Key Points
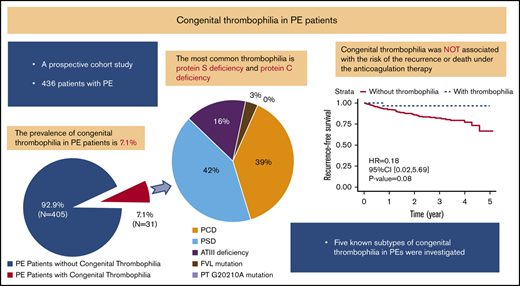
Approximately 7.1% patients with PE had congenital thrombophilia.
Congenital thrombophilia did not affect the risk of death and recurrent venous thromboembolism in PE patients receiving anticoagulation therapy.
Abstract
The prevalence and distribution of congenital thrombophilia is still unclear in patients with pulmonary embolism (PE). We aimed to determine the prevalence and clinical characteristics of congenital thrombophilia in PE patients and their subsequent outcomes. A prospective observational study was conducted from May 2013 to June 2018. A total of 436 consecutive patients with PE were enrolled. All patients were tested for protein C, protein S, antithrombin III (ATIII), factor V Leiden, and prothrombin G20210A mutations. The median follow-up duration was ∼800 days (range, 11-1872 days). Congenital thrombophilia was diagnosed in 31 of 436 (7.1%) patients; 12 patients had protein C deficiency (2.8%), 13 had protein S deficiency (3.0%), 5 had ATIII deficiency (1.1%), and 1 had (0.2%) factor V Leiden. Age ≤50 years at the first episode (odds ratio [OR], 5.43; 95% confidence interval [CI], 2.35-13.52; P < .001) and male sex (OR, 2.67; 95% CI, 1.15-6.78; P = .03) were 2 independent predictors of congenital thrombophilia in PE patients. There was no statistically significant difference in the prevalence of congenital thrombophilia between PE patients with and without risk factors (P = .58). We also found no significant difference in the risk of having a composite outcome of death or recurrent venous thromboembolism between patients with and without congenital thrombophilia (hazard ratio, 0.18; 95% CI, 0.02-5.69; P = .08). These results suggest that age and male sex are independently associated with the occurrence of congenital thrombophilia in PE patients but that congenital thrombophilia is not associated with the risk of recurrence or death with anticoagulation therapy.
Introduction
Pulmonary embolism (PE), one of the major types of venous thromboembolism (VTE), is a life-threatening disease. It is also an important public health issue that has become the third most common fatal cardiovascular disease worldwide.1 Although the association between congenital thrombophilia and deep vein thrombosis (DVT) and their ethnic difference has been clarified,2-4 the role of congenital thrombophilia in patients with PE remains to be determined, regardless of ethnicity.
The most common types of congenital thrombophilia include factor V Leiden mutation, prothrombin G20210A mutation, protein C deficiency (PCD), protein S deficiency (PSD), and antithrombin III (ATIII) deficiency. The prevalence of congenital thrombophilia is diverse and ethnicity specific.5 Factor V Leiden and prothrombin G20210A mutations account for >60% of congenital thrombophilia cases in White patients,3,6,7 while anticoagulant deficiencies such as protein C, protein S, and ATIII are major subtypes of congenital thrombophilia in Asian patients.8-11
However, most previous studies of congenital thrombophilia have primarily focused on DVT patients. Only 1 small-scale study from South Korea investigated the prevalence and genetic background of thrombophilia in PE patients.12 The influence of congenital thrombophilia on PE patients with or without risk factors, as well as the outcome of PE patients, was not determined. This current study aims to investigate the prevalence and distribution of 5 known subtypes of congenital thrombophilia in a large cohort of PE patients and assess the effects of congenital thrombophilia on the morbidity and outcome of PE.
Methods
Study design and patients
This study was conducted in the Thrombosis and Vascular Medicine Center of FuWai Hospital, Chinese Academy of Medical Sciences (Beijing, China), a national referral center for PE. The study was conducted between May 2013 and June 2018. Patients with PE were consecutively enrolled in the study when PE was diagnosed. The PE diagnosis was based on a standardized diagnostic workup for PE as follows: patients were assessed by computed tomography pulmonary angiography (CTPA) or ventilation/perfusion scan for PE and compression ultrasonography for DVT. Patients who declined to participate in the genetic testing or those who did not qualify for congenital anticoagulants testing were excluded. This study was approved by the review board of FuWai Hospital.
The sample consisted of PE patients with risk factors and PE patients without risk factors. Risk factors were based on the 2019 European Society of Cardiology guidelines,13 including active cancer, congestive heart failure, myocardial infarction, paralytic stroke, bed rest >3 days, pregnancy, puerperium, use of oral contraceptives, major trauma, surgery, use of a plaster cast within 3 months before the event, antiphospholipid syndrome (APS), and other autoimmune diseases.
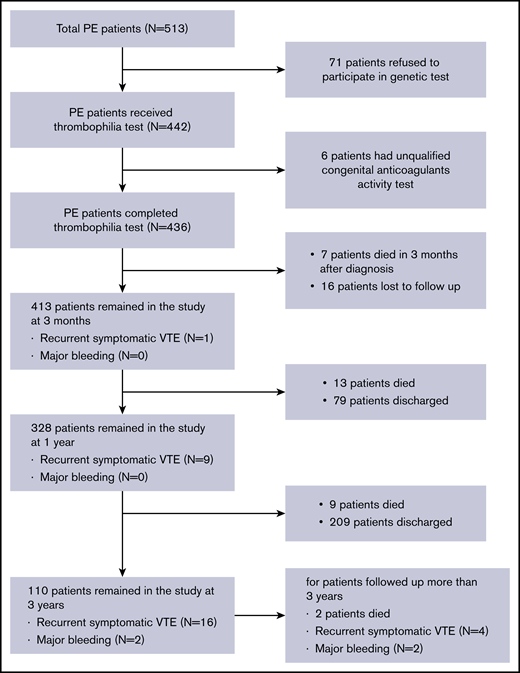
Data regarding patient medical history of VTE, the site of thromboembolism (with or without DVT), and initial treatments were collected. All patients were promptly diagnosed and followed up for ≥3 months. Patients diagnosed with thrombophilia needed to receive long-term anticoagulation, while patients without thrombophilia were required to complete 6 months of anticoagulation therapy. The overall study design is depicted in Figure 1.
Factor V Leiden and prothrombin G20210A mutation tests
Congenital thrombophilia was diagnosed in patients with a homozygous or a heterozygous mutation of factor V Leiden or prothrombin G20210A.7,14 The genetic test was performed by Sanger sequencing. The results of sequencing were analyzed by 2 experienced technicians. Genomic DNA was extracted from each patient’s peripheral blood through a salting-out method. The primer and experimental conditions of polymerase chain reaction are described in supplemental Methods.
Diagnosis of congenital anticoagulant deficiency
Congenital anticoagulant deficiency was defined by reduced anticoagulant activity <2 standard deviation (SD) after potential acquired factors, such as application of dicoumarol and heparin, autoimmune diseases, malignancy, and pregnancy, were ruled out. The tests included screening for protein C activity (HemosIL Protein C, Instrumentation Laboratory Company), protein S activity (ProS, Instrumentation Laboratory Company), and antithrombin III activity (HemosIL Liquid Antithrombin, Instrumentation Laboratory Company). Protein C and ATIII were analyzed with chromogenic substrate assays, while protein S was determined with a clotting assay using ACL TOP 700 (Instrumentation Laboratory Company). The reference ranges were determined according to our laboratory data, and the tests were repeated twice, at an interval of at least 1 week. A minimum of 1 test was performed 1 month after a new VTE event.4,15 The first test for thrombophilia was performed the day after the PE diagnosis. If there was a positive result of thrombophilia testing, the test was repeated 7 days later. Patients with continuous decreased anticoagulant activity were rechecked at a 3-month follow-up.
Follow-up and outcomes
Patients were followed up after the occurrence of index PE event in the anticoagulation outpatient at FuWai Hospital. A patient-specific WeChat group was organized by clinicians from the Thrombosis and Vascular Medicine Center for rapid communication. The patient was interviewed for the first time 3 months after the diagnosis of PE, and the second follow-up was scheduled 1 year after the diagnosis of PE. Subsequently, the patient was followed up once a year. During this period, if the patient did not have an outcome event, the follow-up continued.
The main outcome was a composite of death or recurrent symptomatic PE and DVT. Major bleeding was registered to evaluate the bleeding risk of anticoagulant therapy. Recurrent symptomatic PE was defined as the development of a new intraluminal filling defect in ≥1 segmental or proximal branches through CTPA testing or the development of a new perfusion defect of ≥75% of 1 segment compared with normal baseline. Recurrent DVT was defined by an abnormal intraluminal filling defect on venography by compression ultrasound. Major bleeding was defined as life-threatening bleeding requiring transfusion of ≥2 U packed red blood cells or resulting in an absolute decrease in hematocrit of >10% or death or hemorrhagic/subdural hematoma.
Statistical analysis
Continuous variables are presented as mean ± SD and were compared using an unpaired Student t test or 1-way analysis of variance. Categorical variables were summarized by number (percentage) and compared with χ2 test or Fisher's exact test. Both univariable and multivariable logistic regression models were used to assess the predictors of having congenital thrombophilia in PE patients. The time from admission to the first occurrence of composite outcome of death, recurrent symptomatic PE, and DVT was plotted using the Kaplan-Meier method. A Cox proportional-hazards regression was used to compare the cumulative event rates of composite outcome between PE patients with and without congenital thrombophilia. A value of P < .05 was considered as statistically significant. Point and interval estimates of a variable effect together with P value from regression model analysis are presented. All analyses were performed with R 3.5.0 (https://www.r-project.org/).
Results
Patients
A total of 513 PE patients hospitalized in the Thrombosis and Vascular Medicine Center of FuWai Hospital between May 2013 and June 2018 were screened for eligibility for participating in the study. A total of 436 patients were enrolled into the study, with 77 patients excluded due to incomplete thrombophilia testing (Figure 1). Of the 436 patients enrolled, the mean (SD) age was 57.9 (15.8) years. The majority of patients were women (46.1% male, 53.9% female). A total of 277 patients (63.5%) had both DVT and PE at diagnosis, and 87 patients (20%) had a history of recurrent PE. Non–vitamin K-dependent new oral anticoagulants (NOACs) as an initial therapy were used by 80.7% of patients in our center. Of the 436 enrolled patients, 117 (26.8%) were diagnosed as PE with a risk factor and the remaining 319 (73.2%) as PE without a risk factor (Table 1). The most common predisposing factor was recent surgery or trauma, followed by immobilization, cancer, APS and other connective tissue diseases, estrogen therapy, puerperium, stroke, and congestive heart failure (supplemental Table 1).
Clinical characteristics of PE patients in the study
| Characteristics . | PE patients (N = 436) . |
|---|---|
| Age, mean ± SD, y | 57.9 ± 15.8 |
| Age at the first episode,y | |
| ≤50 | 134 (30.7) |
| >75 | 40 (9.2) |
| Male | 201 (46.1) |
| BMI, kg/m2* | |
| ≤18.5 | 13 (3.0) |
| >18.5-28 | 273 (62.6) |
| >28 | 93 (21.3) |
| With DVT | 277 (63.5) |
| Previous VTE | 87 (20.0) |
| Initial treatment† | |
| NOACs | 352 (80.7) |
| Standard VKA regimen | 81 (18.6) |
| Patients with thrombolytic therapy | 25 (5.7) |
| Characteristics . | PE patients (N = 436) . |
|---|---|
| Age, mean ± SD, y | 57.9 ± 15.8 |
| Age at the first episode,y | |
| ≤50 | 134 (30.7) |
| >75 | 40 (9.2) |
| Male | 201 (46.1) |
| BMI, kg/m2* | |
| ≤18.5 | 13 (3.0) |
| >18.5-28 | 273 (62.6) |
| >28 | 93 (21.3) |
| With DVT | 277 (63.5) |
| Previous VTE | 87 (20.0) |
| Initial treatment† | |
| NOACs | 352 (80.7) |
| Standard VKA regimen | 81 (18.6) |
| Patients with thrombolytic therapy | 25 (5.7) |
Data are presented as n (%) of patients unless otherwise indicated.
BMI, body mass index; VKA, vitamin K antagonist.
Some percentages may not total 100, because 57 patients did not have body mass index data.
Three patients did not receive anticoagulation treatment because of major bleeding in the acute phase or low platelet counts.
Prevalence and clinical characteristics
Of the 436 PE patients, 31 (7.1%) met the diagnostic criteria for congenital thrombophilia (Figure 2). The most common types of congenital thrombophilia were deficiencies in protein S (13/436 [3.0%]; 13/31 [42%]) and protein C (12/436 [2.8%]; 12/31 [39%]). These 2 subtypes accounted for >80% of congenital thrombophilia patients, followed by ATIII deficiency (5/436 [1.1%]; 5/31 [16%]). Only 1 patient (1/436 [0.2%]; 1/31 [3%]) carried factor V Leiden mutations, and none (0/436 [0%]; 0/31 [0%]) had prothrombin G20102A mutations (Figure 2).
Prevalence of 5 known congenital thrombophilia subtypes in Chinese patients with PE. (A) Prevalence of congenital thrombophilia in symptomatic PE patients. (B) Distribution of 5 known congenital thrombophilia subtypes. FVL, factor V Leiden; PT, prothrombin.
Prevalence of 5 known congenital thrombophilia subtypes in Chinese patients with PE. (A) Prevalence of congenital thrombophilia in symptomatic PE patients. (B) Distribution of 5 known congenital thrombophilia subtypes. FVL, factor V Leiden; PT, prothrombin.
When comparing the prevalence of thrombophilia between PE patients with and without risk factors, no statistically significant difference between the 2 groups was observed (7.5% vs 6.0%, P = .68). In a multivariable logistic regression analysis, variables independently associated with predicting the occurrence of congenital thrombophilia were the age of first PE episode ≤50 years (odds ratio [OR], 5.43, 95% confidence interval [CI], 2.35-13.52; P < .001) and male sex (OR, 2.67; 95% CI, 1.15-6.78; P = .03) (Table 2).
Characteristics of PE patients with congenital thrombophilia and risk factors of having congenital thrombophilia in PE
| Characteristics . | Patients with thrombophilia (n = 31), n (%) . | P . | Univariable analysis . | Multivariable analysis* . | ||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |||
| PE without risk factor | .68 | |||||
| Yes | 24/319 (7.5) | 1.28 (0.56-3.29) | .58 | 3.70 (0.14-61.82) | .36 | |
| No | 7/117 (6.0) | 1 (ref) | 1 (ref) | |||
| Age ≤50 y at first episode | <.01 | |||||
| Yes | 22/134 (16.4) | 6.39 (2.95-15.04) | <.001 | 5.43 (2.35-13.52) | <.001 | |
| No | 9/302 (3.0) | 1 (ref) | 1 (ref) | |||
| Sex | <.01 | |||||
| Male | 23/201 (11.4) | 3.67 (1.67-8.92) | .002 | 2.67 (1.15-6.78) | .03 | |
| Female | 8/235 (3.4) | 1 (ref) | 1 (ref) | |||
| With DVT | .08 | |||||
| Yes | 25/277 (9.0) | 2.53 (1.08-6.93) | .05 | 1.73 (0.66-5.13) | .29 | |
| No | 6/159 (3.8) | 1 (ref) | 1 (ref) | |||
| Cause of PE† | ||||||
| Recent surgery or trauma | 1.00 | .64 | ||||
| Yes | 2/38 (5.3) | 0.71 (0.11-2.48) | 2.32 (0.08-38.70) | .55 | ||
| No | 29/398 (7.3) | 1 (ref) | 1 (ref) | |||
| Immobilization | 1.00 | .57 | ||||
| Yes | 1/24 (4.2) | 0.55 (0.03-2.77) | 2.62 (0.09-26.63) | .46 | ||
| No | 30/412 (7.3) | 1 (ref) | 1 (ref) | |||
| Active cancer | 1.00 | NE | .99 | |||
| Yes | 0/12 (0.0) | NE | .99 | |||
| No | 31/424 (7.3) | |||||
| APS and other connective tissue disease | 1.00 | .57 | ||||
| Yes | 1/24 (4.2) | 0.55 (0.03-2.77) | 0.86 (0.02-15.15) | .92 | ||
| No | 30/412 (7.3) | 1 (ref) | 1 (ref) | |||
| Estrogen therapy and puerperium | 1.00 | NE | .99 | |||
| Yes | 0/2 (0.0) | NE | .99 | |||
| No | 31/434 (7.5) | |||||
| Stroke | 1.00 | NE | .99 | |||
| Yes | 0/4 (0.0) | NE | .99 | |||
| No | 31/432 (7.2) | |||||
| Congestive heart failure | .17 | .15 | ||||
| Yes | 3/19 (15.8) | 2.60 (0.58-8.42) | 6.96 (0.24-120.40) | .18 | ||
| No | 28/417 (6.7) | 1 (ref) | 1 (ref) | |||
| Acute coronary syndrome | 1.00 | .95 | ||||
| Yes | 1/15 (6.7) | 0.93 (0.05-4.88) | 2.84 (0.07-36.22) | .47 | ||
| No | 30/421 (7.1) | 1 (ref) | 1 (ref) | |||
| Previous VTE | .06 | |||||
| Yes | 11/87 (12.6) | 2.38 (1.06-5.10) | .03 | 1.64 (0.65-3.98) | .28 | |
| No | 20/349 (5.7) | 1 (ref) | ||||
| Thrombolytic therapy | .70 | |||||
| Yes | 2/25 (8.0) | 2.71 (0.75-7.75) | .09 | 2.31 (0.57-7.73) | .20 | |
| No | 29/411 (7.1) | 1 (ref) | 1 (ref) | |||
| Characteristics . | Patients with thrombophilia (n = 31), n (%) . | P . | Univariable analysis . | Multivariable analysis* . | ||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |||
| PE without risk factor | .68 | |||||
| Yes | 24/319 (7.5) | 1.28 (0.56-3.29) | .58 | 3.70 (0.14-61.82) | .36 | |
| No | 7/117 (6.0) | 1 (ref) | 1 (ref) | |||
| Age ≤50 y at first episode | <.01 | |||||
| Yes | 22/134 (16.4) | 6.39 (2.95-15.04) | <.001 | 5.43 (2.35-13.52) | <.001 | |
| No | 9/302 (3.0) | 1 (ref) | 1 (ref) | |||
| Sex | <.01 | |||||
| Male | 23/201 (11.4) | 3.67 (1.67-8.92) | .002 | 2.67 (1.15-6.78) | .03 | |
| Female | 8/235 (3.4) | 1 (ref) | 1 (ref) | |||
| With DVT | .08 | |||||
| Yes | 25/277 (9.0) | 2.53 (1.08-6.93) | .05 | 1.73 (0.66-5.13) | .29 | |
| No | 6/159 (3.8) | 1 (ref) | 1 (ref) | |||
| Cause of PE† | ||||||
| Recent surgery or trauma | 1.00 | .64 | ||||
| Yes | 2/38 (5.3) | 0.71 (0.11-2.48) | 2.32 (0.08-38.70) | .55 | ||
| No | 29/398 (7.3) | 1 (ref) | 1 (ref) | |||
| Immobilization | 1.00 | .57 | ||||
| Yes | 1/24 (4.2) | 0.55 (0.03-2.77) | 2.62 (0.09-26.63) | .46 | ||
| No | 30/412 (7.3) | 1 (ref) | 1 (ref) | |||
| Active cancer | 1.00 | NE | .99 | |||
| Yes | 0/12 (0.0) | NE | .99 | |||
| No | 31/424 (7.3) | |||||
| APS and other connective tissue disease | 1.00 | .57 | ||||
| Yes | 1/24 (4.2) | 0.55 (0.03-2.77) | 0.86 (0.02-15.15) | .92 | ||
| No | 30/412 (7.3) | 1 (ref) | 1 (ref) | |||
| Estrogen therapy and puerperium | 1.00 | NE | .99 | |||
| Yes | 0/2 (0.0) | NE | .99 | |||
| No | 31/434 (7.5) | |||||
| Stroke | 1.00 | NE | .99 | |||
| Yes | 0/4 (0.0) | NE | .99 | |||
| No | 31/432 (7.2) | |||||
| Congestive heart failure | .17 | .15 | ||||
| Yes | 3/19 (15.8) | 2.60 (0.58-8.42) | 6.96 (0.24-120.40) | .18 | ||
| No | 28/417 (6.7) | 1 (ref) | 1 (ref) | |||
| Acute coronary syndrome | 1.00 | .95 | ||||
| Yes | 1/15 (6.7) | 0.93 (0.05-4.88) | 2.84 (0.07-36.22) | .47 | ||
| No | 30/421 (7.1) | 1 (ref) | 1 (ref) | |||
| Previous VTE | .06 | |||||
| Yes | 11/87 (12.6) | 2.38 (1.06-5.10) | .03 | 1.64 (0.65-3.98) | .28 | |
| No | 20/349 (5.7) | 1 (ref) | ||||
| Thrombolytic therapy | .70 | |||||
| Yes | 2/25 (8.0) | 2.71 (0.75-7.75) | .09 | 2.31 (0.57-7.73) | .20 | |
| No | 29/411 (7.1) | 1 (ref) | 1 (ref) | |||
NE, not estimable; ref, reference.
Multivariable logistic regression model was used to estimate ORs of having congenital thrombophilia and 95% CIs for all factors (age at the first episode of PE, sex, DVT, PE predisposing factors, VTE history, and thrombolytic therapy).
Percentages may not total 100, because patients could have multiple causes of PE.
The thrombophilia group was younger compared with patients without thrombophilia (45.4 vs 58.9, P < .01). More than a half of patients with thrombophilia had their first PE attack at age <50 years (71.0% vs 27.7%, P < .01). Additionally, >30% of patients in the thrombophilia group had previous VTE history, which was significantly higher compared with the nonthrombophilia group (35.5% vs 18.8%, P = .04). The majority of patients with thrombophilia were male (74.2% vs 44.0%, P < .01) (supplemental Table 2).
There was no statistically significant difference in the clinical characteristics among patients with various subtype of thrombophilia except that PE patients resulting from ATIII deficiency were rarely found to have DVT (P < .01) (supplemental Table 3).
Death and recurrence
During the follow-up period, 7 patients died within 3 months after diagnosis and 16 patients were lost to follow-up; the remaining patients were followed up for ≥3 months. The median follow-up was ∼800 days (range, 11-1872 days). A total of 31 patients (7.1%) died, and 30 patients (6.8%) experienced recurrence of thrombotic events. Of the 30 patients with a recurrence event, 10 occurred during anticoagulation treatment and 20 occurred after treatment discontinuation. Four patients experienced major bleeding requiring hospitalization and alteration of anticoagulation treatment (Figure 1).
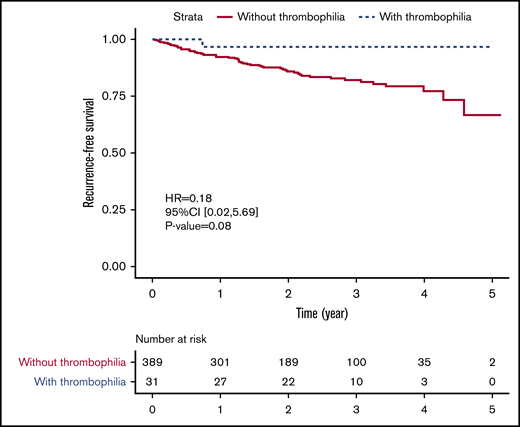
After comparing PE patients with and without thrombophilia, we found no statistically significant difference in all-cause mortality (7.7% vs 0.0%, P = .15) or recurrence rate (7.2% vs 3.2%, P = .71). For thrombophilia patients, only 1 female patient with PCD had a recurrent PE confirmed by CTPA, and no patient died during the follow-up (supplemental Table 2). Although there was no statistically significant difference, the risk of having a composite outcome of death and recurrent VTE tended to be lower among patients with congenital thrombophilia than among those without congenital thrombophilia (hazard ratio, 0.18; 95% CI, 0.02-5.69; P = .08) (Figure 3). Meanwhile, the bleeding risk was also similar between 2 groups (1.0% vs 0.0%, P = 1.00). Congenital thrombophilia patients did not suffer from a major bleeding event despite receiving extended anticoagulation therapy (supplemental Table 2).
Recurrence-free survival in PE patients with and without congenital thrombophilia.
Recurrence-free survival in PE patients with and without congenital thrombophilia.
Discussion
Thrombophilia is a condition with an abnormality in the coagulation system that increases the risk of thrombosis. Congenital thrombophilia refers to a hypercoagulable state resulting from various genetic risk factors plus environmental triggers. The prevalence of congenital thrombophilia varies widely among different ethnic groups.5 In White patients, impaired downregulation of procoagulant is most common, resulting from factor V Leiden mutations and prothrombin G20210A mutations.7,16 In Asians, a deficiency in plasma natural anticoagulants, including protein C, protein S, and ATIII, is more prevalent in patients with VTE. The reported prevalence of PSD, PCD, and ATIII deficiency in Japanese patients with DVT is 17.7%, 8.0%, and 5.6%, respectively.11,17 In Han Chinese patients with VTE, the reported prevalence of PCD, PSD, and ATIII deficiency is 10.3%, 13.9%, and 1.9%, respectively.18
In the present study, 7.1% of PE patients were diagnosed with congenital thrombophilia, including PCD, PSD, and ATIII deficiency, as well as factor V Leiden. Interestingly, PSD (3.0%) is the most frequent subtype of congenital thrombophilia, followed by PCD (2.8%) and ATIII deficiency (1.1%). Only 1 patient (0.2%) was diagnosed having a factor V Leiden heterozygous mutation. Therefore, the prevalence of congenital thrombophilia in our center is much lower than that previously reported for the Asian VTE cohort, while the distribution of thrombophilia is similar in 2 groups. The reduction of prevalence may be attributed to the different characteristics between PE and DVT patients. In our research, PE patients with congenital thrombophilia showed a trend of comorbid DVT (80.6% vs 62.2%, P = .06), especially in the most common thrombophilia subtypes, PCD (83.3%) and PSD (100%) (supplemental Tables 2 and 3). This implied a possible explanation that patients with PCD and PSD were prone to have DVT. Other potential evidence included the prevalence of PCD and PSD being >3 times higher in VTE patients than in PE patients, while the prevalence of ATIII deficiency was similar in the 2 cohorts. Moreover, according to the factor V Leiden paradox,19-22 congenital thrombosis, such as factor V Leiden mutation, is a risk factor for DVT, but not PE. As the largest screening of PE patients with thrombosis, our study verified this conclusion by finding a low incidence of factor V Leiden and prothrombin G20210A mutations in PE patients. This result indicates that the genetic background of isolated PE is not consistent with DVT. There is irreplaceable clinical value of genetic research on isolated PE.
Many previous studies have focused on VTE patients, reporting a younger age at presentation and a higher percentage of men among patients with congenital thrombophilia.4,23 Our data are consistent with these conclusions. In our cohort, we found patients with premature PE (age <50 years) and male predominance (74.2%), and these 2 factors could influence thrombophilia independently. The prevalence of congenital thrombophilia between PE patients with and without risk factors was not significantly different in our study (6.0% vs 7.5%, P > .05). This is different from what Weingarz et al4 reported in which thrombophilia is more common among patients with VTE without risk factors (47.7% vs 57.7%, P = .001). Such a discrepancy may lie in the differences in the thrombophilia spectrum between the 2 studies. The majority of thrombophilias in the Weingarz et al study were caused by factor V Leiden mutations and elevated factor VIII, whereas plasma anticoagulant deficiency was the major cause of congenital thrombophilia. Our results indicate that patients with congenital thrombophilia are predisposed to develop PE spontaneously or PE explained by the presence of a risk factor, emphasizing the importance of thrombophilia screening.
While congenital thrombophilia does not appear to increase the risk of recurrence,24,25 the current guidelines recommend anticoagulation treatment regardless of presence or absence of congenital thrombophilia.26 The role of natural anticoagulant deficiencies in recurrence is still unclear. In the Baglin et al study,24 the recurrence rate in patients with natural anticoagulant deficiency was 17.5%, which is higher than the overall recurrence rate of 11% but did not reach statistical significance. The outcomes of patients with congenital thrombophilia in the present study were not inferior to those of patients without thrombophilia, including all-cause mortality (0.0% vs 7.7%, P = .15) and the recurrence rate (3.2% vs 7.2%, P = .71). Obviously, patients with congenital thrombophilia usually had their first PE attack at a younger age and without comorbidities, which reduced the risk of death. As for the recurrence rate, the decrease in thrombophilia patients may be associated with our anticoagulation therapy (the use of NOACs and extended treatment beyond 3 months, without increased risk of bleeding). In addition, the 16 patients who were lost to follow-up were all nonthrombophilia patients, which might have underestimated the incidence of outcome events in nonthrombophilia patients.
There are some limitations to our study. Firstly, this is a single-center study with a more selected patient population at FuWai Hospital, the national center for the diagnosis and treatment of cardiovascular diseases in China. The results from a single-center study are usually subject to selection bias. However, the patients in our study were from all over China and were enrolled consecutively, which should provide for a good representation of PE patients in China. As a cardiovascular disease–specialized hospital, PE patients with risk factors after general surgery or trauma may be less represented in FuWai Hospital, which may lead to some selective bias in the results of this study. Secondly, the present study is an exploratory observational study in which many statistical tests have been performed. As a result, there may be some false-positive results. As an open-label study, the clinicians involved in the study were aware of the diagnosis of thrombophilia. Therefore, patients with thrombophilia may receive more attention and be more inclined to report outcome events. However, the outcome of this study is a clinical hard end point, and its measurement may suffer less such reporting bias. In addition, different doctors were responsible for the diagnosis and follow-up, and the subsequent doctors may not be aware of a patient’s thrombophilia status, which may have reduced the potential reporting bias. Thirdly, we took >10 potential predictors into the multivariable analysis, but possible unobserved confounding factors cannot be ignored. Nevertheless, our findings do provide novel insight into the prevalence and underlying mechanisms of congenital thrombophilia in Chinese patients and their subsequent outcomes. This will aid in the design of a therapeutic strategy that is tailored to the world’s largest patient population.
For data sets reported in this article, e-mail the corresponding author, Zhi-Cheng Jing (jingzhicheng@vip.163.com).
Acknowledgments
The authors gratefully thank the patients and families for their involvement in this study.
This work was supported by grants from the National Key Research and Development Program of China (2016YFC0901502), the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-002, 2017-12M-3- 003, 2017-I2M-B&R-02, 2017-F16-SYS, 2017-I2M-1-004, 2017-I2M-2-001), the Capital Characteristic Clinic Project of Beijing Municipal (Z171100001017195 and Z181100001718203), and the Fundamental Research Funds for the Central Universities Peking Union Medical College (PUMC) Youth Fund (2017310006).
Authorship
Contribution: Z.-C.J. has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including any adverse effects; T.-Y.L., D.L., X.-X.Y., and Z.-C.J. contributed to study design; D.L., T.W., Y.-J.Z., Y.-P.W., J.-S.T., F.-H.P. and X.J. were responsible for patient recruitment and data collection; T.-Y.L. performed experiments; T.-Y.L. and K.S. did the data analysis and wrote the first draft of the report; Z.-C.J. and L.H. helped write and revise the manuscript; and all authors contributed to and approved the final report.
Conflict-of-interest disclosure: Z.-C.J. received a travel sponsorship from GlaxoSmithKline for the ISTH 2019 Congress. T.-Y.L. received a sponsorship for an ISTH 2019 Congress poster board from Bayer. The remaining authors declare no competing financial interests.
Correspondence: Zhi-Cheng Jing, Department of Cardiology–Key Laboratory of Pulmonary Vascular Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences–Peking Union Medical College, No.1, Shuai-Fu-Yuan, Dong-Cheng District, Beijing 100730, China; e-mail: jingzhicheng@vip.163.com.
References
Author notes
T.-Y.L. and D.L. contributed equally to this study.
The full-text version of this article contains a data supplement.