 |
| Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR* (95% CI) . | P . | |
| IPE location | ||||
| Distal PE | Reference | |||
| Central PE | 1.35 (1.14-1.60) | <.001 | 1.28 (1.08-1.53) | .006 |
| Associated VTE | ||||
| None | Reference | |||
| Peripheral VTE | 1.38 (1.11-1.72) | .003 | 1.30 (1.04-1.63) | .019 |
| Central VTE | 1.65 (1.21-2.25) | .001 | 1.47 (1.07-2.01) | .017 |
Controlling for age, sex, race, Charlson comorbidity index, cancer type, cancer stage, and time from cancer diagnosis.
Discussion
PEs that are discovered incidentally during imaging studies obtained for a different indication are common in cancer patients.22,23 Data from a comprehensive cancer center were collected and used to identify clinical characteristics, management, and outcomes of patients with IPE presenting to the ED. Active chemotherapy or radiotherapy, active cancer, and presentation within 1 year after cancer diagnosis were the main risk factors associated with IPE. Most of the patients (63.6%) had lobar or segmental PE as their most proximal IPE identified. Symptoms were observed in 20% of the patients upon their ED presentation. VTE was discovered in 20.9% of the patients with IPE as a concurrent incidental finding, and it was associated with poor overall survival. In most patients, LMWH was prescribed as the initial (84.2%) or the discharge (88.1%) medication, reflecting the practice pattern during the period studied. Seven-day mortality was ninefold higher in patients with IPE (1.8%) than in patients without IPE (0.2%). Significantly poorer overall survival was also observed during the follow-up period, especially in patients with a central IPE.
Cancer-associated thrombosis is a common event that develops in cancer patients during their disease course. This comorbidity has a negative effect on patient outcomes. In symptomatic PEs, physicians use validated clinical decision rules7,8 that rely on clinical predictors in combination with D-dimer, the main biomarker of thrombosis, to stratify patients according to their risk of having a PE.9 Despite the lower predictive values of D-dimer in cancer patients compared with the general population,24,25 the combination approach is capable of identifying patients in whom a CT pulmonary angiography is required.26 This highly sensitive imaging study can accurately diagnose patients with PE, allowing for the next management step. With the continual improvement in imaging studies, specifically multidetector CT, the clear visualization of the pulmonary arteries has made it possible to diagnose PE incidentally using routine contrast-enhanced CT studies.5,27 In these patients, in which PE is unsuspected, a chest CT study with contrast is usually performed as part of the patient’s baseline staging or surveillance workup.
Despite being asymptomatic or clinically unsuspected, IPE can be an emergent/urgent event, depending on the extent of the thrombosis and the clinical condition of the patient. Radiologic findings, including the PE location, clot burden, presence of pulmonary infarction, and any other warning radiologic signs, such as right heart strain, can help to determine the severity of the IPE. A combination of clinical and radiologic assessments of the patient should guide the ED or the treating physicians in determining the management plan.
The reported incidence of IPE among cancer patients has varied significantly among studies.3,28-30 The 2 major factors that account for this variation include a lack of interobserver agreement and differences in CT scanner or slice thickness.31 Because the chest CT study is requested for reasons other than PE, visualization of the pulmonary arterial tree is of a lesser priority; thus, the discovery of IPE is radiologist dependent. Overdiagnosis and underdiagnosis of IPE have been reported by radiologists. Overdiagnosis was found in as many as 25% of cases upon review by subspecialty thoracic radiologists.32 Most false-positive IPEs were solitary, and the main reason for the false reading was artifacts. In contrast, many studies have reported that reassessment of the CT by an expert radiologist results in the discovery of an IPE that was not reported initially.2 Most of these missed IPEs were solitary or distal PEs.
In the current study, active cancer treatment with cytotoxic chemotherapy, diagnosis of cancer within 1 year of index event, and active cancer were risk factors for IPE; they are also risk factors for PE in different clinical contexts.33,34 IPE risk factors identified were similar to those known for clinically suspected PE. Also, most IPEs were treated with LMWH, which is consistent with the findings of recent studies on the long-term treatment options for cancer-associated thrombosis, which reflects the standard treatment used during the period studied.35 PE-associated complications, including sudden death, pulmonary infarction, pulmonary hypertension, pleural effusion, cardiac arrest and arrhythmias, and recurrent VTE, can all account for poor outcomes in patients.36 In addition, side effects of anticoagulants and embolectomy complications can further intensify the poor prognosis.37 A recent large prospective study showed that the risk of recurrent VTE is significant despite anticoagulant therapy in patients with IPE, including in patients with subsegmental IPE. Major bleeding occurred in 5.7% of patients in the first year of follow-up. Both VTE and bleeding contributed to poor outcomes, in which 43% of the study population died within 1 year.35 This is consistent with a previous 1:2 ratio case-control study of 70 patients with IPE, reporting HR of 1.51 for death (95% CI, 1.01-2.27; P = .048).15 In the current study, we have shown that patients with IPE have worse short-term and long-term survival outcomes than matched patients without IPE, especially those with a central IPE. This is similar to prior studies that also identified an increased risk associated with central PEs.13 A key finding in the current study is the frequent discovery of incidental VTE as a concurrent finding in patients with IPE; when present, VTE was associated with even poorer patient outcomes.
Certain limitations accompanied our study. Although retrospective studies may have limitations in data collection, the well-established medical electronic records at The University of Texas MD Anderson Cancer Center made it possible to accurately identify PEs that were found incidentally on a chest CT study and collect related clinical and management data. The retrospective data collected about discharge medications were confirmed by the physician and discharge notes and the pharmacy database, on and around the ED visit. Changes in the medication regimen (including switching to warfarin, stopping the medication, or changing treatment duration) are expected. Therefore, only descriptive analysis of the discharge medication was shown here. The significant changes in medical practice over time, and the recent introduction of direct oral anticoagulants, require a future study of the shift in the initial and discharged medications being used. Also, retrospective studies have limitations in collecting other factors that may influence the outcome of the patients with IPE, including the effect of IPE diagnosis on the cancer management, such as delaying chemotherapy or scheduled surgeries. A future prospective study can include and accurately control for these factors. Another important limitation is the incidence of other concurrent VTEs. This incidence could be underestimated, because many patients had only a chest CT study. The lack of any other imaging study could cause physicians to miss an incidental VTE, if present.
In summary, we found that cancer-associated IPE is a common comorbidity in cancer patients. PEs that are found incidentally share similar risk factors with symptomatic PEs.34,38,39 Despite being asymptomatic or clinically unsuspected, IPE is associated with poor short- and long-term outcomes in cancer patients. Proper management plans similar to those of symptomatic PEs are essential. Further investigations of the predictors of poor patient outcome are needed to improve management of IPEs. The treatment of IPEs in cancer patients remains controversial. We also showed that concurrent incidental VTE is common and leads to poorer outcomes. Identifying other concurrent incidental VTEs is necessary and can improve patient outcomes.
Anonymized patient-level data that are compliant with Health Insurance Portability and Accountability Act regulations will be shared upon acquiring MD Anderson Institutional Review Board approval. Please contact syeung@mdanderson.org.
Acknowledgments
The authors thank Erica Goodoff for editorial support.
This work was supported by Bristol-Myer Squibb/Pfizer American Thrombosis Investigator Initiated Research Program (ARISTA-USA).
The funder had no role in study design, data collection and analysis, or preparation of the manuscript.
Authorship
Contribution: S.-C.J.Y. and A.Q. conceived and designed the study and developed the methods; A.Q., M.K., A.A.-B., and B.G. acquired data; C.C.W. reviewed questionable diagnostic images; S-C.J.Y. and S.Z. supervised statistical analysis; A.Q. and S.Z. analyzed and interpreted the data; A.Q. created the figures and tables; A.Q., S.-C.J.Y., T.W.R., and K.A. wrote the manuscript; and all authors reviewed and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sai-Ching Jim Yeung, Department of Emergency Medicine, Unit 1468, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: syeung@mdanderson.org.
References
Author notes
The full-text version of this article contains a data supplement.