Key Points
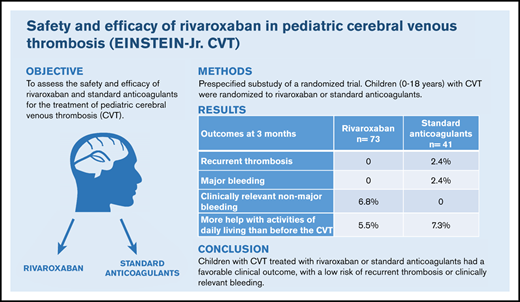
Children with CVT treated with rivaroxaban or standard anticoagulants had a low risk of recurrent VTE or clinically relevant bleeding.
Abstract
Anticoagulant treatment of pediatric cerebral venous thrombosis has not been evaluated in randomized trials. We evaluated the safety and efficacy of rivaroxaban and standard anticoagulants in the predefined subgroup of children with cerebral venous thrombosis (CVT) who participated in the EINSTEIN-Jr trial. Children with CVT were randomized (2:1), after initial heparinization, to treatment with rivaroxaban or standard anticoagulants (continued on heparin or switched to vitamin K antagonist). The main treatment period was 3 months. The primary efficacy outcome, symptomatic recurrent venous thromboembolism (VTE), and principal safety outcome, major or clinically relevant nonmajor bleeding,were centrally evaluated by blinded investigators. Sinus recanalization on repeat brain imaging was a secondary outcome. Statistical analyses were exploratory. In total, 114 children with confirmed CVT were randomized. All children completed the follow-up. None of the 73 rivaroxaban recipients and 1 (2.4%; CVT) of the 41 standard anticoagulant recipients had symptomatic, recurrent VTE after 3 months (absolute difference, 2.4%; 95% confidence interval [CI], −2.6% to 13.5%). Clinically relevant bleeding occurred in 5 (6.8%; all nonmajor and noncerebral) rivaroxaban recipients and in 1 (2.5%; major [subdural] bleeding) standard anticoagulant recipient (absolute difference, 4.4%; 95% CI, −6.7% to 13.4%). Complete or partial sinus recanalization occurred in 18 (25%) and 39 (53%) rivaroxaban recipients and in 6 (15%) and 24 (59%) standard anticoagulant recipients, respectively. In summary, in this substudy of a randomized trial with a limited sample size, children with CVT treated with rivaroxaban or standard anticoagulation had a low risk of recurrent VTE and clinically relevant bleeding. This trial was registered at clinicaltrials.gov as #NCT02234843.
Introduction
Cerebral venous thrombosis (CVT) is a rare type of stroke in childhood with an estimated incidence of 0.7 per 100 000 children per year.1 CVT typically causes severe headache and may result in vision problems, seizures, focal neurologic deficits, decreased level of consciousness, and death.2-4 Commonly reported risk factors for pediatric CVT include birth complications, infection of the head or neck, cancer, traumatic head injury, acquired or inherited thrombophilia, and use of hormonal contraceptive methods.1,3-7
In adults with CVT, therapeutic anticoagulation is widely considered the mainstay of treatment,8,9 although only supported by data from a few small, randomized trials,10-12 which included only a few patients with infectious CVT. A unique challenge of anticoagulation in CVT compared with other types of venous thromboembolism (VTE) is the high proportion of patients who are at risk for development of intracranial hemorrhage. In children with CVT (ie, neonates and older children), use of anticoagulant therapy, with the potential for intracranial hemorrhage, is more controversial because of the absence of clinical trials and the infectious nature of CVT in many children.13-16 However, the potential short-term risk of withholding anticoagulants is extension of the thrombus with associated neurological deterioration.17,18
Recently, the direct factor Xa inhibitor rivaroxaban was compared with standard anticoagulation for the treatment of acute VTE of all types in 500 children of all ages (EINSTEIN-Jr).19,20 The body weight–adjusted oral rivaroxaban treatment regimens successfully targeted the adult rivaroxaban exposure range without the need for laboratory monitoring.20-22 Recurrent VTE occurred infrequently with both rivaroxaban and standard anticoagulation, and no major bleeding events were observed with rivaroxaban.19 The absolute incidences of study outcomes and relative treatment effects observed with rivaroxaban were similar to those in the large rivaroxaban VTE studies in adults.19,23-25
We present a predefined subgroup analysis of the safety and efficacy of rivaroxaban and standard anticoagulants in the 114 children who presented with CVT in the EINSTEIN-Jr phase 3 trial.19
Methods
Study design and patients
This is a substudy of the EINSTEIN-Jr phase 3 trial, which was a multicenter, open-label, randomized trial that compared the efficacy and safety of rivaroxaban with those of standard anticoagulants for the treatment of pediatric VTE. The study protocol, the clinical efficacy and safety results, and the rivaroxaban dose-exposure response have been published.19,20,25 The statistical analysis plan for this prespecified substudy can be found in the supplemental Data. The institutional review board at each participating center approved the study protocol. Written permission from a parent or guardian and, when appropriate, the child’s assent, were obtained. An independent data-monitoring committee periodically reviewed the study outcomes. The substudy was conducted at 51 sites in 20 countries (Australia, Turkey, Israel, and China and other countries in Europe, South America, and North America). The main study treatment period was 3 months.
CVT was defined as thrombosis of any of the cerebral veins or sinuses diagnosed by computed tomography (CT)–venography, or magnetic resonance imaging (MRI) with magnetic resonance venography. Children with isolated jugular vein thrombosis did not qualify for this subgroup. Children with CVT were considered if treatment had been initiated with unfractionated heparin, low-molecular-weight heparin, or fondaparinux. Children younger than 0.5 year were required to have a gestational age at birth of at least 37 weeks and body weight above 2600 g and to have received oral feeding for at least 10 days. The main exclusion criteria were active bleeding or a high risk of bleeding that contraindicated anticoagulant therapy; an estimated glomerular filtration rate <30 mL/min per 1.73 m2; or, if aged <1 year, serum creatinine >97.5th percentile. CVT-associated intracerebral hemorrhage was not an exclusion criterion. The full list of eligibility criteria is provided in supplemental Table 1.
Enrollment started with children aged 12 to 17 years followed by those aged 6 to 11 and 2 to 5 years and 0.5 to 1 and <0.5 year, as described previously.19
Randomization and study medication
After completion of 5 to 9 days of anticoagulation with unfractionated heparin, low-molecular-weight heparin, or fondaparinux, the children were randomized, by using an interactive Web response system and permuted blocks of 3, in a 2:1 ratio to receive either open-label rivaroxaban or standard anticoagulation. Randomization was stratified per age group and site of VTE (extremity venous thrombosis, pulmonary embolism, caval or catheter-related thrombosis vs CVT or jugular, renal, or portal vein thrombosis). Children in the standard anticoagulation group continued with heparin treatment or were switched to a vitamin K antagonist, at the discretion of the treating physician, at therapeutic doses, according to international guidelines.13-15 Children allocated to rivaroxaban received a body weight–adjusted 20-mg equivalent dose, given 1, 2, or 3 times daily for body weights of ≥30, 12 to <30, and <12 kg, respectively (supplemental Table 2). Rivaroxaban was administered as immediate-release, film-coated tablets available in strengths of 5, 10, 15, or 20 mg, or as a newly developed suspension for oral use.20
Follow-up and outcomes
All children who stopped study treatment earlier than scheduled were observed until the end of the 3-month treatment period. Patients and their parents were instructed to report to the study center if the patients had symptoms suggestive of recurrent thrombosis or bleeding. The primary efficacy outcome was symptomatic recurrent VTE (extremity deep venous thrombosis; pulmonary embolism; CVT; or jugular, caval, renal, portal vein, or catheter-related thrombosis). The principal safety outcome was the composite of overt major and clinically relevant nonmajor bleeding, according to the criteria defined by the International Society of Thrombosis and Haemostasis (supplemental Table 3).26,27 Repeat imaging of the cerebral venous system was performed at the end of the study treatment period and compared with baseline images. The degree of cerebral vein and sinus recanalization was classified as normalized (ie, no residual thrombus observed), improved (ie, thrombus still present, but partly recanalized or involving fewer venous segments), no relevant change (ie, not recanalized and similar in extent), or deteriorated (ie, new venous segment involved). The degree of recanalization was classified as uncertain if repeat imaging was not performed or not evaluable, or if repeat imaging was performed <7 days before or >7 days after cessation of the study medication. In addition, the baseline and repeat imaging tests were evaluated to describe the extent and anatomical location of the CVT and to assess the presence of focal edema or intracerebral hemorrhage. Intracerebral hemorrhage was defined as either intraparenchymal hemorrhage or hemorrhagic infarction. Secondary outcome measures were a composite of symptomatic recurrent VTE and asymptomatic deterioration on repeat imaging and a composite of symptomatic recurrent VTE and major bleeding. A blinded, independent adjudication committee of experienced neurologists and thrombosis experts (study committees are listed in the supplemental Data) evaluated the initial diagnosis, suspected primary and secondary outcomes, and all repeat imaging. Two experienced neurologists from the adjudication committee independently assessed the baseline and repeat imaging, and disagreements were resolved by a third reviewer. In addition, the adjudication committee classified the individual risk factor profile for VTE as unprovoked or provoked by a permanent or transient risk factor. Thrombophilia screening was performed locally at the discretion of the local treating physician. Details on the total number of children screened for thrombophilia and method of thrombophilia screening were not routinely collected. Local investigators classified the CVT as symptomatic or asymptomatic and recorded the occurrence of headache or seizures during the study period, as well as the presence of focal neurologic deficits, visual disturbances, and increased requirement of help with activities of daily living (ADL) at 3 months vs before the CVT.
Statistical analysis
The EINSTEIN-Jr trial and, consequently, this substudy were not powered for confirmatory analyses, because the incidence of venous thrombosis in children is ∼100 times lower than in adults, which renders well-powered studies unfeasible.19 Efficacy outcomes were considered during the 3-month study treatment period (intention to treat), whereas safety outcomes were considered for the same period but only during the time from administration of the first dose of study medication to 48 hours after the administration of the last dose. For comparison of the ordered categories of change in thrombotic burden (ie, normalized, improved, uncertain, no relevant change, asymptomatic deterioration, or symptomatic recurrent VTE), the Wilcoxon rank-sum test was used. Confidence intervals (CIs) for incidences and for absolute risk differences were calculated by exact methods. Calculations were performed by M.M. using SAS v9.2 (SAS Institute Inc., Cary, NC) and StatXact (r) PROCs (v10.0) (Cytel Inc, Cambridge, MA).
Results
From 2 March 2015 through 26 November 2018, 117 children with CVT were randomized (Figure 1). In 3 children, the presence of CVT was not confirmed after randomization. Demographics, clinical, and radiological characteristics of the remaining 114 children (rivaroxaban, 73; standard anticoagulation, 41) are shown in Table 1. The most common presenting symptoms were headache (n = 81; 71%), focal neurological deficits (n = 34; 30%), diplopia (n = 22; 19%), and seizures (n = 12; 11%), and 4 (3.5%) children were in a coma. Baseline head CT or MRI scan revealed focal edema, intracerebral hemorrhage, or both in 12 (11%), 6 (5.3%), and 4 (3.5%) children, respectively. CVT limited to a single sinus was found in 32 (28%) children. None of the children had isolated cortical vein thrombosis. Overall, CVT was unprovoked in 5 (4.4%) children and was provoked by transient or permanent risk factors in 84 (74%) and 25 (22%) children, respectively (Table 2). Infectious disease was observed in 76 (67%) children, of whom 74 (97%) had a local infection of the head or neck. Active cancer and (acquired or inherited) thrombophilia occurred in 9 (7.9%) and 8 (7.0%) children, respectively.
Flowchart of selection of patients for the EINSTEIN-Jr CVT substudy. *Withdrew from the study before any of the outcomes had occurred.
Flowchart of selection of patients for the EINSTEIN-Jr CVT substudy. *Withdrew from the study before any of the outcomes had occurred.
Demographics, clinical presentation, baseline imaging, and study treatment
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Demographics, n (%) | ||
| Male sex | 46 (63) | 23 (56) |
| Age groups | ||
| Birth to 1 y | 4 (5.5) | 5 (12) |
| 2-5 y | 22 (30) | 12 (29) |
| 6-11 y | 31 (42) | 15 (37) |
| 12-17 y | 16 (22) | 9 (22) |
| Clinical presentation, n (%) | ||
| Headache | 51 (70) | 30 (73) |
| Focal neurological deficits* | 25 (34) | 9 (22) |
| Epileptic seizure | 6 (8.2) | 6 (15) |
| Papilledema | 23 (32) | 11 (27) |
| Diplopia | 15 (21) | 7 (17) |
| Decreased vision | 8 (11) | 2 (4.9) |
| Coma† | 3 (4.1) | 1 (2.4) |
| Asymptomatic presentation CVT | 11 (15) | 7 (17) |
| CT-/MR-venography, n (%) | ||
| Parenchymal lesions | ||
| Focal edema | 9 (12) | 7 (17) |
| Intracerebral hemorrhage | 7 (10) | 3 (7.3) |
| Thrombosis location(s) | ||
| Superior sagittal sinus | 13 (18) | 8 (19.5) |
| Lateral sinus‡ | 63 (86) | 35 (85) |
| Straight sinus | 7 (9.6) | 4 (9.8) |
| Deep venous system | 5 (6.8) | 3 (7.3) |
| Cortical veins | 7 (9.6) | 2 (4.9) |
| Cavernous sinus | 4 (5.5) | 3 (7.3) |
| Jugular vein | 39 (53) | 19 (46) |
| Rivaroxaban group, n (%)§ | ||
| Tablet | 19 (26) | NA |
| Suspension | 54 (74) | NA |
| Standard anticoagulation group, n (%)§ | ||
| LMWH | NA | 35 (85) |
| LMWH followed by vitamin K antagonists | NA | 6 (15) |
| Study treatment duration, median (IQR), d | 92 (87-95) | 92 (90-95) |
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Demographics, n (%) | ||
| Male sex | 46 (63) | 23 (56) |
| Age groups | ||
| Birth to 1 y | 4 (5.5) | 5 (12) |
| 2-5 y | 22 (30) | 12 (29) |
| 6-11 y | 31 (42) | 15 (37) |
| 12-17 y | 16 (22) | 9 (22) |
| Clinical presentation, n (%) | ||
| Headache | 51 (70) | 30 (73) |
| Focal neurological deficits* | 25 (34) | 9 (22) |
| Epileptic seizure | 6 (8.2) | 6 (15) |
| Papilledema | 23 (32) | 11 (27) |
| Diplopia | 15 (21) | 7 (17) |
| Decreased vision | 8 (11) | 2 (4.9) |
| Coma† | 3 (4.1) | 1 (2.4) |
| Asymptomatic presentation CVT | 11 (15) | 7 (17) |
| CT-/MR-venography, n (%) | ||
| Parenchymal lesions | ||
| Focal edema | 9 (12) | 7 (17) |
| Intracerebral hemorrhage | 7 (10) | 3 (7.3) |
| Thrombosis location(s) | ||
| Superior sagittal sinus | 13 (18) | 8 (19.5) |
| Lateral sinus‡ | 63 (86) | 35 (85) |
| Straight sinus | 7 (9.6) | 4 (9.8) |
| Deep venous system | 5 (6.8) | 3 (7.3) |
| Cortical veins | 7 (9.6) | 2 (4.9) |
| Cavernous sinus | 4 (5.5) | 3 (7.3) |
| Jugular vein | 39 (53) | 19 (46) |
| Rivaroxaban group, n (%)§ | ||
| Tablet | 19 (26) | NA |
| Suspension | 54 (74) | NA |
| Standard anticoagulation group, n (%)§ | ||
| LMWH | NA | 35 (85) |
| LMWH followed by vitamin K antagonists | NA | 6 (15) |
| Study treatment duration, median (IQR), d | 92 (87-95) | 92 (90-95) |
IQR interquartile range; LMWH, low-molecular-weight heparin; NA, not applicable.
Motor weakness, aphasia, sensory deficits, hemianopia.
Coma was defined as a Glasgow Coma Scale score of <9.
Composite of the sigmoid and transverse sinuses.
One child in each treatment arm also received thrombolytic therapy, both uncomplicated.
Risk factor profile at baseline
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Etiology of index CVT, n (%) | ||
| Transient risk factor only | 50 (69) | 34 (83) |
| Persistent risk factor* | 21 (29) | 4 (9.8) |
| Unprovoked | 2 (2.7) | 3 (7.3) |
| Type of risk factor, n (%)† | ||
| Infectious disease | 46 (63) | 30 (73) |
| Otitis media and/or mastoiditis‡ | 39 (53) | 20 (49) |
| CNS infection‡ | 12 (16) | 9 (22) |
| Sinusitis‡ | 12 (16) | 6 (15) |
| Infection other than head or neck | 1 (1.4) | 1 (2.4) |
| Major head trauma | 5 (6.8) | 3 (7.3) |
| Major surgery§ | 4 (5.5) | 1 (2.3) |
| Use of estrogens or progestins | 3 (60) || | 2 (50) || |
| Active cancer¶ | 7 (9.6) | 2 (4.9) |
| Hematologic cancer# | 5 (6.8) | 2 (4.9) |
| Solid tumor** | 2 (2.7) | 0 |
| Renal inflammatory disease†† | 5 (6.8) | 0 |
| Cerebral arteriovenous malformation | 1 (1.4) | 1 (2.4) |
| Known inherited thrombophilia | 5 (6.8) | 0 |
| Antithrombin, protein C, or S deficiency | 3 (4.1) | 0 |
| Factor V Leiden or prothrombin mutation | 2 (2.7) | 0 |
| Acquired thrombophilia‡‡ | 2 (2.7) | 1 (2.4) |
| Familial venous thrombosisa | 1 (1.4) | 0 |
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Etiology of index CVT, n (%) | ||
| Transient risk factor only | 50 (69) | 34 (83) |
| Persistent risk factor* | 21 (29) | 4 (9.8) |
| Unprovoked | 2 (2.7) | 3 (7.3) |
| Type of risk factor, n (%)† | ||
| Infectious disease | 46 (63) | 30 (73) |
| Otitis media and/or mastoiditis‡ | 39 (53) | 20 (49) |
| CNS infection‡ | 12 (16) | 9 (22) |
| Sinusitis‡ | 12 (16) | 6 (15) |
| Infection other than head or neck | 1 (1.4) | 1 (2.4) |
| Major head trauma | 5 (6.8) | 3 (7.3) |
| Major surgery§ | 4 (5.5) | 1 (2.3) |
| Use of estrogens or progestins | 3 (60) || | 2 (50) || |
| Active cancer¶ | 7 (9.6) | 2 (4.9) |
| Hematologic cancer# | 5 (6.8) | 2 (4.9) |
| Solid tumor** | 2 (2.7) | 0 |
| Renal inflammatory disease†† | 5 (6.8) | 0 |
| Cerebral arteriovenous malformation | 1 (1.4) | 1 (2.4) |
| Known inherited thrombophilia | 5 (6.8) | 0 |
| Antithrombin, protein C, or S deficiency | 3 (4.1) | 0 |
| Factor V Leiden or prothrombin mutation | 2 (2.7) | 0 |
| Acquired thrombophilia‡‡ | 2 (2.7) | 1 (2.4) |
| Familial venous thrombosisa | 1 (1.4) | 0 |
With or without transient risk factor(s).
CVT was not related to the use of central venous catheters, hyperthyroidism, dural arteriovenous fistula, or paroxysmal nocturnal hemoglobinuria.
The subcategories of head or neck infections overlap substantially; 41 of 74 (55%) children with a head or neck infection underwent a surgical intervention (26 of 45 [58%] in the rivaroxaban group and 15 of 29 [52%] in the standard anticoagulant group).
Mastoidectomy (n = 4) and medulloblastoma (n = 1).
Percentages calculated for girls aged 12 to 17 years in the rivaroxaban group (n = 5) and in the standard anticoagulation group (n = 4).
Active cancer is defined as presence of metastases or cancer recently (<6 months) diagnosed or treated.
Acute lymphoblastic leukemia (n = 5) and non-Hodgkin lymphoma (n = 2).
Medulloblastoma and rhabdomyosarcoma.
Nephrotic syndrome (n = 4) and lupus nephritis (n = 1).
Antiphospholipid syndrome (lupus anticoagulant, anti-cardiolipin, and/or anti-β2-glycoprotein antibodies); none of the children were triple positive for antiphospholipid antibodies.
First degree (parent or sibling).
All children received initial anticoagulant therapy with low-molecular-weight heparin (n = 106, 93%) or unfractionated heparin (n = 8, 7.0%). Two children (1.8%) received thrombolytic therapy. All 41 children allocated to standard anticoagulation treatment continued with low-molecular-weight heparin, of whom 6 (15%) transitioned to vitamin K antagonist therapy. Following completion of the 3-month study duration, 53 (46%) of the 114 children continued anticoagulant therapy. The median study treatment duration was 92 days with an interquartile range of 87 to 95 and 90 to 95 days for rivaroxaban and standard anticoagulation, respectively. All children completed the follow-up.
Efficacy
Symptomatic recurrent VTE occurred in none of the 73 children in the rivaroxaban group (95% CI, 0.0-4.9) and in 1 of the 41 children in the standard anticoagulation group (recurrent CVT, 2.4%; 95% CI, 0.1-12.4), for an absolute risk difference in favor of rivaroxaban of 2.4% (95% CI −2.6% to 13.5%; Table 3). The composite of recurrent VTE and asymptomatic deterioration on repeat imaging was found in none of the rivaroxaban recipients and in 2 (4.9%) of the standard anticoagulation recipients (absolute risk difference in favor of rivaroxaban, 4.9%; 95% CI −0.4% to 17.1%). Overall, repeat imaging demonstrated a similar effect on clot resolution with rivaroxaban as compared with standard anticoagulation (Table 4; Wilcoxon rank-sum test, P = .20). Complete recanalization of the index thrombosis occurred in 18 children (25%) in the rivaroxaban group, as compared with 6 children (15%) in the standard anticoagulation group (unadjusted odds ratio [OR], 1.63; 95% CI, 0.78-3.41). Incomplete recanalization occurred in a further 39 (53%) and 24 (59%) children, respectively, whereas asymptomatic deterioration of the CVT was found in a single child (2.4%) in the standard anticoagulation group.
Prespecified efficacy and safety outcomes
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . | Absolute difference (95% CI) . |
|---|---|---|---|
| Efficacy population, n (%) | |||
| Symptomatic recurrent VTE | 0 | 1 (2.4)* | 2.4% (−2.6% to 13.5%) |
| Symptomatic recurrent VTE or asymptomatic deterioration on repeat imaging | 0 | 2 (4.9) | 4.9% (−0.4% to 17.1%) |
| Symptomatic recurrent VTE or major bleeding | 0 | 2 (4.9) | 4.9% (−0.4% to 17.1%) |
| Safety population, n (%) | |||
| Major or clinically relevant nonmajor bleeding | 5 (6.8) | 1 (2.4) | 4.4% (−6.7% to 13.4%) |
| Major bleeding | 0 | 1 (2.4)† | |
| Clinically relevant nonmajor bleeding | 5 (6.8)‡ | 0 |
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . | Absolute difference (95% CI) . |
|---|---|---|---|
| Efficacy population, n (%) | |||
| Symptomatic recurrent VTE | 0 | 1 (2.4)* | 2.4% (−2.6% to 13.5%) |
| Symptomatic recurrent VTE or asymptomatic deterioration on repeat imaging | 0 | 2 (4.9) | 4.9% (−0.4% to 17.1%) |
| Symptomatic recurrent VTE or major bleeding | 0 | 2 (4.9) | 4.9% (−0.4% to 17.1%) |
| Safety population, n (%) | |||
| Major or clinically relevant nonmajor bleeding | 5 (6.8) | 1 (2.4) | 4.4% (−6.7% to 13.4%) |
| Major bleeding | 0 | 1 (2.4)† | |
| Clinically relevant nonmajor bleeding | 5 (6.8)‡ | 0 |
Recurrent CVT; occurred in a 6-year-old child with otitis media during the index event. No thrombophilia was reported.
Subdural hematoma; occurred in a 6-month-old child with bacterial meningitis during the index event.
One case each of Mallory-Weiss bleeding in a 15-year-old child with lymphoma, hemorrhoidal bleeding in a 14-year-old child with factor VII deficiency and homocystinuria, and hematuria in a 10-year-old child with lupus nephritis; 2 cases of nasal bleeding (1 in a 4-year-old child with otitis media during the index event and 1 in an 8-year-old child with nephrotic syndrome).
Clinical course during the 3-month study period and results of repeat imaging
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Clinical course, n (%) | ||
| During the 3-mo treatment period | ||
| Headache lasting >2 wk* | 6 (8.2) | 2 (4.9) |
| Epileptic seizure | 1 (1.4) | 0 |
| At 3 mo | ||
| Mortality | 0 | 0 |
| Persisting focal neurological deficits† | 5 (6.8) | 3 (7.3) |
| Persisting decreased vision | 4 (5.5) | 1 (2.4) |
| Inability to walk/move as before the CVT | 1 (1.4)‡ | 0 |
| Requirement of more help with ADL than before the CVT | 4 (5.5)§ | 3 (7.3) |
| Results of repeat imaging at 3 mo, n (%) | ||
| New nonhemorrhagic infarct or intracerebral hemorrhage | 0 | 0 |
| Change in thrombotic burden as compared with index event | ||
| Normalized | 18 (25) | 6 (15) |
| Improved | 39 (53) | 24 (59) |
| No relevant change | 5 (6.8) | 4 (9.8) |
| Asymptomatic deterioration | 0 | 1 (2.4) |
| Uncertain|| | 11 (15) | 5 (12) |
| . | Rivaroxaban (n = 73) . | Standard anticoagulation (n = 41) . |
|---|---|---|
| Clinical course, n (%) | ||
| During the 3-mo treatment period | ||
| Headache lasting >2 wk* | 6 (8.2) | 2 (4.9) |
| Epileptic seizure | 1 (1.4) | 0 |
| At 3 mo | ||
| Mortality | 0 | 0 |
| Persisting focal neurological deficits† | 5 (6.8) | 3 (7.3) |
| Persisting decreased vision | 4 (5.5) | 1 (2.4) |
| Inability to walk/move as before the CVT | 1 (1.4)‡ | 0 |
| Requirement of more help with ADL than before the CVT | 4 (5.5)§ | 3 (7.3) |
| Results of repeat imaging at 3 mo, n (%) | ||
| New nonhemorrhagic infarct or intracerebral hemorrhage | 0 | 0 |
| Change in thrombotic burden as compared with index event | ||
| Normalized | 18 (25) | 6 (15) |
| Improved | 39 (53) | 24 (59) |
| No relevant change | 5 (6.8) | 4 (9.8) |
| Asymptomatic deterioration | 0 | 1 (2.4) |
| Uncertain|| | 11 (15) | 5 (12) |
Headache that interfered with normal daily activities.
Motor weakness, aphasia, sensory deficits, hemianopia.
Advanced cancer.
Advanced cancer in 2 children.
Repeat imaging not performed, not evaluable, or performed <7 days before or >7 days after cessation of study medication.
Safety
Clinically relevant bleeding was observed in 5 of the 73 rivaroxaban recipients (6.8%; all were nonmajor extracranial bleeding events) and in 1 of the 41 standard anticoagulation recipients (2.4%; major [subdural] bleeding event), for an absolute risk difference in favor of standard anticoagulation of 4.4% (95% CI, −6.7% to 13.4%; Table 3). The composite of recurrent VTE or major bleeding occurred in none of the 73 children in the rivaroxaban group and in 2 of the 41 children (4.9%) in the standard anticoagulation group (absolute risk difference in favor of rivaroxaban, 4.9%; 95% CI, −0.4% to 17.1%).
Clinical course during the 3-month treatment period
During the 3-month treatment period, headache lasting for more than 2 weeks occurred in 6 (8.2%) of the rivaroxaban recipients and in 2 (4.9%) of the standard anticoagulation recipients (Table 4). Epileptic seizures occurred in 1 (1.4%) child in the rivaroxaban group.
At the end of the 3-month study treatment period, none of the children had died. Focal neurologic deficits were still present in 5 (6.8%) and 3 (7.3%) children in the rivaroxaban and standard anticoagulation group, respectively (Table 4). Thus, baseline focal neurologic deficits had resolved in 20 (27%) children in the rivaroxaban group and in 6 (15%) children in the standard anticoagulant group (Tables 1 and 4). Four (5.5%) rivaroxaban recipients and 3 (7.3%) standard anticoagulation recipients required more help with ADL than before the CVT. None of the children had a new intracerebral hemorrhage or nonhemorrhagic infarct. No important differences were found between children who presented with symptomatic CVT and those who had asymptomatic CVT (supplemental Table 4).
Discussion
In children with CVT treated with initial heparin followed by 3 months of treatment with either rivaroxaban or standard anticoagulation, we observed low rates of symptomatic recurrent thrombosis and major bleeding, no new nonhemorrhagic infarcts or intracerebral hemorrhages, high rates of complete or partial sinus recanalization, and good clinical outcome in most children. CVT was associated with transient or permanent risk factors for VTE in >95% of the children, with head and neck infection being the main risk factor in almost two-thirds of them. Our study represents, to our knowledge, the first prospective trial of anticoagulation in pediatric CVT and further substantiates the efficacy and safety of anticoagulant therapy in pediatric CVT.
The current suggestion for the use of anticoagulant therapy in pediatric CVT is based on results from small randomized trials in adult CVT,10-12 cohort studies of pediatric CVT,1,17,28,29 as well as pathophysiological plausibility.13-15 In the pediatric cohort studies, anticoagulant treatment was associated with a reduced risk of severe neurologic deficit or death at hospital discharge (OR, 0.32; 95% CI, 0.14-0.73),29 reduced mortality (1.8% vs 10%),15 lower risk of recurrent VTE (3.6% vs 7.3% at a median treatment duration of 6 months),17 and lower rates of thrombus propagation on repeat imaging within 2 weeks (6.0% vs 32%),28 whereas intracranial bleeding rates with anticoagulant treatment varied from 0% to 6%.1,28 Thus, the observed frequencies of these outcomes in both arms of our study compare well with the reported frequencies among children treated with anticoagulants in the cited cohort studies.
Only a few adults presented with septic CVT in the previous randomized trials,10-12 and some physicians have voiced concerns about a possible increased risk of intracerebral hemorrhage in this patient group.16 In an observational study on adult CVT, 57 of the 624 (9.1%) patients had an associated head or neck infection.30 Those with head or neck infection had an increased risk of new intracerebral hemorrhages (12.3%) than did those without a local infection (5.3%). The use of therapeutic anticoagulation with heparin did not seem to influence the risk of new intracranial hemorrhages, but the number of patients who did not receive anticoagulation was too small to draw firm conclusions. In our pediatric study, 74 of the 114 (65%) children had CVT associated with head or neck infections, and none of those children (all of whom received therapeutic anticoagulation) developed an intracerebral hemorrhage. A single child with infectious CVT in the standard anticoagulation group had a subdural hematoma while receiving the study medication. Thus, although the absolute group sizes of adults (n = 47) and children (n = 74) with CVT and a head or neck infection who received anticoagulant treatment were comparable, a minimum of 6 (12.8%) of the adults compared with none of the children developed an intracerebral hemorrhage during anticoagulant treatment, providing important safety information on the use of anticoagulant treatment of this patient group.
Strengths of our study include the randomized, multicenter design; the central blinded end point evaluation; and the absence of loss to follow-up. Randomization occurred early, within 5 to 9 days after diagnosis of CVT. Moreover, the 2:1 randomization ratio led to more precise estimates of outcomes in the rivaroxaban group. In addition, standard anticoagulation was administered according to current guidelines.13-15
Several limitations also warrant comment. First, although it was predefined, the current study was a subgroup analysis and was not powered for hypothesis testing. Therefore, we refrained from formal statistical comparisons between rivaroxaban and standard anticoagulation. Second, the EINSTEIN-Jr study was an open-label trial, because of the practical and ethical difficulties of administering long-term placebo injections and taking blood samples for sham therapeutic monitoring in children. However, efficacy, safety, and imaging outcomes were evaluated by a blinded adjudication committee, and standardized predefined criteria were used. Third, for ethical reasons, term neonates and infants were included only after demonstration of the absence of safety issues with rivaroxaban in older children. Hence, the number of neonates and infants we included is relatively low. Neonates with CVT have different risk factors and comorbidities than older children with CVT, and their outcome is significantly worse.6,28 Therefore, the results of the present study can only be applied to a limited extent to term neonates and infants. Fourth, our cohort may represent a less ill population than those included in the previous pediatric cohort studies.3,17,28 In our study, all children were required to have started therapeutic heparinization, whereas the pediatric cohort studies included not only those who received anticoagulation but also those in whom anticoagulation was thought not to be indicated or contraindicated. It is possible that individual study physicians chose to exclude children with severe manifestations of CVT because of a perceived contraindication for anticoagulation.13,14 In addition, 18 children with asymptomatic CVT (as assessed locally) were included in our study. However, no important differences were found between children who presented with symptomatic CVT and those who had asymptomatic CVT with regard to rates of symptomatic recurrent VTE, major bleeding, sinus recanalization on repeat imaging after 3 months, and increased need for assistance with ADL after 3 months (supplemental Table 4). Finally, our study evaluated anticoagulant therapy for a period of 3 months, whereas extended treatment durations are often given in CVT. Indeed, almost half of the 114 children continued anticoagulant therapy after the main study treatment period. The optimal duration of anticoagulant treatment of CVT has thus far not been established in dedicated clinical trials and, therefore, the decision to stop anticoagulants at 3 months or to extend treatment thereafter should be based on an individual assessment weighing the perceived long-term risk of recurrence against the risk of bleeding.
What are the clinical implications of our study? The low point estimate of recurrent thrombosis and intracranial bleeding in the rivaroxaban group excludes with 97.5% confidence an incidence higher than 4.9%, suggesting that in children with CVT in whom therapeutic anticoagulation is considered necessary, rivaroxaban may be a good treatment option. Moreover, whereas standard anticoagulation requires either parenteral administration or frequent venipunctures for laboratory monitoring, rivaroxaban can be administered orally, even in the youngest patients, and does not require routine laboratory monitoring. Indeed, in the current study, 85% of children in the standard anticoagulation group received long-term, twice-daily parenteral low-molecular-weight heparin, given that the alternative standard of care of using vitamin K antagonists is usually very problematic in young children.
In summary, most children with acute CVT had favorable clinical outcomes. Both rivaroxaban and standard anticoagulants were associated with low risks of recurrent thrombosis and major bleeding.
Data will be shared with academic researchers upon request to anthonie.lensing@bayer.com. Further information regarding data sharing is provided in the supplemental Data.
Acknowledgments
Data from the University Children’s Hospital, Bern, Switzerland, were gathered with the help of the clinical trial unit PEDNET. The authors thank Laurien E. Lensing for editorial assistance.
This study was supported by Bayer AG and Janssen Research and Development.
M.S.v.K. is a doctoral candidate at the University of Amsterdam. This work is submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: A.W.A.L., C.M., M.H.P., and P.M. designed the study; M.S.v.K. and A.W.A.L. drafted the manuscript; P.C. and J.M.C. revised the manuscript; and all authors contributed to data collection, data analysis, and data interpretation.
Conflict-of-interest disclosure: P.C., K.K., P. Simioni, T.B., S.G., R.B., A.V.D., J. Palumbo, P. Saracco, J. Payne, S.B., K.G., V.L., I.M., M.M.S., J.M., S.S., H.L.H., P.M., and J.M.C. report fees paid to their institutions from Bayer. A.W.A.L., D.K., W.T.S., S.D.B., A.F.P. and M.M. are employees of Bayer. E.C. has received personal fees from Boehringer Ingelheim and Bristol-Myers Squibb and reports fees paid to her institution from Bayer. D.B. has received personal fees and grant support from Actelion Pharmaceuticals and Novartis and reports fees paid to his institution from Bayer. O.L. has received personal fees from Bayer and reports fees paid to her institution from Bayer, Pfizer, and Boehringer Ingelheim. C.M. has received personal fees and reports fees paid to his institution from Bayer, Bristol-Myers Squibb, and Pfizer and reports fees paid to his institution from Boehringer-Ingelheim. K.H., R.K., and A.S. have received personal fees and report fees paid to their institution from Bayer. M.H.P. has received personal fees from Bayer. M.S.v.K. and F.B. declare no competing financial interests.
A complete list of the EINSTEIN-Jr CVT trial investigators appears in the Appendix.
Correspondence: Jonathan M. Coutinho, Department of Neurology, Amsterdam University Medical Center, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: j.coutinho@amsterdamumc.nl.
Appendix: study group members
EINSTEIN-Jr CVT trial investigators by country and number of randomized children
| Australia | Jeremy Robertson, Lady Cilento Children’s Hospital, South Brisbane (1) |
| Austria | Christoph Male, Katharina Thom, Universitätsklinik für Kinder und Jugendheilkunde, Medizinische Universität Vienna (2); Werner Streif, Landeskrankenhaus-Universitätskliniken Innsbruck, Innsbruck (1) |
| Belgium | An Van Damme, Saint Luc University Hospital, Brussels (3); Philip Maes, UZ Antwerpen, Edegem (1); Veerle Labarque, UZ Leuven Gasthuisberg, Leuven (2) |
| Canada | Leonardo Brandao, Hospital for Sick Children, Toronto (1); Christine Sabapathy, Montreal Children’s Hospital-MUHC, Montreal (1); Patricia M. Massicotte, University of Alberta Hospital, Edmonton (1) |
| China | Wenyan Huang, Children’s Hospital of Shanghai, Shangai (1); Jianhua Mao, The Children’s Hospital Zhengjiang University School of Medicine, Hangzhou (1) |
| France | Damien Bonnet, Fanny Bajolle, Hôpital Necker les Enfants Malades, Paris (4) |
| Germany | Susanne Holzhauer, Charité Campus Virchow-Klinikum (CVK), Berlin (1); Toralf Bernig, Medizinische Fakultät der Martin-Luther-Universität Halle-Wittenberg, Halle (1) |
| Hungary | Krisztian Kally, DPCKh Orszagos Hematologiai es Infektologiai Intezet, Budapest (6) |
| Ireland | Beatrice Nolan, Our Lady’s Hospital for Sick Children, Crumlin (1) |
| Israel | Hannah Tamary, Schneider Children’s Medical Center of Israel, Petach Tikva (1) |
| Italy | Paolo Simioni, Daniela Tormene, A.O. di Padua, Padua (5); Ida Martinelli, Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, Milano (2); Paola Saracco, A.O.U. Città della Salute e della Scienza di Torino, Torino (3) |
| Mexico | Michelle Morales Soto, Antiguo Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara (2) |
| Netherlands | Marije Bartels, University Medical Center Utrecht, Utrecht (1); Hélène L Hooimeijer, Universitair Medisch Centrum Groningen, Groningen (2) |
| Russia | Olga Lvova, Ural State Medical University, Yekaterinburg (4) |
| Slovakia | Ludmila Podracka, Narodny Ustav Detskych Chorob, Bratislava (1) |
| Spain | Amparo Santamaría, Ciutat Sanitària i Universitaria de la Vall d Hebron, Barcelona (3) |
| Switzerland | Sebastian Grunt, Inselspital Universitätsspital Bern, Bern (1); Johannes Rischewski, Kinderspital Luzern, Luzern (1); Manuela Albisetti Pedroni, Universitätskinderspital Zürich, Zürich (1) |
| Turkey | Ali Antmen, Acibadem Adana Hastanesi, Adana (1); Huseyin Tokgoz, Necmettin Erbakan Universitesi Meram Tip Fakultesi Hastanesi, Konya (1) |
| United Kingdom | Tina Biss, Royal Victoria Infirmary, Newcastle Upon Tyne (4); Elizabeth Chalmers, Royal Hospital for Children, Glasgow (6); Georgina Hall (1) and Jayashree Motwani (2), Birmingham Children’s Hospital, Birmingham; John Grainger, Royal Manchester Children’s Hospital, Manchester (1); Philip Connor, University Hospital of Wales, Cardiff (7); Susan Baird, Royal Hospital for Sick Children, Edinburgh (3); Jeanette Payne, Department of Pediatric Haematology, Sheffield Children’s Hospital, Western Bank, Sheffield (3) |
| United States | Tung Wynn, University of Florida, Gainesville, FL (1); Shannon Carpenter, Children’s Mercy Hospital and Clinics, Kansas City, MO (1); Kerry Hege, Riley Hospital for Children, Indianapolis, IN (6); Marcela Torres, Cook Children’s Medical Center, Fort Worth, TX (1); Kamar Godder, Miami Children’s Hospital, Miami, FL (2); Joseph Palumbo, Cincinnati Children’s Hospital and Medical Center, Cincinnati OH (3); Rukhmi Bhat, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL (3); Riten Kumar, Nationwide Children’s Hospital, Columbus, OH (4); Donald L. Yee, Texas Children’s Hospital, Houston, TX (2); Sanjay Shah, Phoenix Children’s Hospital, Phoenix, AZ (2); Courtney Thornburg, Rady Children’s Hospital San Diego, San Diego, CA (1) |
| Australia | Jeremy Robertson, Lady Cilento Children’s Hospital, South Brisbane (1) |
| Austria | Christoph Male, Katharina Thom, Universitätsklinik für Kinder und Jugendheilkunde, Medizinische Universität Vienna (2); Werner Streif, Landeskrankenhaus-Universitätskliniken Innsbruck, Innsbruck (1) |
| Belgium | An Van Damme, Saint Luc University Hospital, Brussels (3); Philip Maes, UZ Antwerpen, Edegem (1); Veerle Labarque, UZ Leuven Gasthuisberg, Leuven (2) |
| Canada | Leonardo Brandao, Hospital for Sick Children, Toronto (1); Christine Sabapathy, Montreal Children’s Hospital-MUHC, Montreal (1); Patricia M. Massicotte, University of Alberta Hospital, Edmonton (1) |
| China | Wenyan Huang, Children’s Hospital of Shanghai, Shangai (1); Jianhua Mao, The Children’s Hospital Zhengjiang University School of Medicine, Hangzhou (1) |
| France | Damien Bonnet, Fanny Bajolle, Hôpital Necker les Enfants Malades, Paris (4) |
| Germany | Susanne Holzhauer, Charité Campus Virchow-Klinikum (CVK), Berlin (1); Toralf Bernig, Medizinische Fakultät der Martin-Luther-Universität Halle-Wittenberg, Halle (1) |
| Hungary | Krisztian Kally, DPCKh Orszagos Hematologiai es Infektologiai Intezet, Budapest (6) |
| Ireland | Beatrice Nolan, Our Lady’s Hospital for Sick Children, Crumlin (1) |
| Israel | Hannah Tamary, Schneider Children’s Medical Center of Israel, Petach Tikva (1) |
| Italy | Paolo Simioni, Daniela Tormene, A.O. di Padua, Padua (5); Ida Martinelli, Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, Milano (2); Paola Saracco, A.O.U. Città della Salute e della Scienza di Torino, Torino (3) |
| Mexico | Michelle Morales Soto, Antiguo Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara (2) |
| Netherlands | Marije Bartels, University Medical Center Utrecht, Utrecht (1); Hélène L Hooimeijer, Universitair Medisch Centrum Groningen, Groningen (2) |
| Russia | Olga Lvova, Ural State Medical University, Yekaterinburg (4) |
| Slovakia | Ludmila Podracka, Narodny Ustav Detskych Chorob, Bratislava (1) |
| Spain | Amparo Santamaría, Ciutat Sanitària i Universitaria de la Vall d Hebron, Barcelona (3) |
| Switzerland | Sebastian Grunt, Inselspital Universitätsspital Bern, Bern (1); Johannes Rischewski, Kinderspital Luzern, Luzern (1); Manuela Albisetti Pedroni, Universitätskinderspital Zürich, Zürich (1) |
| Turkey | Ali Antmen, Acibadem Adana Hastanesi, Adana (1); Huseyin Tokgoz, Necmettin Erbakan Universitesi Meram Tip Fakultesi Hastanesi, Konya (1) |
| United Kingdom | Tina Biss, Royal Victoria Infirmary, Newcastle Upon Tyne (4); Elizabeth Chalmers, Royal Hospital for Children, Glasgow (6); Georgina Hall (1) and Jayashree Motwani (2), Birmingham Children’s Hospital, Birmingham; John Grainger, Royal Manchester Children’s Hospital, Manchester (1); Philip Connor, University Hospital of Wales, Cardiff (7); Susan Baird, Royal Hospital for Sick Children, Edinburgh (3); Jeanette Payne, Department of Pediatric Haematology, Sheffield Children’s Hospital, Western Bank, Sheffield (3) |
| United States | Tung Wynn, University of Florida, Gainesville, FL (1); Shannon Carpenter, Children’s Mercy Hospital and Clinics, Kansas City, MO (1); Kerry Hege, Riley Hospital for Children, Indianapolis, IN (6); Marcela Torres, Cook Children’s Medical Center, Fort Worth, TX (1); Kamar Godder, Miami Children’s Hospital, Miami, FL (2); Joseph Palumbo, Cincinnati Children’s Hospital and Medical Center, Cincinnati OH (3); Rukhmi Bhat, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL (3); Riten Kumar, Nationwide Children’s Hospital, Columbus, OH (4); Donald L. Yee, Texas Children’s Hospital, Houston, TX (2); Sanjay Shah, Phoenix Children’s Hospital, Phoenix, AZ (2); Courtney Thornburg, Rady Children’s Hospital San Diego, San Diego, CA (1) |
The number of patients enrolled is in parentheses after each investigator.
References
Author notes
P.C. and M.S.v.K. contributed equally to this study.
The full-text version of this article contains a data supplement.