Key Points
sC5b9 plasma levels are elevated in children with SARS-CoV-2 infection, even if they have minimal symptoms of COVID-19.
A high proportion of children with SARS-CoV-2 infection met clinical criteria for TMA.
Abstract
Most children with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have mild or minimal disease, with a small proportion developing severe disease or multisystem inflammatory syndrome in children (MIS-C). Complement-mediated thrombotic microangiopathy (TMA) has been associated with SARS-CoV-2 infection in adults but has not been studied in the pediatric population. We hypothesized that complement activation plays an important role in SARS-CoV-2 infection in children and sought to understand if TMA was present in these patients. We enrolled 50 hospitalized pediatric patients with acute SARS-CoV-2 infection (n = 21, minimal coronavirus disease 2019 [COVID-19]; n = 11, severe COVID-19) or MIS-C (n = 18). As a biomarker of complement activation and TMA, soluble C5b9 (sC5b9, normal 247 ng/mL) was measured in plasma, and elevations were found in patients with minimal disease (median, 392 ng/mL; interquartile range [IQR], 244-622 ng/mL), severe disease (median, 646 ng/mL; IQR, 203-728 ng/mL), and MIS-C (median, 630 ng/mL; IQR, 359-932 ng/mL) compared with 26 healthy control subjects (median, 57 ng/mL; IQR, 9-163 ng/mL; P < .001). Higher sC5b9 levels were associated with higher serum creatinine (P = .01) but not age. Of the 19 patients for whom complete clinical criteria were available, 17 (89%) met criteria for TMA. A high proportion of tested children with SARS-CoV-2 infection had evidence of complement activation and met clinical and diagnostic criteria for TMA. Future studies are needed to determine if hospitalized children with SARS-CoV-2 should be screened for TMA, if TMA-directed management is helpful, and if there are any short- or long-term clinical consequences of complement activation and endothelial damage in children with COVID-19 or MIS-C.
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), three distinct clinical phenotypes have emerged in children. Most children with acute SARS-CoV-2 infection are asymptomatic or develop mild symptoms.1-3 A small proportion of acutely infected children, typically adolescents with significant comorbidities, develop severe respiratory symptoms requiring hospitalization or admission to the pediatric intensive care unit.1,2,4 In addition, some children develop multisystem inflammatory syndrome in children (MIS-C), a hyperinflammatory syndrome characterized by fever, inflammation, and organ dysfunction in the setting of recent SARS-CoV-2 infection. With few exceptions, MIS-C seems to uniquely affect children.5-8 Severe cases of MIS-C present with shock and cardiovascular collapse, with 60% to 80% of children requiring pediatric intensive care unit care in recent large studies.5,6,9 MIS-C seems to be a postinfectious immune-mediated phenomenon distinct from other manifestations of SARS-CoV-2.10,11 The pathophysiology of these presentations is currently unknown.
High rates of thrombosis and thrombotic-related complications have been reported in adult patients with severe COVID-19.12,13 These complications have been reported in healthy young people, without prior comorbidities, raising the concern that thrombotic complications could be directly caused or exacerbated by SARS-CoV-2 infection.12 Studies in adults have invoked thrombotic microangiopathy (TMA) as a potential cause for severe manifestations of COVID-19.14-16 TMA results from endothelial cell damage to small blood vessels, leading to hemolytic anemia, thrombocytopenia, and, in some cases, organ damage.17-21 TMA has been reported in postmortem studies of adult patients with COVID-19.22
One proposed mechanism for SARS-CoV-2–mediated TMA is via complement activation.15-18 Complement dysregulation results in unregulated formation of the C5b9 membrane attack complex, leading to the clinical manifestations of TMA. Soluble C5b9 (sC5b9) is a clinically available biomarker and has been implicated as an indicator of severity in hematopoietic stem cell transplant–associated TMA (HSCT-TMA), as patients with markedly elevated sC5b9 have increased mortality.23 In mouse models of the related coronaviruses (SARS-CoV and Middle East respiratory syndrome coronavirus), knockout or blockade of components of the alternative complement pathway led to amelioration of severe respiratory syndromes and a decrease in cytokine production.24,25
In an initial small cohort of children, we previously showed that sC5b9 levels may be elevated across the spectrum of manifestations of SARS-CoV-2 infections.11 We did not, however, evaluate whether these children met the clinical criteria for diagnosis of TMA nor did we compare these values vs those of healthy control subjects. We hypothesized that sC5b9 was a marker of TMA in patients with evidence of SARS-CoV-2 infection. Here, we present serum levels of sC5b9 in healthy control subjects and in children with acute SARS-CoV-2 or MIS-C, and evaluate these cohorts for the clinical and laboratory findings of TMA.
Methods
Study design and population
We prospectively enrolled patients admitted to the Children’s Hospital of Philadelphia (CHOP) during the COVID-19 pandemic who had a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) from upper respiratory tract mucosa, or who met clinical criteria for MIS-C. The enrollment criteria have been previously described.11 Patients were classified into 3 groups: minimal COVID-19, severe COVID-19, or MIS-C. Minimal COVID-19 was defined as patients with an incidental finding of COVID-19 during routine testing before admission or a procedure, or those with mild COVID-19 symptoms that did not require noninvasive mechanical ventilation (including high-flow nasal cannula, continuous positive airway pressure, or bilevel positive airway pressure). Severe COVID-19 was defined as patients requiring new noninvasive or invasive mechanical ventilation, or escalation above their baseline invasive or noninvasive mechanical ventilation. MIS-C was defined per the Centers for Disease Control and Prevention criteria as patients who had fever, laboratory evidence of inflammation, illness with at least 2 organ systems involved (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurologic), no alternative plausible diagnosis and positive SARS-CoV-2 infection by RT-PCR, serology (performed in a Clinical Laboratory Improvement Amendments/College of American Pathologists [CLIA/CAP] laboratory), or proven COVID-19 exposure to a close contact within 4 weeks before onset of symptoms.26 Patients were prospectively classified into one of these groups by physicians with expertise in pediatric hematology/oncology (C.D. and D.T.T.), pediatric infectious diseases (H.B.), and pediatric rheumatology (E.M.B.). Adjudicators were blinded to sC5b9 levels and TMA clinical criteria at the time of classification into these groups. All patients who consented were enrolled in a consecutive manner. If patients were subsequently found to not meet criteria (ie, false-positive SARS-CoV-2 infection or different infectious etiology determined as cause of MIS-C symptoms), they remained enrolled but were excluded from analyses.
Subject samples were compared with those of normal control samples obtained from the coagulation laboratory at CHOP. Discarded plasma collected from otherwise healthy children who had been evaluated for symptoms of a bleeding disorder (eg, epistaxis, menorrhagia) was used for the control groups. A limited chart review was performed to confirm that these patients had no comorbid medical illness or medical problems. Children who were found to have comorbid medical issues, other than underlying bleeding disorders, were excluded.
Patients were categorized as having TMA based on the following 7 criteria, adapted from Gloude et al27 : elevated lactate dehydrogenase (LDH) levels greater than the upper limit of normal for age, schistocytes on blood smear, new thrombocytopenia below the normal range for age, new anemia below the normal range for age, evidence of proteinuria (≥1+ proteinuria [30 mg/dL] on random urinalysis or random urine protein/creatinine ratio ≥2 mg/mg), hypertension (blood pressure >99th percentile for age, sex, and height if aged <18 years, ≥140 mm Hg systolic or 90 mm Hg diastolic if aged ≥18 years, at least twice during the admission, or if receiving antihypertensive therapy for new hypertension), and elevated sC5b9. To meet criteria for TMA, patients had to meet at least 5 of the 7 criteria during their hospital admission for COVID-19 or MIS-C. Hematoxylin and eosin–stained peripheral blood smears were independently examined for schistocytes by a hematologist and hematopathologist, who were blinded to the categorization and clinical histories of patients, as well as to the other examiners’ findings. In a subsequent analysis, a simple set of clinical criteria was proposed to define TMA in settings in which sC5b9 and other diagnostics may be unavailable. These criteria included the presence of thrombocytopenia, microangiopathic hemolytic anemia (anemia for age and schistocytes on a peripheral blood smear), and organ dysfunction. Organ dysfunction was defined as renal dysfunction, liver dysfunction (bilirubin levels >2 times the upper limit of normal for age, alanine aminotransferase or aspartate aminotransferase levels >3 times the upper limit of normal), or cardiac dysfunction (troponin levels greater than the upper limit of normal or requirement for inotropic support). The glomerular filtration rate (GFR) was calculated by using the original Schwartz formula.28 Patients were classified as having renal dysfunction according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria.29 Baseline creatinine was defined as the lowest creatinine level in the 3 months before admission or, if unavailable, was calculated per KDIGO definitions. Acute kidney injury (AKI) was defined as KDIGO stage 2 or higher (at least a twofold increase from baseline).
Two different sensitivity analyses with highly stringent criteria were performed. First, we evaluated all patients included in the clinical cohort for TMA based on the aforementioned criteria. In this analysis, we imputed all missing data as negative. In the second sensitivity analysis, sC5b9 was excluded in the criteria for TMA. We used the remaining 6 clinical criteria to classify all patients as having clinical TMA (met 5 or 6 criteria), being indeterminate (4 criteria), or not meeting criteria for TMA (≤3 criteria). In patients who were deemed indeterminate, we looked at whether sC5b9 elevation would lead them to meet criteria for TMA.
Data collection
Data were abstracted from the patient chart on to a standardized case report form using a Research Electronic Data Capture database (Vanderbilt University, Nashville, TN) hosted at CHOP.30 Data were abstracted by a physician or a research assistant. All data elements extracted were validated by a physician. Data collected included demographic information, comorbid conditions, and most extreme laboratory values for laboratory studies of interest. Clinical criteria for TMA were abstracted as outlined earlier.
Blood collection and assays
Blood draws were obtained in conjunction with the first clinical blood draw after consent was obtained, within the first 2 weeks of the positive SARS-CoV-2 test or admission for MIS-C. For most patients, blood samples were obtained within 48 hours of admission. Blood was collected in a lithium heparin tube and processed into plasma and cell pellets. Separated components were frozen for batched analysis.
Proinflammatory cytokine profiling
Ten proinflammatory cytokines were measured (interferon-γ, interleukin IL-1β [IL-1β], IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and tumor necrosis factor-α) using V-Plex Pro-inflammatory Panel 1 Human Kits (catalog #K15049D; Meso Scale Diagnostics, Rockville, MD). All assays were performed in duplicate, according to the manufacturer’s protocol. Assays were read and analyzed on a QuickPlex SQ120 (Meso Scale Diagnostics). Of particular interest to TMA is the neutrophil chemotactic factor IL-8, due to its role as a marker of endothelial damage.31
sC5b9 assay
Plasma samples were assayed for sC5b9 at 2 dilutions by using a human C5b9 enzyme-linked immunosorbent assay (ELISA) set (#558315; BD Biosciences, San Jose, CA) according to the manufacturer’s protocols. All samples were assayed in triplicate. Standard curves were used to derive sC5b9 levels from best fit curves.
The upper limit of normal for the sC5b9 assay was set as 247 ng/mL, based on the upper limit of normal defined by two CLIA/CAP clinical assays (250 ng/mL, Machaon Diagnostics, Oakland, CA; 244 ng/mL, Cincinnati Children’s Hospital, Cincinnati, OH). We validated this upper limit of normal on our research-based ELISA by running samples from 26 healthy control subjects; the mean value from these controls was 84.5 ng/mL, and two standard deviations above this mean was 247 ng/mL.
SARS-CoV-2–specific antibodies
Complement is believed to be activated by coronaviruses in large part via the alternative pathway.24,25 Furthermore, in the complement-mediated TMA syndromes, complement is activated by the loss of negative regulation of the alternative pathway.30 We previously showed that most patients, and specifically patients with MIS-C, had elevations in anti–SARS-CoV-2 antibody titers.31 We considered whether sC5b9 elevations may be related to classical pathway activation from antiviral antibody–antigen complexes rather than the alternative complement activation of TMA. SARS-CoV-2–specific antibodies were measured by using ELISA as previously described.32 Serum IgG, IgA, and IgM antibodies against the receptor-binding domain (RBD) of the spike protein were measured.32,33 Optical densities at the 450 nm wavelength were obtained on a SpectraMax 190 Microplate Reader (Molecular Devices, San Jose, CA). Serum antibody titers are expressed as the reciprocal serum dilution at a set optical density based on a standard from the monoclonal antibody CR3022 starting at 0.5 μg/mL.
Statistical analyses
Analysis and visualizations were performed by using Prism 8 (GraphPad, San Diego, CA) and Stata/IC (StataCorp, College Station, TX). Descriptive statistics were used to summarize characteristics in each group. Pairwise comparisons were performed by using Kruskal-Wallis testing. The Mann-Whitney U test was used to compute P values between 2 groups, and post hoc Dunn’s test for multiple comparisons was used to compute P values. Pearson’s r was used to calculate correlations between sC5b9 and clinical variables and to examine the relationship between sC5b9 and log-transformed viral titers. The χ2 test was used to compare count data.
Study approval
This study was conducted according to the guidelines of the Declaration of Helsinki. Due to the COVID-19 pandemic, verbal consent was obtained from a legally authorized representative, with consent forms signed by the consenting physician or research assistant and a physical copy provided to the consenting party. Assent was obtained from children aged >7 years, if appropriate. The study protocol was approved by the Institutional Review Board (IRB) at CHOP. For healthy control subjects, protected health information was not recorded. The CHOP IRB determined that the limited chart review of this control cohort met the IRB exemption criteria per 45 CFR 46.104(d) 4(ii) and waiver of Health Insurance Portability and Accountability Act of 1996 authorization.
Results
Between 3 April 2020, and 7 July 2020, a total of 112 patients were screened and 58 were enrolled. Fifty patients were included in this analysis for whom complete sC5b9 data were available (supplemental Figure 1). All included patients had either a positive SARS-CoV-2 PCR or a positive SARS-CoV-2 antibody test. Patients were classified into the 3 categories described earlier: minimal COVID-19 (n = 21), severe COVID-19 (n = 11), or MIS-C (n = 18). In addition, remnant plasma on healthy control subjects was collected (n = 26). Demographic characteristics of the patients are presented in Table 1. Clinical characteristics were consistent with previously reported pediatric patients with MIS-C and COVID-19.5,7 Twenty of these patients (n = 5, minimal COVID-19; n = 9, severe COVID-19; and n = 6, MIS-C) were included in a previously published analysis, focused on comparing clinical features and cytokines between groups.11 Study identifiers have been included to allow for comparison across reports.
Demographic data associated with patients included in the sample
| Variable . | Minimal COVID-19 (n = 21) . | Severe COVID-19 (n = 11) . | MIS-C (n = 18) . |
|---|---|---|---|
| Age, median (IQR), y | 13 (5-17) | 15 (14-17) | 9 (7-13) |
| Sex, n (%) | |||
| Female | 9 (43) | 6 (55) | 9 (50) |
| Male | 12 (57) | 5 (45) | 9 (50) |
| Race, n (%) | |||
| White | 8 (38) | 5 (45) | 8 (44) |
| Black | 10 (48) | 3 (27) | 7 (39) |
| Other | 3 (14) | 2 (18) | 2 (11) |
| Declined or not reported | 0 | 1 (9) | 1 (6) |
| Ethnicity, n (%) | |||
| Hispanic | 4 (19) | 3 (27) | 3 (17) |
| Not Hispanic | 17 (81) | 7 (64) | 14 (78) |
| Declined | 0 | 1 (9) | 1 (6) |
| BMI percentile, median (IQR) | 65 (19.9-90.23); n = 19 | 82 (65-95) | 93 (80-98) |
| Pediatric intensive care unit admission, n (%) | |||
| Yes | 2 (10) | 11 (100) | 13 (72) |
| No | 19 (90) | 0 | 5 (28) |
| Extracorporeal membrane oxygenation, n (%) | |||
| Yes | 0 | 2 (18) | 0 |
| No | 21 (100) | 9 (82) | 18 |
| Inotropic support, n (%) | |||
| Yes | 0 | 8 (73) | 12 (67) |
| No | 21 (100) | 3 (27) | 6 (33) |
| Invasive mechanical ventilation, n (%) | |||
| Yes | 1 (5)* | 9 (82) | 4 (22) |
| No | 20 (95) | 2 (18) | 14 (78) |
| Liver dysfunction,†n (%) | |||
| Yes | 5 (24) | 5 (45) | 3 (17) |
| No | 16 (76) | 6 (55) | 15 (83) |
| Renal dysfunction,‡n (%) | |||
| Yes | 2 (10) | 4 (36) | 5 (28) |
| No | 16 (76) | 7 (64) | 13 (72) |
| Not available | 3 (14) | ||
| Dialysis, n (%) | |||
| Yes | 0 | 1 (9) | 0 |
| No | 21 (100) | 10 (91) | 18 (100) |
| Hypertension,§n (%) | |||
| Yes | 11 (52) | 9 (82) | 10 (56) |
| No | 10 (48) | 2 (18) | 8 (44) |
| Proteinuria, n (%) | |||
| Yes | 7 (33) | 8 (72) | 12 (66) |
| No | 7 (33) | 3 (27) | 4 (22) |
| Not available | 7 (33) | 0 | 4 (22) |
| Outcome, n (%) | |||
| Discharged | 21 (100) | 7 (64) | 18 (100) |
| Remains hospitalized | 0 | 2 (18) | 0 |
| Death | 0 | 2 (18) | 0 |
| Variable . | Minimal COVID-19 (n = 21) . | Severe COVID-19 (n = 11) . | MIS-C (n = 18) . |
|---|---|---|---|
| Age, median (IQR), y | 13 (5-17) | 15 (14-17) | 9 (7-13) |
| Sex, n (%) | |||
| Female | 9 (43) | 6 (55) | 9 (50) |
| Male | 12 (57) | 5 (45) | 9 (50) |
| Race, n (%) | |||
| White | 8 (38) | 5 (45) | 8 (44) |
| Black | 10 (48) | 3 (27) | 7 (39) |
| Other | 3 (14) | 2 (18) | 2 (11) |
| Declined or not reported | 0 | 1 (9) | 1 (6) |
| Ethnicity, n (%) | |||
| Hispanic | 4 (19) | 3 (27) | 3 (17) |
| Not Hispanic | 17 (81) | 7 (64) | 14 (78) |
| Declined | 0 | 1 (9) | 1 (6) |
| BMI percentile, median (IQR) | 65 (19.9-90.23); n = 19 | 82 (65-95) | 93 (80-98) |
| Pediatric intensive care unit admission, n (%) | |||
| Yes | 2 (10) | 11 (100) | 13 (72) |
| No | 19 (90) | 0 | 5 (28) |
| Extracorporeal membrane oxygenation, n (%) | |||
| Yes | 0 | 2 (18) | 0 |
| No | 21 (100) | 9 (82) | 18 |
| Inotropic support, n (%) | |||
| Yes | 0 | 8 (73) | 12 (67) |
| No | 21 (100) | 3 (27) | 6 (33) |
| Invasive mechanical ventilation, n (%) | |||
| Yes | 1 (5)* | 9 (82) | 4 (22) |
| No | 20 (95) | 2 (18) | 14 (78) |
| Liver dysfunction,†n (%) | |||
| Yes | 5 (24) | 5 (45) | 3 (17) |
| No | 16 (76) | 6 (55) | 15 (83) |
| Renal dysfunction,‡n (%) | |||
| Yes | 2 (10) | 4 (36) | 5 (28) |
| No | 16 (76) | 7 (64) | 13 (72) |
| Not available | 3 (14) | ||
| Dialysis, n (%) | |||
| Yes | 0 | 1 (9) | 0 |
| No | 21 (100) | 10 (91) | 18 (100) |
| Hypertension,§n (%) | |||
| Yes | 11 (52) | 9 (82) | 10 (56) |
| No | 10 (48) | 2 (18) | 8 (44) |
| Proteinuria, n (%) | |||
| Yes | 7 (33) | 8 (72) | 12 (66) |
| No | 7 (33) | 3 (27) | 4 (22) |
| Not available | 7 (33) | 0 | 4 (22) |
| Outcome, n (%) | |||
| Discharged | 21 (100) | 7 (64) | 18 (100) |
| Remains hospitalized | 0 | 2 (18) | 0 |
| Death | 0 | 2 (18) | 0 |
One patient with minimal COVID-19 symptoms was admitted during a trauma team activation and was intubated for airway protection.
Defined as bilirubin levels >2 times the upper limit of normal for age, alanine aminotransferase or aspartate aminotransferase levels >3 times the upper limit of normal.
Defined according to KDIGO criteria.
Defined as blood pressure >99th percentile for age, height, and sex.
Laboratory data are presented in Table 2. If <50% of the patients in the group had data available for a given laboratory value, these data were excluded from the table. Median D-dimer values tended to be higher in patients with MIS-C than in those with severe COVID-19. Patients with severe COVID-19 and MIS-C had highly elevated inflammatory markers, including ferritin and C-reactive protein. Patients with MIS-C also had evidence of acute cardiac injury with elevated troponin and B-type natriuretic peptide protein levels.
Median, IQR, and number of patients of the most extreme laboratory values during the admission for patients included in the sample
| Value (reference range) . | Minimal COVID-19 . | Severe COVID-19 . | MIS-C . | |||
|---|---|---|---|---|---|---|
| Median (IQR) . | n . | Median (IQR) . | n . | Median (IQR) . | n . | |
| Coagulation | ||||||
| D-dimer, highest (0.27-0.60 μg/mL FEU) | — | 2.53 (0.6-20.5) | 11 | 4.93 (3.42-5.75) | 17 | |
| PT, highest (11.6-13.8 s) | — | 16 (13.2-31) | 11 | 15.4 (14.4-17.4) | 18 | |
| PTT, highest (22-36 s) | — | 52.4 (37.5-78.8) | 11 | 31.8 (28.4-36.6) | 17 | |
| Fibrinogen (172-471 mg/dL) | ||||||
| Lowest | — | 297 (239-332) | 10 | 297 (237-370) | 17 | |
| Highest | — | 488 (305-890) | 10 | 572 (484-721) | 17 | |
| Chemistry | ||||||
| AST, highest (10-30 U/L) | 59 (40-72) | 17 | 123 (54-627) | 11 | 88 (63-104) | 18 |
| Creatinine, highest (0.3-0.8 mg/dL) | 0.4 (0.3-0.8) | 19 | 0.7 (0.3-2.3) | 11 | 0.6 (0.5-1.3) | 18 |
| GFR, minimum (mL/min) | 193 (108-221) | 18 | 111 (40-243) | 11 | 136 (65-182) | 18 |
| BUN, highest (7-18 mg/dL) | 12 (7-23) | 19 | 23 (13-34) | 11 | 21.5 (16-39) | 18 |
| Hematology | ||||||
| Platelets (150-400 K/μL) | ||||||
| Lowest | 220 (127-295) | 21 | 128 (78-165) | 11 | 141 (121-194) | 18 |
| Highest | 414 (165-363) | 21 | 311 (223-347) | 11 | 414 (275-483) | 18 |
| Hemoglobin, lowest (12-16 g/dL) | 9.2 (7.4-11.9) | 21 | 8.9 (6.7-11.1) | 11 | 8.9 (7.9-10) | 18 |
| Inflammatory and cardiac | ||||||
| Ferritin, highest (10.0-82.0 ng/ml) | — | 419 (164-2747) | 10 | 806 (665-1162) | 17 | |
| CRP, highest (0-0.9 mg/dL) | 13.9 (3.1-18.3) | 11 | 30.3 (7-34.9) | 11 | 23.7 (19-34.6) | 18 |
| ESR, highest (0-20 mm/h) | — | – | 59 (43-82) | 17 | ||
| BNP, highest (≤100 pg/mL) | — | 307 (46-542) | 8 | 997 (510-1242) | 17 | |
| Troponin, highest (<0.3 ng/ml) | — | 0.13 (0.02-1.79) | 9 | 0.39 (0.07-1.34) | 17 | |
| IL-8 | 10 (5-16.6) | 15 | 32.7 (12.3-124) | 11 | 37.3 (20.4-56.7) | 17 |
| Interferon-γ | 14 (4-150) | 15 | 54 (12-100) | 11 | 191 (41-481) | 17 |
| sC5b9 (≤257 ng/mL) | 392 (244-622) | 21 | 646 (203-728) | 11 | 630 (359-932) | 18 |
| Value (reference range) . | Minimal COVID-19 . | Severe COVID-19 . | MIS-C . | |||
|---|---|---|---|---|---|---|
| Median (IQR) . | n . | Median (IQR) . | n . | Median (IQR) . | n . | |
| Coagulation | ||||||
| D-dimer, highest (0.27-0.60 μg/mL FEU) | — | 2.53 (0.6-20.5) | 11 | 4.93 (3.42-5.75) | 17 | |
| PT, highest (11.6-13.8 s) | — | 16 (13.2-31) | 11 | 15.4 (14.4-17.4) | 18 | |
| PTT, highest (22-36 s) | — | 52.4 (37.5-78.8) | 11 | 31.8 (28.4-36.6) | 17 | |
| Fibrinogen (172-471 mg/dL) | ||||||
| Lowest | — | 297 (239-332) | 10 | 297 (237-370) | 17 | |
| Highest | — | 488 (305-890) | 10 | 572 (484-721) | 17 | |
| Chemistry | ||||||
| AST, highest (10-30 U/L) | 59 (40-72) | 17 | 123 (54-627) | 11 | 88 (63-104) | 18 |
| Creatinine, highest (0.3-0.8 mg/dL) | 0.4 (0.3-0.8) | 19 | 0.7 (0.3-2.3) | 11 | 0.6 (0.5-1.3) | 18 |
| GFR, minimum (mL/min) | 193 (108-221) | 18 | 111 (40-243) | 11 | 136 (65-182) | 18 |
| BUN, highest (7-18 mg/dL) | 12 (7-23) | 19 | 23 (13-34) | 11 | 21.5 (16-39) | 18 |
| Hematology | ||||||
| Platelets (150-400 K/μL) | ||||||
| Lowest | 220 (127-295) | 21 | 128 (78-165) | 11 | 141 (121-194) | 18 |
| Highest | 414 (165-363) | 21 | 311 (223-347) | 11 | 414 (275-483) | 18 |
| Hemoglobin, lowest (12-16 g/dL) | 9.2 (7.4-11.9) | 21 | 8.9 (6.7-11.1) | 11 | 8.9 (7.9-10) | 18 |
| Inflammatory and cardiac | ||||||
| Ferritin, highest (10.0-82.0 ng/ml) | — | 419 (164-2747) | 10 | 806 (665-1162) | 17 | |
| CRP, highest (0-0.9 mg/dL) | 13.9 (3.1-18.3) | 11 | 30.3 (7-34.9) | 11 | 23.7 (19-34.6) | 18 |
| ESR, highest (0-20 mm/h) | — | – | 59 (43-82) | 17 | ||
| BNP, highest (≤100 pg/mL) | — | 307 (46-542) | 8 | 997 (510-1242) | 17 | |
| Troponin, highest (<0.3 ng/ml) | — | 0.13 (0.02-1.79) | 9 | 0.39 (0.07-1.34) | 17 | |
| IL-8 | 10 (5-16.6) | 15 | 32.7 (12.3-124) | 11 | 37.3 (20.4-56.7) | 17 |
| Interferon-γ | 14 (4-150) | 15 | 54 (12-100) | 11 | 191 (41-481) | 17 |
| sC5b9 (≤257 ng/mL) | 392 (244-622) | 21 | 646 (203-728) | 11 | 630 (359-932) | 18 |
Results were not reported if >50% of data were missing.
AST, aspartate transaminase; BNP, B-type natriuretic peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEU, fibrinogen equivalent units; PT, prothrombin time; PTT, partial thromboplastin time.
The median sC5b9 level in the healthy control subjects (57 ng/mL; interquartile range [IQR], 9-163 ng/mL) differed significantly from that in patients with minimal disease (392 ng/mL; IQR, 244-622 ng/mL), severe disease (646 ng/mL; IQR, 203-728 ng/mL), and MIS-C (630 ng/mL; IQR, 359-932 ng/mL) (P < .001 in each case) (Figure 1A). There were no statistically significant differences between patients with minimal disease, severe disease, or MIS-C. One patient with minimal symptoms of COVID-19 but a very high sC5b9 (1568 ng/mL) was concomitantly diagnosed with systemic lupus erythematosus. To ensure that comorbidities such as lupus that can be associated with complement dysfunction were not confounding this relationship, we repeated this analysis but excluded all patients with a diagnosis of lupus, cancer, sickle cell disease, renal disease, or inflammatory disease. Levels of sC5b9 in patients with minimal COVID-19, severe COVID-19, and MIS-C remained elevated compared with levels from healthy control subjects (P < .0001) (supplemental Figure 2).
Elevations in sC5b9 correlate with renal dysfunction. (A) Elevated sC5b9 levels in patients with minimal COVID-19 (n = 21), severe COVID-19 (n = 11), and MIS-C (n = 18) are significantly different relative to those of healthy control subjects (n = 26). (B) sC5b9 levels are significantly higher in those with AKI (n = 9) than without AKI (n = 38). Increases in sC5b9 levels in patients with all 3 manifestations of disease (N = 48) correlate in a statistically significant manner with elevations in creatinine (C) and in elevations of blood urea nitrogen (BUN; D) and GFR (E). Dotted line indicates upper limit of normal cutoff for sC5b9 of 247 ng/mL.
Elevations in sC5b9 correlate with renal dysfunction. (A) Elevated sC5b9 levels in patients with minimal COVID-19 (n = 21), severe COVID-19 (n = 11), and MIS-C (n = 18) are significantly different relative to those of healthy control subjects (n = 26). (B) sC5b9 levels are significantly higher in those with AKI (n = 9) than without AKI (n = 38). Increases in sC5b9 levels in patients with all 3 manifestations of disease (N = 48) correlate in a statistically significant manner with elevations in creatinine (C) and in elevations of blood urea nitrogen (BUN; D) and GFR (E). Dotted line indicates upper limit of normal cutoff for sC5b9 of 247 ng/mL.
Clinical TMA and sC5b9 elevations are closely associated with renal damage.17 We compared plasma sC5b9 levels between those with AKI (defined as KIDGO stage 2 or above) and those without AKI (KDIGO stage 1 or less), and found significantly higher sC5b9 levels in those with AKI (717 ng/mL; IQR, 404-1232 ng/mL) than in those without AKI (433 ng/mL; IQR, 232-706 ng/mL) (P = .0374) (Figure 1B). We next evaluated correlations between sC5b9, creatinine, GFR, and blood urea nitrogen in the entire combined cohort with SARS-CoV-2 or MIS-C. Because creatinine levels in children increase with age, the correlation between sC5b9 and age was also examined. Elevations in sC5b9 correlated in a statistically significant manner with the maximum creatinine (r = 0.36; P = .01), blood urea nitrogen (r = 0.29; P = .04), and GFR (r = –0.36; P = .01) measured during hospitalization (Figure 1C-E) but not with age (P = .512). In our cohort, 10% of minimal COVID-19, 36% of severe COVID-19, and 28% of MIS-C patients had evidence of AKI (Table 1).
In patients with severe COVID-19 and MIS-C, we examined correlations between sC5b9 and markers of inflammation, hemolysis, and coagulopathy, including ferritin, C-reactive protein, LDH, prothrombin time, partial thromboplastin time, fibrinogen, D-dimer, aspartate transaminase, hemoglobin, and platelets. Peak values were used for all except hemoglobin, in which the lowest value was used, and for fibrinogen and platelets, in which both peak value and lowest value were calculated. This analysis was limited to those with severe COVID-19 and MIS-C, due to a high proportion of missing data in children with minimal disease. Heatmaps of Pearson r correlations and P values are shown in Figure 2. sC5b9 did not correlate significantly with any of these variables. Clinical variables, including markers of coagulopathy and cytopenias, clustered together.
Heatmaps of Pearson r correlations demonstrate clusertings of laboratory findings. Heatmaps of Pearson r correlations (A) and associated P values (B) for ancillary findings of thrombotic microangiopathy in patients with severe COVID-19 (n = 11) and patients with MIS-C (n = 18).
Heatmaps of Pearson r correlations demonstrate clusertings of laboratory findings. Heatmaps of Pearson r correlations (A) and associated P values (B) for ancillary findings of thrombotic microangiopathy in patients with severe COVID-19 (n = 11) and patients with MIS-C (n = 18).
Peripheral blood smears were available on 34 patients. Schistocytes were present in 5 (45%) of 11 patients with minimal COVID-19, 7 (87%) of 8 patients with severe COVID-19, and 13 (87%) of 15 patients with MIS-C (χ2 = 6.59; P = .037) (supplemental Figure 3). We retrospectively evaluated if patients met expanded clinical criteria for TMA in the subgroup of patients who had a blood smear, complete blood count, and LDH level available (n = 19). Of those patients, 17 (89%) met clinical criteria for TMA. sC5b9 levels were elevated both in patients who did and did not meet criteria for TMA (supplemental Figure 4). Some patients in the severe COVID-19 and minimal COVID-19 cohorts had comorbidities; these are listed in Table 3. Of note, all patients in the MIS-C group were previously healthy or had minimal comorbidities. Of those who met criteria for AKI in Figure 1B, 7 of 9 patients had all criteria for TMA assessed, and all 7 met criteria for TMA.
Criteria for TMA in patients with MIS-C, minimal COVID-19, and severe COVID-19
| ID . | Age, y . | Other conditions . | Elevated LDH . | Schistocytes . | Low platelets . | Anemia . | HTN . | Protein in urine . | Elevated sC5b9 . | Met criteria for TMA? . | No. of criterion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimal COVID-19 | |||||||||||
| 13 | 4 | Sickle cell disease | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 38 | 17 | Obesity, asthma | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| Summary: fraction (%) | 2/2 (100) | 1/2 (50) | 2/2 (100) | 1/2 (50) | 2/2 (100) | 1/2 (50) | 1/2 (50) | 2/2 (100) | |||
| Severe COVID-19 | |||||||||||
| 10 | 10 | Panhypopituitarism | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 33 | 15 | Obesity, PCOS | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 37 | 15 | Obesity | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 4 | 18 | Obesity, hypertension, diabetes mellitus | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| Summary: fraction (%) | 4/4 (100) | 4/4 (100) | 2/4 (67) | 4/4 (100) | 3/4 (75) | 3/4 (75) | 4/4 (100) | 4/4 (100) | |||
| MIS-C | |||||||||||
| 19 | 5 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 22 | 6 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 18 | 6 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| 24 | 7 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 29 | 8 | Asthma | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 44 | 8 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 55 | 8 | Precocious puberty | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 28 | 9 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 50 | 9 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| 48 | 11 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 26 | 13 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 27 | 14 | Previously healthy | ✓ | ✓ | ✓ | ✓ | No | 4 | |||
| 51 | 17 | Asthma | ✓ | ✓ | ✓ | ✓ | No | 4 | |||
| Summary: fraction (%) | 8/13(61) | 12/13 (92) | 8/13(61) | 12/13 (92) | 10/13 (76) | 9/13 (69) | 12/13 (92) | 11/13 (85) | |||
| ID . | Age, y . | Other conditions . | Elevated LDH . | Schistocytes . | Low platelets . | Anemia . | HTN . | Protein in urine . | Elevated sC5b9 . | Met criteria for TMA? . | No. of criterion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimal COVID-19 | |||||||||||
| 13 | 4 | Sickle cell disease | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 38 | 17 | Obesity, asthma | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| Summary: fraction (%) | 2/2 (100) | 1/2 (50) | 2/2 (100) | 1/2 (50) | 2/2 (100) | 1/2 (50) | 1/2 (50) | 2/2 (100) | |||
| Severe COVID-19 | |||||||||||
| 10 | 10 | Panhypopituitarism | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 33 | 15 | Obesity, PCOS | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 37 | 15 | Obesity | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 4 | 18 | Obesity, hypertension, diabetes mellitus | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| Summary: fraction (%) | 4/4 (100) | 4/4 (100) | 2/4 (67) | 4/4 (100) | 3/4 (75) | 3/4 (75) | 4/4 (100) | 4/4 (100) | |||
| MIS-C | |||||||||||
| 19 | 5 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 22 | 6 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 18 | 6 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| 24 | 7 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 29 | 8 | Asthma | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 44 | 8 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 55 | 8 | Precocious puberty | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 6 | |
| 28 | 9 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 50 | 9 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 7 |
| 48 | 11 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 26 | 13 | Previously healthy | ✓ | ✓ | ✓ | ✓ | ✓ | Yes | 5 | ||
| 27 | 14 | Previously healthy | ✓ | ✓ | ✓ | ✓ | No | 4 | |||
| 51 | 17 | Asthma | ✓ | ✓ | ✓ | ✓ | No | 4 | |||
| Summary: fraction (%) | 8/13(61) | 12/13 (92) | 8/13(61) | 12/13 (92) | 10/13 (76) | 9/13 (69) | 12/13 (92) | 11/13 (85) | |||
Criteria for TMA as defined by Gloude et al.27 Meeting at least 5 of the 7 criteria are required to meet definition. Protein in urine defined as random urine protein measurement ≥30 mg/dL or urine protein/creatinine ratio ≥2 mg/mg. Hypertension defined as >99th percentile for age, height, and sex. Check marks indicate that the criterion was met; blank spaces indicate that the criterion was not met.
B-ALL, B-cell acute lymphoblastic leukemia; HTN, hypertension; PCOS, polycystic ovarian syndrome.
We found that 13 (38%) of 34 patients met simple clinical criteria for TMA (microangiopathic hemolytic anemia, thrombocytopenia, and evidence of organ damage). In patients who met simple clinical criteria for TMA, the median sC5b9 level was 420 ng/mL, compared with 634 ng/mL in patients who did not meet simple criteria for TMA (P = .60). Urinalyses were available on 28 of these patients, and proteinuria was present in 9 of 13 patients who met criteria for simple TMA and 10 of 15 patients who did not (χ2 = 2.92; P = .09).
Supplemental Table 1 presents similar data on patients in whom full peripheral blood smears, LDH levels, and complete blood count were not available. We performed a sensitivity analysis and evaluated whether all patients, regardless of whether complete criteria were available, met criteria for TMA. For this analysis, sC5b9 elevations were included in the clinical criteria for TMA. All missing data were counted as negative. In total, 24 (48%) of 50 patients met criteria for TMA, including 21% of patients with minimal disease, 82% of patients with severe disease, and 61% of patients with MIS-C (supplemental Table 2). Summary data for patients according to disease category are presented in supplemental Tables 3-5.
We considered that sC5b9 elevations could be caused by an unknown pathophysiological process in SARS-CoV-2 patients other than TMA. We therefore performed a sensitivity analysis in which sC5b9 was not included in the criteria for TMA. To test the most stringent hypothesis, missing data were imputed to be negative. Fifteen (30%) of 50 patients still met criteria for TMA, 9 patients were indeterminate, and 26 patients did not meet criteria for TMA. Of the 9 indeterminate patients, all 9 had elevations in sC5b9 that would have led them to meet criteria for TMA if sC5b9 had been included.
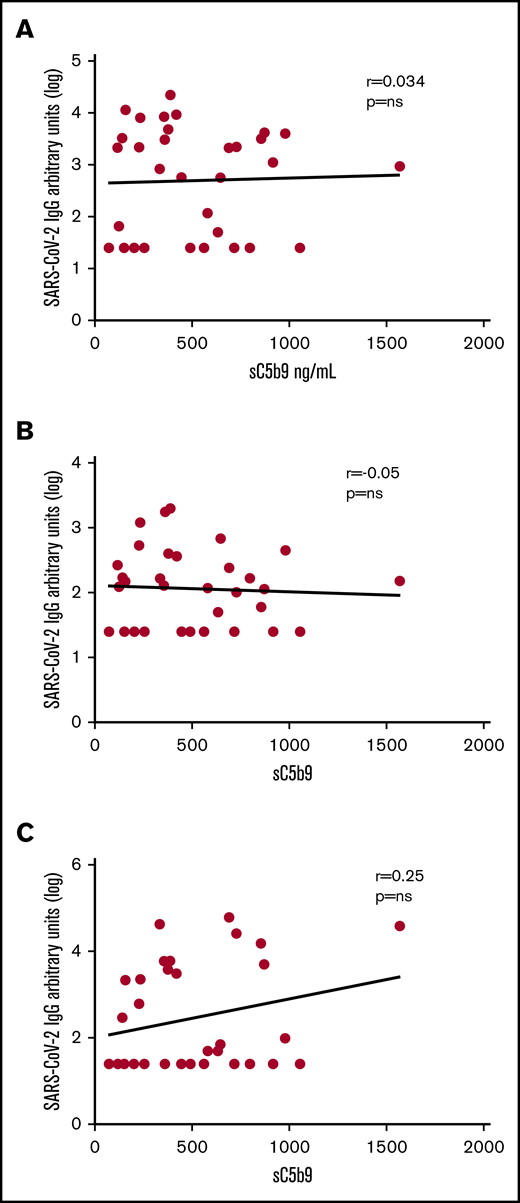
To probe if the complement activation of SARS-CoV-2 infection could be related to immune complex and classical activation, we measured antibody response in the first 33 patients enrolled (12 minimal COVID-19, 11 severe COVID-19, and 10 MIS-C) and tested whether antibody titers correlated with sC5b9 levels. IgG, IgM, and IgA levels against the SARS-CoV-2 spike protein RBD did not significantly correlate with sC5b9 (Figure 3).
Absence of correlation between sC5b9 elevatons and antibodies against SARS-CoV-2. Correlation between sC5b9 vs the logarithmically transformed value of IgG (A), IgM (B), and IgA (C) against the SARS-CoV-2 RBD protein for a subset of included patients with MIS-C (n = 10), severe disease (n = 11), and minimal disease (n = 12).
Absence of correlation between sC5b9 elevatons and antibodies against SARS-CoV-2. Correlation between sC5b9 vs the logarithmically transformed value of IgG (A), IgM (B), and IgA (C) against the SARS-CoV-2 RBD protein for a subset of included patients with MIS-C (n = 10), severe disease (n = 11), and minimal disease (n = 12).
Levels of IL-8, measured as a marker of endothelial dysfunction, differed significantly between patients with MIS-C (P = .0166) and patients with severe COVID-19 (P = .0079), when compared with minimal COVID-19 patients, but not between patients with MIS-C and severe disease (P = .99) (supplemental Figure 5). Levels of IL-8 did not correlate with elevations in sC5b9 (P = .184). Levels of interferon-γ differed significantly between patients with minimal disease and MIS-C (P = .01) but not between those with MIS-C and severe disease (P = .13) or between those with minimal disease and severe disease (P > .99).
Discussion
We shows activation of the final common pathway of complement activation in the 3 most commonly described presentations of SARS-CoV-2 in pediatric patients. Strikingly, sC5b9 levels were abnormal even in children with minimal disease or an incidental finding of SARS-CoV-2 infection, suggesting that any exposure to SARS-CoV-2 may be sufficient to induce elevations in this biomarker. In addition, schistocytes were prevalent in blood smears of patients with minimal COVID-19, severe COVID-19, and MIS-C. IL-8, a marker of endothelial damage, was also significantly higher in patients with MIS-C and severe COVID-19 compared with the minimal COVID-19 group.34 These findings together suggest more severe endothelial dysfunction in MIS-C and severe COVID-19. The majority (19 of 22) of children who had all laboratory and clinical criteria for TMA measured met criteria for TMA, as did 8 of 9 patients with AKI. Even in children on whom all criteria were not measured, and when missing values were assumed to be negative, nearly one-half (48%) met criteria for TMA.
Elevations in sC5b9 correlated with evidence of renal dysfunction, implying that higher levels of terminal complement activation were associated with renal injury. Elevations in sC5b9 occurred independently of laboratory features associated with TMA such as elevations in LDH and decreases in platelets and hemoglobin. sC5b9 was also elevated independently of inflammatory markers. The presence of elevated sC5b9 levels in all SARS-CoV-2 groups compared with healthy control subjects suggests that complement activation and thrombotic microangiopathy are prevalent in pediatric patients with SARS-CoV-2 infections.
The presence of elevated sC5b9 even in children with minimal symptoms of COVID-19 disease is particularly striking. This finding implies that SARS-CoV-2 clinical syndromes are associated with robust complement activation, even when symptoms are minimal. We also found higher levels of complement activation in patients with more severe manifestations of COVID-19 than in those with minimal COVID-19, implying that some degree of complement activation may be necessary to combat the virus, but excessive complement activation may lead to an overly robust immune response. Notably, patients with MIS-C, who have generally cleared SARS-CoV-2 infection at the time of presentation, also had high levels of complement activation. We would therefore expect that the particle which incites complement activation is no longer present. This implies that prolonged and excessive activation in the host may be what leads to pathology. sC5b9 elevations did not correlate with SARS-CoV-2 RBD antibody production, which suggests that anti–SARS-CoV-2 immune complex is likely not the driving force of complement activation in these patients. Previous studies have shown that proteins on SARS-CoV-2 contain mannose groups recognized by the mannose-binding lectin complement pathway, and autopsy specimens of lungs from patients who have died of COVID-19 have exhibited immunohistochemistry staining for mannose-binding lectin, C3, C4, and sC5b9.35 Direct viral activation of the complement cascade by the alternative pathway has been previously shown in mouse models of the related coronaviruses SARS-CoV-1 and Middle East respiratory syndrome coronavirus.24,25 SARS-CoV-2 has been shown to infect tissues with angiotensin-converting enzyme 2 receptor expression, including the lungs, heart, kidneys, intestines, and endothelial cells, suggesting a mechanism of viral infection, complement activation, and vascular and organ injury.35,36 MIS-C is hypothesized to be a postinfectious etiology; it is not clear if complement activation in MIS-C occurs by the same mechanism as in acute SARS-CoV-2 infection.10,11 Future work will need to better elucidate the role of the complement cascade in the pathogenesis of MIS-C and COVID-19 in children, particularly given the high rates of TMA seen in this population.
Regardless of the mechanism of complement activation in SARS-CoV-2 infection, the finding of elevated sC5b9 in children across the spectrum of presentations of SARS-CoV-2 is an important area for future inquiry. Elevations in sC5b9 have been shown to be associated with an increased risk of death in pediatric HSCT-TMA.23 The pathophysiology of complement activation in pediatric patients is likely similar to that in adult patients.37 A key treatment of HSCT-TMA in pediatric patients is eculizumab, a monoclonal antibody against the complement protein C5.34,38 Although eculizumab has been shown to improve survival in children with HSCT-TMA, it can cause vulnerability to encapsulated bacteria and lead to serious meningococcal infections even with appropriate vaccinations and prophylaxis.39-41 Thus, in pediatric patients with SARS-CoV-2, the consideration of eculizumab should likely be limited to only those patients with the most severe manifestations of disease with organ-threatening evidence of TMA. Other complement-targeted therapies for treatment of COVID-19 are currently being explored in adult patients, including possible inhibition of the lectin pathway, blockade of C3, and utilization of eculizumab for blockade of C5.42-45 The degree to which complement activation may be necessary for control of SARS-CoV-2 infection and the factors that lead to inappropriate complement overactivation are not yet clear. This represents an important area for future investigations. Future studies are also needed to establish the prognostic implications of elevated sC5b9 in children with SARS-CoV-2 and to define the potential role and appropriateness of complement blockade in this population.
The short- and long-term implications of complement activation in children with SARS-CoV-2 are unclear, especially in children with minimal or no symptoms. HSCT recipients who develop TMA can develop life-long clinical issues, including hypertension, pulmonary hypertension, stroke, and chronic kidney disease.41 It is therefore possible that there could be unrecognized long-term consequences of TMA due to SARS-CoV-2 infection. Future work is needed to better understand the long-term sequelae of SARS-CoV-2 infection and SARS-CoV-2–related TMA. Children with elevated sC5b9 and evidence of TMA should arguably be monitored for resolution of findings and for potential long-term sequelae. It is unclear whether children with elevated sC5b9 but no other evidence of TMA require monitoring. These are important areas for future study.
Our study is limited by several important factors. First, we enrolled patients prospectively in the described biospecimen repository; however, some clinical data were collected retrospectively. The high incidence of TMA seen in patients with complete laboratory evaluation for TMA (19 of 22 patients) is confounded by ascertainment bias, as LDH was more frequently obtained in sicker children. Markers of hemolysis, including LDH, unfortunately cannot be measured accurately on banked specimens. Future work should prospectively examine children with SARS-CoV-2 infection for clinical signs of TMA, particularly hypertension and proteinuria. Second, we compared hospitalized patients with severe COVID-19 and MIS-C vs hospitalized patients with either mild symptoms or who were asymptomatic. By definition, these hospitalized children had other comorbidities leading to their admission, which could have caused elevations in sC5b9. However, even when excluding patients with potentially confounding comorbidities, there were still significant elevations in sC5b9 relative to healthy control subjects. Future studies should seek to characterize the role of terminal complement activation and TMA in pediatric patients who have asymptomatic SARS-CoV-2 infection and who are without comorbidities. Our healthy control subjects were also patients who were being evaluated for a bleeding disorder. Although we do not believe that this affected complement activation, future studies should seek to include children without any clinical comorbidities as healthy control subjects. Of note, the normal range of sC5b9 in our assay was in concordance with that of sC5b9 measured in CLIA/CAP clinical laboratories. Practical considerations associated with the ethical conduct of research during a pandemic precluded inclusion of these comparator groups and limited our study to a relatively small sample size. Finally, although measures of renal function included in this study were all based on creatinine, future investigations should use cystatin C as a potentially more precise measure of renal function in children.39
We have shown that terminal complement activation is present in children across the spectrum of SARS-CoV-2 infection and that a high proportion of these children met clinical criteria for TMA. Elevations in sC5b9 occur independently of other laboratory markers associated with COVID-19 and MIS-C, and are associated with evidence of renal dysfunction. Although additional studies are clearly needed, evaluation for clinical TMA and complications of TMA should be considered in hospitalized children with SARS-CoV-2. The long-term implications of complement activation and TMA in these children need to be studied.
Presented in abstract form at the 62nd annual meeting of the American Society of Hematology, 5-8 December 2020.
Requests for data sharing may be submitted to the corresponding author (Hamid Bassiri; e-mail: bassiri@email.chop.edu).
Acknowledgments
Financial support for this project was provided by CHOP Frontiers Program Immune Dysregulation Team (D.T.T., E.M.B., and H.B.), National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (R01AI121250 [E.M.B.]; R01AI103280, R01AI123433, and R21AI144472 [A.R.O.J.]; K08 AI136660 [L.A.V.]; and K08AI135091 [S. E. Henrickson]), NIH/National Cancer Institute (R01CA193776, X01HD100702-01, 5UG1CA233249, and R01A1123538) (D.T.T.), the Leukemia and Lymphoma Society (D.T.T.), Cookies for Kids Cancer (D.T.T.), Alex’s Lemonade Stand Foundation for Childhood Cancer (D.T.T.), Children’s Oncology Group (D.T.T.), Stand UP 2 Cancer (D.T.T.), Team Connor Childhood Cancer Foundation (H.B.), Burroughs Wellcome Fund CAMS (S. E. Henrickson and A.R.O.J.), Clinical Immunology Society (S. E. Henrickson), the American Academy of Allergy, Asthma, and Immunology (S. E. Henrickson), and the Agency for Healthcare Research and Quality (K12HS026393) (K.C.). C.D. is supported by an Institute for Translation Medicine and Therapheutics (ITMAT) scholarship and by the CHOP Gail Slap Fellowship Award. A.M.B. is supported by NIH/National Institute of General Medical Sciences (T32-GM075766). J.C.F. is supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (K23DK119463). E.M.A. was supported by the NIH Training in Virology T32 Program (T32-AI-007324).
Authorship
Contribution: H.B., E.M.B., and D.T.T. contributed equally to the manuscript and are joint last authors; C.D., K.O.M., M.L., M.P., E.M.A., F.B.B., K.G., C.W., K.E.S., B.L.L., S. E. Henrickson, H.B., E.M.B., and D.T.T. designed the research; C.D., K.O.M., J.C., J.H.L., T.L., W.P., S. E. Henrickson, K.C., L.A.V., A.R.O.J., and A.M.B. assisted in the consenting and recruitment of patients; C.D., K.O.M., J.H.L., and J.C. enrolled patients; K.O.M., E.M.A., and C.B. performed the assays described; C.D., K.O.M., M.L., M.P., S. E. Henrickson, J.C., E.J.L., J.H.L., L.A.V., C.J., T.L., H.B., E.M.B., and D.T.T. performed data abstraction and validation; C.D., K.O.M., F.B.B., A.M.B., K.C., J.C.F., T.M.G., K.G., A.R.O.J., W.P., L.A.V., C.W., K.E.S., B.L.L., S. E. Hensley, H.B., E.M.B., and D.T.T. contributed to data analysis and helped write the manuscript; and all authors contributed intellectually and reviewed and revised the manuscript.
Conflict-of-interest disclosure: D.T.T. serves on advisory boards for Janssen, Amgen, La Roche, Sobi, and Humanigen. H.B. has stock ownership in CSL Behring and is a consultant for Kriya Therapeutics. S. E. Henrickson served on the advisory board for Horizon Pharma. A.R.O.J. serves on the advisory board of Pluton Biosciences. M.L. is an advisory board member for Octapharma and Shionogi; a consultant for Amgen, Novartis, Shionogi, Dova, Principia, Argenz, and Bayer; and has received research funding from Sysmex, Novartis, and AstraZeneca. K.E.S. received personal fees from Elsevier and the Immune Deficiency Foundation. B.L.L. has a patent application under review (Compositions and Methods for Treatment of HSCT-Associated Thrombotic Microangiopathy. United States Patent Number PCT/US2014/055922, 2014). S.E.H. has received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck for work unrelated to this report. The remaining authors declare no competing financial interests.
Correspondence: Hamid Bassiri, Children’s Hospital of Philadelphia, 3401 Civic Center Blvd, Philadelphia, PA 19104; e-mail: bassiri@email.chop.edu.
References
Author notes
C.D. and K.O.M. contributed equally to this work.
H.B., E.M.B., and D.T.T. contributed equally to this work.
The full-text version of this article contains a data supplement.