Key Points
IBL-202 is highly synergistic with venetoclax via a proapoptotic shift in the balance of proteins of the Bcl-2 family.
IBL-202 and venetoclax in combination are highly effective against TP53-deficient CLL cells.
Abstract
The B-cell receptor signaling pathway and dysregulation of the Bcl-2 family of proteins play crucial roles in the pathogenesis of chronic lymphocytic leukemia (CLL). Despite significant advances in the treatment of the disease, relapse and drug resistance are not uncommon. In the current study, we investigated the dual PI3/PIM kinase inhibitor IBL-202 in combination with venetoclax as a treatment option for CLL using both primary CLL cells and TP53-deficient OSU-CLL cells generated using the CRISPR-Cas9 system. IBL-202 and venetoclax were highly synergistic against primary CLL cells cocultured with CD40L fibroblasts (combination index [CI], 0.4, at a fractional effect of 0.9) and TP53-knockout (KO) OSU-CLL cells (CI, 0.5, at a fractional effect of 0.9). Synergy between the drugs was consistent, with a significant (P < .05) reduction in the 50% inhibitory concentration for both drugs. IBL-202 and venetoclax in combination induced cell-cycle arrest and slowed the proliferation of both wild-type and TP53-KO cell lines. The drug combination inhibited AKT phosphorylation, reduced expression of Bcl-xL and NF-κB, and increased the Noxa/Mcl-1 ratio. Downregulation of CXCR4 was consistent with inhibition of the SDF-1α–induced migratory capacity of CLL cells. Synergy between IBL-202 and venetoclax against primary CLL cells cultured under conditions that mimic the tumor microenvironment suggests this drug combination may be effective against CLL cells within the lymph nodes and bone marrow. Furthermore, the efficacy of the combination against the TP53-KO OSU-CLL cell line suggests the combination may be a highly effective treatment strategy for high-risk CLL.
Introduction
The interaction of chronic lymphocytic leukemia (CLL) cells with the cells that comprise the bone marrow and lymph node microenvironments and subsequent activation of B-cell receptor (BCR)–mediated signaling plays a crucial role in the pathogenesis of the disease (as reviewed by Chiorazzi et al1 ). Several key proteins mediate the effects of BCR activation, including Bruton tyrosine kinase (BTK), phosphoinositol-3 kinase (PI3K), and mitogen-activated protein kinase (MAPK). These pathways prevent apoptosis both in vivo and under certain in vitro culture conditions.2,3
The class I PI3Ks catalyze the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to PIP3 after activation of receptor tyrosine kinases, G-protein–coupled receptors, or BCR via tyrosine protein kinase.4-6 Although the α and β isoforms of PI3Ks are expressed in most cell types, the δ and γ isoforms are primarily found in leukocytes and play crucial roles in B-cell survival7 and immune response.8 PI3K in turn activates signaling and promotes cell survival via protein kinase B (AKT), mammalian target of rapamycin, nuclear factor κB (NF-κB), glycogen synthase kinase 3, and forkhead box O.9
Trials of ibrutinib and idelalisib, which target BTK and the δ isoform of PI3K, respectively, have revolutionized the treatment of CLL.10,11 Inhibition of BTK and PI3K inhibits the chemotaxis and adhesion of CLL cells, resulting in the liberation of the leukemic cells from sanctuary sites.12-14 Although idelalisib was approved for the treatment of CLL, its clinical development was largely halted because of frequent transaminitis, colitis, and pneumonitis,15,16 whereas clinical trials of ibrutinib are ongoing17 and have shown high overall response rates, even for relapsed and refractory disease.18,19 However, mutations in BTK or PLCγ2 are associated with relapse and the evolution of ibrutinib-resistant disease.20
Clinical trials of the Bcl-2 inhibitor venetoclax have also shown promising results against several hematological malignancies, including CLL,17,21,22 resulting in its approval as a treatment option for CLL in many countries. Studies have shown that venetoclax is effective against aggressive disease associated with TP53 lesions23,24 and ibrutinib resistance.17 Despite remarkable response rates, a significant proportion of patients either do not respond or relapse after treatment with venetoclax.
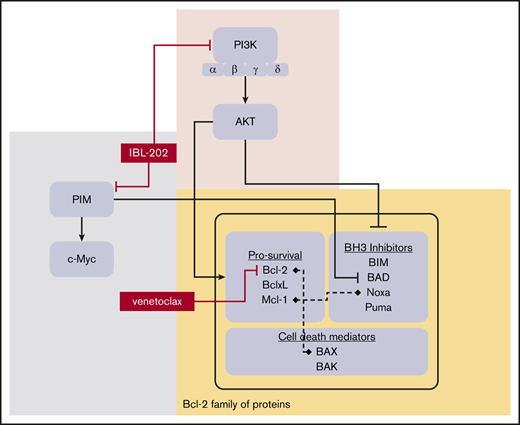
The Bcl-2 family of proteins comprises antiapoptotic proteins, including Bcl-2, Mcl-1, and Bcl-xL, and proapoptotic proteins, including Bax, Noxa, and Bad. Although CLL cell survival is dependent on Bcl-2 overexpression,25 there is increasing evidence that other members of the Bcl-2 protein family also play significant roles in CLL cells. This is highlighted by recent studies that suggest resistance to venetoclax may be conferred by changes in expression of other members of the Bcl-2 family, such as Mcl-1 and Bcl-xL.26 However, therapies targeting multiple Bcl-2 family proteins may be problematic, as shown by clinical trials of navitoclax, a predecessor to venetoclax, which was associated with dose-limiting thrombocytopenia as a result of Bcl-xL inhibition.27,28
We previously showed that inhibition of MEK1/2 sensitizes CLL cells to venetoclax and ABT-737 through downregulation of Mcl-1,29 and Cervantes-Gomez et al30 reported the synergy of the BH3 mimetics with AZD1208, SGI-1776, and SMI-4a, which inhibit the proviral integration site for Moloney murine leukemia virus (PIM) family of kinases. The PIM kinases are regulated by the JAK-STAT pathway and are involved in the regulation of several oncogenes, including MYC and BCL2.31-33 Studies have also shown that the PIM kinases regulate cell survival by blocking the proapoptotic functions of the Bcl-2 family protein Bad34,35 and that PIM kinase inhibition restricts prosurvival effects of the CLL tumor microenvironment (TME).33 In our recent study of the dual pan-PIM and pan-PI3K inhibitor IBL-202 (Inflection Biosciences, Ltd), we showed that the drug has significant activity against CLL cells cultured under conditions that mimic the TME through modulation of the Noxa/Mcl-1 ratio.36
The effects of IBL-202 on the Noxa/Mcl-1 ratio in CLL cells we demonstrated in our previous study,36 the importance of the PIM kinases in CLL cell survival,33,37,38 and the potential role of other Bcl-2 family proteins in resistance to venetoclax39-41 provided the rationale for the current study. Here, we demonstrate synergy between IBL-202 and venetoclax against primary CLL cells cultured under conditions that mimic the TME and in cells with TP53 lesions, suggesting this drug combination may be an effective treatment strategy for CLL patients with high-risk disease.
Materials and methods
CLL patient samples
All CLL samples were obtained from patients managed at the Royal North Shore Hospital (St Leonards, NSW, Australia) after written consent. CLL was diagnosed according to the international workshop on CLL guidelines.42 Peripheral blood mononuclear cells (PBMCs) were isolated from whole-blood samples by Ficoll density centrifugation. The isolated PBMC fraction was composed of >85% CD5+/CD19+ CLL cells, determined by flow cytometry. All samples were cryopreserved in fetal calf serum containing 10% dimethylsulphoxide. ZAP-70 and CD38 levels and ATM/TP53 functional categorization were assessed as previously described43-45 (Table 1).
CLL patient samples
| CLL patient . | ZAP-70* . | CD38* . | ATM/TP53 function . | 17p−† . | 11q− . | Treatment history . |
|---|---|---|---|---|---|---|
| 1 | 72.1 | 57.7 | ND | +/− (11) | +/+ | FCR |
| 2 | 12.1 | 2.43 | N | +/+ | +/+ | NT |
| 3 | 4.26 | 1.49 | N | +/+ | +/+ | NT |
| 4 | 12.1 | 2.43 | N | +/+ | +/+ | NT |
| 5 | 8.5 | 0.1 | N | +/+ | +/+ | FCR |
| 6 | 77.4 | 2.9 | 3 | +/− (15) | +/+ | FCR |
| 7 | 4.36 | 2.22 | N | +/+ | +/+ | FCR |
| 8 | 17.8 | 57.7 | N/2 | +/+ | +/+ | CLB |
| 9 | 37.3 | 89.6 | 1 | +/− (88) | +/+ | Multiple |
| 10 | 43.7 | 53.1 | N | +/− (62) | +/+ | FCR |
| 11 | 26.1 | 57.7 | N | +/+ | +/+ | CLB, FCR |
| 12 | 66.8 | 84.2 | N | +/− (12) | +/+ | NT |
| 13 | 16.2 | 14.6 | N | +/− (23) | +/+ | Multiple |
| 14 | 3.3 | 0 | 1 | +/+ | +/+ | ND |
| 15 | 3.3 | 17.9 | 3 | ND | ND | NT |
| 16 | ND | 5.38 | 1 | ND | ND | NT |
| 17 | 19.1 | 0.3 | N | ND | ND | ND |
| 18 | 2.84 | 4.26 | N | +/+ | +/+ | FCR |
| 19 | 1.64 | 0.2 | N | +/+ | +/+ | RCVP/CHOP |
| 20 | 62 | 35.6 | ND | +/+ | +/+ | FCR |
| CLL patient . | ZAP-70* . | CD38* . | ATM/TP53 function . | 17p−† . | 11q− . | Treatment history . |
|---|---|---|---|---|---|---|
| 1 | 72.1 | 57.7 | ND | +/− (11) | +/+ | FCR |
| 2 | 12.1 | 2.43 | N | +/+ | +/+ | NT |
| 3 | 4.26 | 1.49 | N | +/+ | +/+ | NT |
| 4 | 12.1 | 2.43 | N | +/+ | +/+ | NT |
| 5 | 8.5 | 0.1 | N | +/+ | +/+ | FCR |
| 6 | 77.4 | 2.9 | 3 | +/− (15) | +/+ | FCR |
| 7 | 4.36 | 2.22 | N | +/+ | +/+ | FCR |
| 8 | 17.8 | 57.7 | N/2 | +/+ | +/+ | CLB |
| 9 | 37.3 | 89.6 | 1 | +/− (88) | +/+ | Multiple |
| 10 | 43.7 | 53.1 | N | +/− (62) | +/+ | FCR |
| 11 | 26.1 | 57.7 | N | +/+ | +/+ | CLB, FCR |
| 12 | 66.8 | 84.2 | N | +/− (12) | +/+ | NT |
| 13 | 16.2 | 14.6 | N | +/− (23) | +/+ | Multiple |
| 14 | 3.3 | 0 | 1 | +/+ | +/+ | ND |
| 15 | 3.3 | 17.9 | 3 | ND | ND | NT |
| 16 | ND | 5.38 | 1 | ND | ND | NT |
| 17 | 19.1 | 0.3 | N | ND | ND | ND |
| 18 | 2.84 | 4.26 | N | +/+ | +/+ | FCR |
| 19 | 1.64 | 0.2 | N | +/+ | +/+ | RCVP/CHOP |
| 20 | 62 | 35.6 | ND | +/+ | +/+ | FCR |
1, TP53 mutated; 2, ATM mutated; 3, evidence of low-level TP53 dysfunction; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CLB, chlorambucil; FCR, fludarabine, cyclophosphamide, and rituximab; N, normal ATM/TP53; ND, no data; NT, no previous treatment; RCVP, rituximab, cyclophosphamide, vincristine, and prednisolone.
Cutoffs for ZAP-70 and CD38 positivity were 10% and 7%, respectively.
Clone sizes for deletions of 17p are shown in parentheses.
Where indicated, primary CLL cells were seeded onto confluent monolayers of mouse L-fibroblasts expressing the human CD40 ligand or HS-5 human bone marrow stromal cells. CLL cells were harvested by gently pipetting them away from the fibroblast layer.
Generation of CRISPR-Cas9 TP53 KO OSU-CLL cell line
The OSU-CLL cell line was derived as previously described46 and obtained from the Human Genetics Sample Bank at Ohio State University under a material transfer agreement. OSU-CLL cell lines were maintained in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal calf serum, 2 mM of L-glutamine, and 1% penicillin/streptomycin. The TP53-knockout (KO) OSU-CLL (OSU-CLL-TP53ko) cell line was generated using a lentiviral CRISPR-Cas9 technique developed at the Walter and Eliza Hall Institute (Melbourne, Australia). The methods employed to generate the OSU-CLL-TP53ko line were as described by Aubrey et al.47 KO of the TP53 gene and loss of protein expression were confirmed by direct sequencing and immunoblotting (supplemental Figure 1E).
Assessment of cytotoxicity and synergy
Cell viability was assessed using the mitochondrial membrane potential dye 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide (DilC1[5]; 50 nM; Thermo Fisher Scientific) and propidium iodide (PI; 10 µg/mL; Sigma-Aldrich, St. Louis, MO) after 48 hours of treatment, with analysis by flow cytometry on an LSR Fortessa (Becton Dickinson, Franklin Lakes, NJ). Contaminating fibroblasts were excluded from the analysis based on their distinct forward/side-scatter properties.
The cytotoxic effects of IBL-202 and venetoclax against CLL cells and autologous T cells were compared by treating PBMC fractions from CLL patients with the indicated doses of the drugs for 24 hours. After treatment, the proportions of viable CLL (CD19+/CD5+) and T cells (CD19−/CD5+) were assessed by flow cytometry using fluorochrome conjugated antibodies (Biolegend, San Diego, CA) and DiIC1(5) and PI, as described earlier.
The 50% inhibitory concentration (IC50) values for IBL-202 and venetoclax were calculated using BioDataFit. For analysis of synergy, IBL-202 and venetoclax were combined at a fixed ratio determined by the IC50 values of the 2 drugs as single agents. Combination indices (CIs) and isobolograms for assessment of synergy were calculated and constructed using Compusyn (ComboSyn, Inc., Paramus, NJ) according to the method of Chou and Talalay.48
Proliferation and cell-cycle analysis
Proliferation of the OSU-CLL and OSU-CLL-TP53ko cells was assessed using carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich) and flow cytometry. Cells were stained with CFSE (2 μM) for 20 minutes at 37°C and cultured overnight before treatment with IBL-202 or venetoclax, alone or in combination, at a density of 5000 cells per mL. Doses were based on the IC50s of the drugs when in combination after a 48-hour treatment. Proliferation was determined by assessing the decay in the mean fluorescence intensity of CFSE over 72 hours, relative to time 0. Apoptotic and dead cells were excluded from the analysis based on their forward/side-scatter properties.
The effects of the drug treatments on the cell-cycle distribution of the OSU-CLL and OSU-CLL-TP53ko lines were assessed using PI and flow cytometry. After a 72-hour incubation, cells were washed in phosphate-buffered saline, resuspended in 50% ethanol, and stored at −20°C for 24 hours. Samples were then stained in 0.02 mg/mL of PI, 10 mg/mL of RNase, and 0.1% Triton in phosphate-buffered saline at 37°C for 30 minutes. Data were acquired by flow cytometry and analyzed using ModFit (Verity Software House, Topsham, ME).
Western blotting
Primary CLL cells (1.5 × 106 per condition) in coculture with CD40L-expressing fibroblasts were treated for 6 hours with concentrations of IBL-202 (1 μM) and/or venetoclax (10 nM), which in combination resulted in 95% cell killing at 48 hours. Methods employed for western blotting and densitometric measurements were as described in our previous study.49 The antibodies used for western blotting and flow cytometry in this study are detailed in supplemental Table 1.
Immunophenotypic changes and cell migration
Primary CLL cells were cultured in medium for 20 hours before treatment with IBL-202 (1 µM), venetoclax (10 nM), or the drugs in combination for 6 hours. Cells were stained with phycoerythrin-conjugated antibodies against CD49d or CXCR4 (Biolegend), with analysis by flow cytometry. The viable CLL cell population was quantified using DiIC1(5)/PI as described. The CD49d antibody used recognizes the open conformation of the CD49d molecule and is the same clone (9F10) as used in the studies by Shanafelt et al50 and Bulian et al,51 which demonstrated the prognostic significance of CD49d expression in CLL.
The migratory capacity of primary CLL cells toward stroma-derived factor-1α (SDF-1α) was assessed using the method described in our previous study.52 CLL cell viability was assessed by flow cytometry using DilC1(5)/PI before initiation of each assay. An equal number of viable CLL cells from each culture condition were then loaded into the upper chambers of the Transwell culture inserts (Merck Millipore, Burlington, MA). Cells were allowed to migrate toward medium containing 200 ng of recombinant human SDF-1α (Peprotech, Rocky Hill, NJ) for 3 hours. Viable (DilC1[5]+/PI−) cells were quantified from 120 s of data acquisition on an LSR Fortessa flow cytometer (Becton Dickinson).
Statistical analyses
Statistical analyses were conducted using the Student t test function of Microsoft Excel, with P < .05 considered significant.
Results
IBL-202 and venetoclax (ABT-199) are synergistic against CLL cells
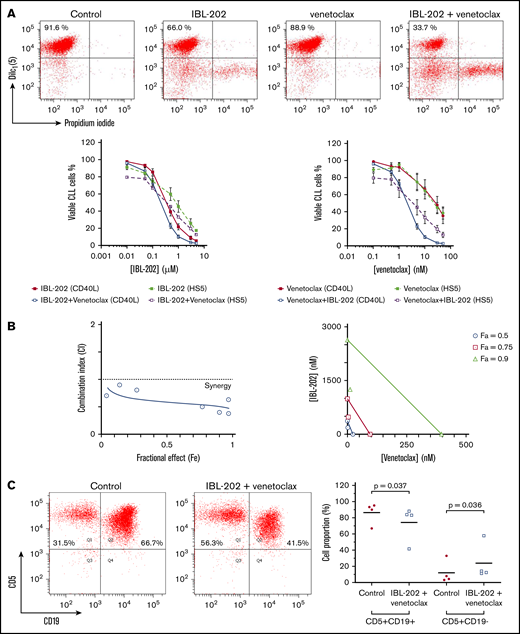
PBMCs from CLL patients were cocultured with CD40L-expressing fibroblasts (n = 10) or HS-5 stromal cells (n = 6) and treated with IBL-202 or venetoclax as single agents or in combination for 48 hours. Viability in the dose-response analyses (Figure 1A) is expressed relative to vehicle-treated control cultures. The mean viability of the CLL cells in the control cultures was 78.2% ± 27.5% at 48 hours. In our previous study,36 the mean viability of CLL cells cultured in the absence of stromal cells was <40% at 48 hours. In CLL cells cocultured with CD40L− fibroblasts, the mean viability after treatment with 50 nM of venetoclax or 5 µM of IBL-202 alone was 33.1% ± 8.7% and 4.9% ± 0.9%, respectively. In coculture with HS-5 stromal cells, the same doses of each drug reduced the viability of the CLL cells to 10.2% ± 3.8% and 4.5% ± 0.7%, respectively.
IBL-202 and venetoclax are synergistic against CLL cells. (A) Dose responses for IBL-202 and venetoclax, alone and in combination, at a ratio of 1:100 against primary CLL cells cocultured with CD40L-expressing fibroblasts (n = 10) or HS-5 stromal cells (n = 6) as indicated. The viability of CLL cells was assessed by DilC1(5)/PI staining and flow cytometry after 48 hours of treatment. Flow cytometric data from 1 representative patient sample illustrates the viability of CLL cells cocultured with CD40L fibroblasts after treatment with IBL-202 and venetoclax at 0.5 µM and 5 nM, respectively, alone or in combination. Results are expressed relative to vehicle-treated control cultures. (B) CI plot (left) and isobologram (right) for IBL-202 and venetoclax in combination against CLL patient samples (n = 10) cocultured with CD40L-expressing fibroblasts illustrates synergy between the 2 drugs. Data are presented as the CI over a range of fractional effects, where a fractional effect value of 0.5, for example, is indicative of 50% cell kill. (C) The proportion of viable CLL and T cells in mixed PBMC fractions from 4 CLL patients before and after drug treatment were determined by flow cytometry using antibodies to CD19 and CD5. PBMC fractions from CLL patients were treated with IBL-202 (1 μM) or venetoclax (10 nM) or the combination for 24 hours in medium. Representative plots from 1 patient are shown.
IBL-202 and venetoclax are synergistic against CLL cells. (A) Dose responses for IBL-202 and venetoclax, alone and in combination, at a ratio of 1:100 against primary CLL cells cocultured with CD40L-expressing fibroblasts (n = 10) or HS-5 stromal cells (n = 6) as indicated. The viability of CLL cells was assessed by DilC1(5)/PI staining and flow cytometry after 48 hours of treatment. Flow cytometric data from 1 representative patient sample illustrates the viability of CLL cells cocultured with CD40L fibroblasts after treatment with IBL-202 and venetoclax at 0.5 µM and 5 nM, respectively, alone or in combination. Results are expressed relative to vehicle-treated control cultures. (B) CI plot (left) and isobologram (right) for IBL-202 and venetoclax in combination against CLL patient samples (n = 10) cocultured with CD40L-expressing fibroblasts illustrates synergy between the 2 drugs. Data are presented as the CI over a range of fractional effects, where a fractional effect value of 0.5, for example, is indicative of 50% cell kill. (C) The proportion of viable CLL and T cells in mixed PBMC fractions from 4 CLL patients before and after drug treatment were determined by flow cytometry using antibodies to CD19 and CD5. PBMC fractions from CLL patients were treated with IBL-202 (1 μM) or venetoclax (10 nM) or the combination for 24 hours in medium. Representative plots from 1 patient are shown.
Combining IBL-202 and venetoclax resulted in a significant reduction in the IC50 values for IBL-202 (P = .013) and venetoclax (P = .045; Table 2). We observed no significant difference (P = .44) in the sensitivity of CLL patient samples with or without TP53 aberrations to IBL-202 in combination with venetoclax (n = 5; supplemental Figure 1A). Because data concerning the IGHV mutational status of the patient samples were not available, we assessed ZAP-70 expression using an assay that demonstrates a reasonable concordance with IGHV status.45 No difference (P = .87) in the sensitivity of samples from ZAP-70+ (n = 6) and ZAP-70− patients (n = 4) to IBL-202 and venetoclax was observed (supplemental Figure 1A).
IC50 values of IBL-202 and venetoclax
| . | IC50 . | P* . | |
|---|---|---|---|
| Alone . | In combination . | ||
| Primary CLL cells + CD40L fibroblasts | |||
| IBL-202 | 0.47 ± 0.32 µM | 0.24 ± 0.03 µM | .01 |
| Venetoclax | 30.2 ± 25.4 nM | 2.40 ± 0.30 nM | <.01 |
| Primary CLL cells + HS-5 fibroblasts | |||
| IBL-202 | 0.94 ± 0.24 µM | 0.53 ± 0.18 µM | <.01 |
| Venetoclax | 36.37 ± 8.34 nM | 5.30 ± 1.80 nM | <.01 |
| OSU-CLL cells | |||
| IBL-202 | 0.61 ± 0.05 µM | 0.07 ± 0.01 µM | .01 |
| Venetoclax | 295 ± 108 nM | 14.3 ± 1.20 nM | <.01 |
| OSU-CLL-TP53ko cells | |||
| IBL-202 | 1.89 ± 0.16 μM | 0.06 ± 0.01 µM | <.01 |
| Venetoclax | 18.5 ± 2.26 μM | 0.63 ± 0.10 µM | .01 |
| . | IC50 . | P* . | |
|---|---|---|---|
| Alone . | In combination . | ||
| Primary CLL cells + CD40L fibroblasts | |||
| IBL-202 | 0.47 ± 0.32 µM | 0.24 ± 0.03 µM | .01 |
| Venetoclax | 30.2 ± 25.4 nM | 2.40 ± 0.30 nM | <.01 |
| Primary CLL cells + HS-5 fibroblasts | |||
| IBL-202 | 0.94 ± 0.24 µM | 0.53 ± 0.18 µM | <.01 |
| Venetoclax | 36.37 ± 8.34 nM | 5.30 ± 1.80 nM | <.01 |
| OSU-CLL cells | |||
| IBL-202 | 0.61 ± 0.05 µM | 0.07 ± 0.01 µM | .01 |
| Venetoclax | 295 ± 108 nM | 14.3 ± 1.20 nM | <.01 |
| OSU-CLL-TP53ko cells | |||
| IBL-202 | 1.89 ± 0.16 μM | 0.06 ± 0.01 µM | <.01 |
| Venetoclax | 18.5 ± 2.26 μM | 0.63 ± 0.10 µM | .01 |
IC50 values for IBL-202 and venetoclax, alone and in combination, against primary CLL cells and OSU-CLL cell lines were calculated from the dose-response analyses.
P values shown indicate that in each of the culture conditions and in the 2 OSU-CLL lines, combining the drugs resulted in a significant decrease in the IC50 value for each drug compared with the IC50 values for the drugs as single agents. P values were calculated using the t test function of GraphPad Prism software.
CIs for IBL-202 and venetoclax against CLL cells cocultured with CD40L-expressing fibroblasts (Figure 1B left) or HS-5 bone marrow stromal cells (supplemental Figure 1B) were <1 at all fractional effect levels, which is suggestive of synergy between the drugs under both coculture conditions. Analysis of the data by isobologram (Figure 1B right) illustrates the significant degree to which combining the drugs (open symbols) reduces the dose of each drug (filled symbols) necessary to induce apoptosis in 50%, 75%, and 90% of the CLL cells.
IBL-202 and venetoclax in combination preferentially induce apoptosis in CLL cells
We investigated the specificity of IBL-202 and venetoclax for CLL cells by measuring the relative proportion of viable CLL (CD19+/CD5+) and T cells (CD19−/CD5+) in mixed PBMC fractions from 4 CLL patients (Figure 1C). IBL-202 and venetoclax, alone and in combination, significantly decreased the proportion of CLL cells, whereas the proportion of T cells significantly increased (P = .04). These data suggest that IBL-202 and venetoclax, as single agents and in combination, preferentially induce apoptosis of CLL cells rather than T cells in PBMC fractions from CLL patients.
IBL-202 and venetoclax in combination downregulate PI3K/AKT and PIM signaling
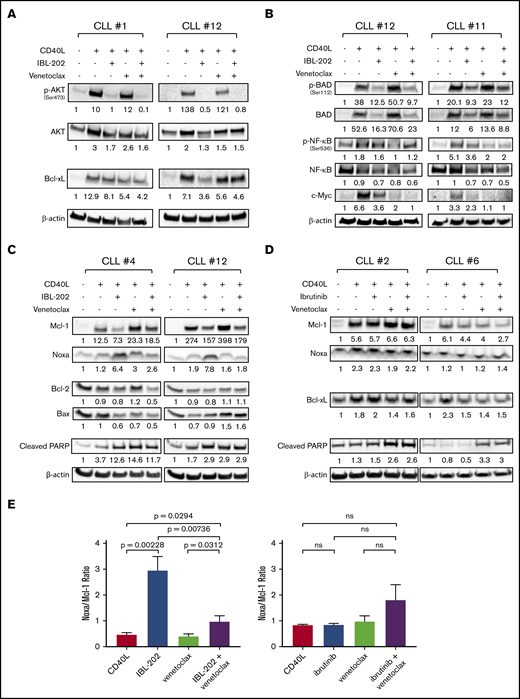
To investigate the mechanisms of the synergy between IBL-202 and venetoclax, we first examined changes in expression and phosphorylation of proteins of the PI3K/AKT and PIM pathways. CD40L coculture increased the levels of phosphorylated AKT (n = 8; Figure 2A), NF-κB, and Bad (n = 6; Figure 2B) and expression of total AKT and c-Myc in CLL cells compared with medium controls, indicating activation of signaling downstream of BCR. Consistent with our previous study,36 IBL-202, as a single agent and in combination with venetoclax, significantly reduced the phosphorylation of AKT, Bad, and NF-κB relative to untreated CLL cells (P < .05). Venetoclax alone reduced the phosphorylation of NF-κB in 5 of the 6 samples, but increased levels of Bad phosphorylation in 4 of the 6 samples. IBL-202 and venetoclax as single agents and in combination reduced the level of c-Myc in all 6 CLL samples assessed (P < .05). IBL-202 as a single agent or in combination with venetoclax reduced the expression of Bcl-xL relative to levels in untreated cells in 7 of the 8 samples (Figure 2A).
IBL-202 and venetoclax inhibit PI3K signaling and downregulate expression of Bcl-2 family proteins. Expression of phosphorylated and total AKT and Bcl-xL (n = 8) (A) and phosphorylated and total Bad and NF-κB and c-Myc (n = 6) (B) was assessed by immunoblotting in primary CLL cells cultured in medium alone or cocultured with CD40L fibroblasts. CLL cells in coculture with fibroblasts were treated with IBL-202 (1 μM) or venetoclax (10 nM), alone or in combination. Levels of Mcl-1, Noxa, Bax, Bcl-2, Bcl-xL, and PARP were assessed by immunoblotting of primary CLL cells treated with IBL-202 (1 μM) and venetoclax (10 nM), alone or in combination (n = 6) (C), and ibrutinib (0.5 μM) or venetoclax (10 nM), alone or in combination (n = 3) (D). Representative data from 2 patient samples are shown in panels A-D. The values shown under each lane indicate the fold change in expression relative to an untreated control for each patient sample. (E) Histograms show the cumulative data of the Noxa/Mcl-1 expression ratio in CLL cells treated with IBL-202 (n = 6) or ibrutinib (n = 3), alone or in combination with venetoclax. ns, not significant.
IBL-202 and venetoclax inhibit PI3K signaling and downregulate expression of Bcl-2 family proteins. Expression of phosphorylated and total AKT and Bcl-xL (n = 8) (A) and phosphorylated and total Bad and NF-κB and c-Myc (n = 6) (B) was assessed by immunoblotting in primary CLL cells cultured in medium alone or cocultured with CD40L fibroblasts. CLL cells in coculture with fibroblasts were treated with IBL-202 (1 μM) or venetoclax (10 nM), alone or in combination. Levels of Mcl-1, Noxa, Bax, Bcl-2, Bcl-xL, and PARP were assessed by immunoblotting of primary CLL cells treated with IBL-202 (1 μM) and venetoclax (10 nM), alone or in combination (n = 6) (C), and ibrutinib (0.5 μM) or venetoclax (10 nM), alone or in combination (n = 3) (D). Representative data from 2 patient samples are shown in panels A-D. The values shown under each lane indicate the fold change in expression relative to an untreated control for each patient sample. (E) Histograms show the cumulative data of the Noxa/Mcl-1 expression ratio in CLL cells treated with IBL-202 (n = 6) or ibrutinib (n = 3), alone or in combination with venetoclax. ns, not significant.
Synergy between IBL-202 and venetoclax is consistent with increase in Noxa/Mcl-1 ratio
We then investigated the effects of the drugs on the expression of other proteins of the Bcl-2 family. The anti-/proapoptotic balance in Bcl-2 family protein levels was assessed by examining the Noxa/Mcl-1 and Bax/Bcl-2 ratios. As we previously demonstrated,36 IBL-202 induced a significant (P = .002; 6.4-fold) increase in the Noxa/Mcl-1 ratio (Figure 2C,E left) but had no effect on the Bax/Bcl-2 ratio (supplemental Figure 1C). Venetoclax alone had no significant effect on either the Noxa/Mcl-1 or Bax/Bcl-2 ratio compared with controls; however, IBL-202 and venetoclax in combination attenuated the increase in the Noxa/Mcl-1 ratio induced by IBL-202. Despite this attenuation, we observed a significant (P = .03; 2.14-fold) increase in the Noxa/Mcl-1 ratio in CLL cells treated with IBL-202 in combination with venetoclax, compared with levels in CD40L fibroblast controls. No significant effect of IBL-202 in combination with venetoclax was observed in the Bax/Bcl-2 ratio (supplemental Figure 1C).
In comparison, ibrutinib, at a clinically achievable dose (0.5 µM)12 as a single agent or in combination with venetoclax, had little or no effect on the expression of Bcl-xL and did not increase the levels of cleaved PARP (Figure 2D) in CLL samples (n = 3) cocultured with CD40L fibrobasts. Ibrutinib, alone and in combination with venetoclax, decreased Mcl-1 in 2 of the 3 samples but, unlike IBL-202, had no effect on expression of Noxa and consequently had no significant effect on the Noxa/Mcl-1 ratio, alone or in combination with venetoclax (Figure 2D-E).
IBL-202 and venetoclax are synergistic in their cytotoxic effects against TP53-KO OSU-CLL cells
To investigate the effects of IBL-202 in combination with venetoclax against cycling CLL cells with a TP53 aberration, we employed the OSU-CLL cell line and a derivative of this line in which TP53 was deleted using the CRISPR-Cas9 technology (OSU-CLL-TP53ko).
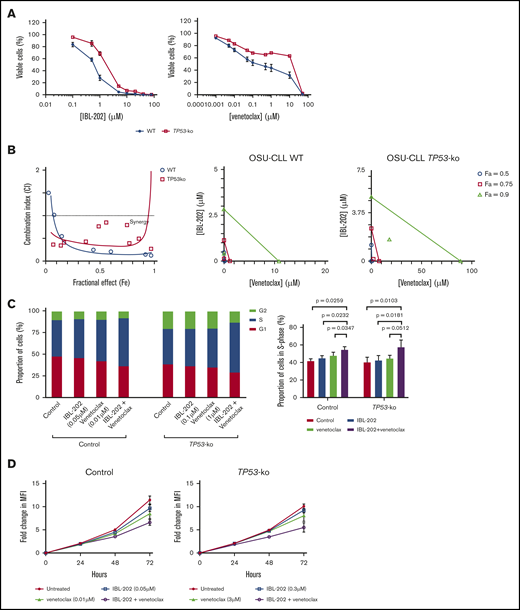
Comparison of the IC50 values (Table 2) suggests TP53 KO significantly (P < .05) reduced the sensitivity of OSU-CLL cells to both IBL-202 and venetoclax. CI values of <1 were observed irrespective of the fraction of cells affected in both the OSU-CLL and OSU-CLL-TP53ko lines (Figure 3B), indicative of synergy between IBL-202 and ventoclax against both lines.
IBL-202 and venetoclax are synergistic against OSU-CLL-TP53ko cells. (A) Dose responses for IBL-202 or venetoclax as single agents toward wild-type (WT) OSU-CLL and OSU-CLL-TP53ko cells. (B) CI plot and isobolograms for IBL-202 and venetoclax in combination against WT and OSU-CLL-TP53ko cells. CI values <1 indicate synergy. (C) Cell-cycle analyses performed on control and OSU-CLL-TP53ko cells after treatment for 72 hours with the indicated dose of each drug. (D) Proliferation analysis of control and OSU-CLL-TP53ko cells treated with IBL-202 or venetoclax alone or in combination over a 72-hour time course. MFI, mean fluorescence intensity.
IBL-202 and venetoclax are synergistic against OSU-CLL-TP53ko cells. (A) Dose responses for IBL-202 or venetoclax as single agents toward wild-type (WT) OSU-CLL and OSU-CLL-TP53ko cells. (B) CI plot and isobolograms for IBL-202 and venetoclax in combination against WT and OSU-CLL-TP53ko cells. CI values <1 indicate synergy. (C) Cell-cycle analyses performed on control and OSU-CLL-TP53ko cells after treatment for 72 hours with the indicated dose of each drug. (D) Proliferation analysis of control and OSU-CLL-TP53ko cells treated with IBL-202 or venetoclax alone or in combination over a 72-hour time course. MFI, mean fluorescence intensity.
IBL-202 and venetoclax in combination reduce proliferation of OSU-CLL cells and induce S-phase cell-cycle arrest
Treatment of OSU-CLL or OSU-CLL-TP53ko cells with IBL-202 or venetoclax as single agents led to an increase in the proportion of cells in S phase, with a concomitant decrease in the proportion in G0/G1 (Figure 3C left). Treatment of both cell lines with the drugs in combination resulted in a further accumulation of cells in S phase (Figure 3C right; P < .05) relative to treatment with IBL-202. Treatment of OSU-CLL, but not OSU-CLL-TP53ko, cells with IBL-202 in combination with venetoclax resulted in a significant (P = .03) accumulation of cells in S phase relative to treatment with venetoclax alone (Figure 3C right).
The effects of IBL-202 and venetoclax, alone and in combination, on the proliferation of control OSU-CLL and OSU-CLL-TP53 cells were assessed using CFSE and flow cytometry over a 72-hour time course (Table 3). At the concentrations used, neither IBL-202 nor venetoclax had any significant effect on the proliferation of control OSU-CLL cells (Figure 3D). In the OSU-CLL-TP53ko cell line, IBL-202, but not venetoclax, as a single agent reduced cell proliferation relative to untreated control cells. However, in both cell lines, combining IBL-202 and venetoclax had a significantly (P < .05) greater inhibitory effect relative to the proliferation of untreated cells.
Fold change in proliferation of OSU-CLL cell lines at 72 h, relative to 0 h
| . | Fold change in proliferation . | |
|---|---|---|
| OSU-CLL . | OCU-CLL-TP53ko . | |
| Untreated | 11.4 ± 1.52 | 10.1 ± 0.83 |
| IBL-202 | 9.70 ± 1.24 | 9.17 ± 0.83 |
| Venetoclax | 8.52 ± 1.85 | 8.05 ± 2.07 |
| IBL-202 + venetoclax | 6.60 ± 1.07 | 5.52 ± 1.76 |
| . | Fold change in proliferation . | |
|---|---|---|
| OSU-CLL . | OCU-CLL-TP53ko . | |
| Untreated | 11.4 ± 1.52 | 10.1 ± 0.83 |
| IBL-202 | 9.70 ± 1.24 | 9.17 ± 0.83 |
| Venetoclax | 8.52 ± 1.85 | 8.05 ± 2.07 |
| IBL-202 + venetoclax | 6.60 ± 1.07 | 5.52 ± 1.76 |
Effect of IBL-202 and venetoclax, alone and in combination, on the proliferation of OSU-CLL and OSU-CLL-TP53ko cell lines after treatment for 72 hours. Results are expressed as fold changes relative to time 0 hours (n = 3).
IBL-202 and venetoclax alone and in combination reduce expression of CD49d and CXCR4 and inhibit CLL cell migration
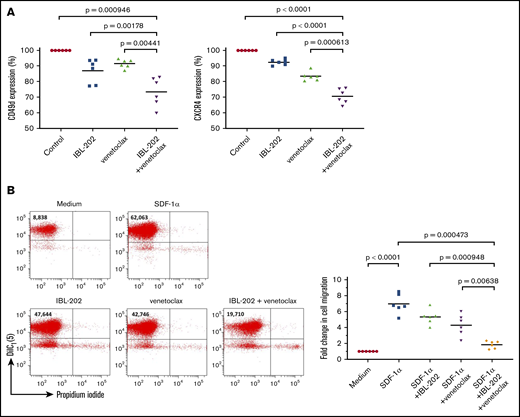
Migration of CLL cells to the lymph nodes and bone marrow and the retention of the leukemic cells in these tissues play crucial roles in the pathogenesis of the disease. The effects of IBL-202 and venetoclax on the adhesive and migratory capacities of CLL cells were assessed by examining expression of CD49d and the chemokine receptor CXCR4 and by measuring the migration of primary CLL cells. IBL-202 treatment of CLL cells from 6 patients significantly (P < .01) reduced the expression of CD49d and CXCR4 by 13.06% ± 7.68%, and 7.52% ± 1.83%, respectively. Venetoclax also had a significant (P < .001) effect, downregulating CD49d and CXCR4 levels by 8.48% ± 2.85%, and 16.6% ± 2.88%, respectively. IBL-202 and venetoclax in combination significantly (P < .001) reduced CD49d (26.57% ± 9.36%) and CXCR4 (29.43% ± 4.70%) expression relative to medium controls, and in combination, they were significantly (P < .01) more effective at downregulating expression of both proteins compared with either drug alone (Figure 4A).
IBL-202 plus venetoclax decreases expression of CD49d and CXCR4 and impairs the migratory capacity of primary CLL cells. Changes in expression of CD49d and CXCR4 (A) and the migration of primary CLL cells down an SDF-1α gradient (n = 6) (B) were determined by flow cytometry after treatment with IBL-202 (1 μM) or venetoclax (10 nM) alone or in combination. Changes in antigen expression are presented using mean fluorescence intensity (MFI) values relative to levels in untreated cells. Flow cytometric plots from 1 representative patient sample illustrating the effects of IBL-202 and venetoclax, alone and in combination, on the number of viable cells migrating across a permeable support are shown. The number shown in the top left quadrant of each plot indicates the viable cell count under each condition.
IBL-202 plus venetoclax decreases expression of CD49d and CXCR4 and impairs the migratory capacity of primary CLL cells. Changes in expression of CD49d and CXCR4 (A) and the migration of primary CLL cells down an SDF-1α gradient (n = 6) (B) were determined by flow cytometry after treatment with IBL-202 (1 μM) or venetoclax (10 nM) alone or in combination. Changes in antigen expression are presented using mean fluorescence intensity (MFI) values relative to levels in untreated cells. Flow cytometric plots from 1 representative patient sample illustrating the effects of IBL-202 and venetoclax, alone and in combination, on the number of viable cells migrating across a permeable support are shown. The number shown in the top left quadrant of each plot indicates the viable cell count under each condition.
Because CXCR4 is the receptor for the chemokine SDF-1α, we sought to determine the functional consequence of CXCR4 downregulation on the migratory capacity of CLL cells in vitro. SDF-1α induced a 6.96- ± 1.22-fold increase in the number of cells migrating through the culture insert, relative to passive migration (P < .0001). Consistent with their effects on CXCR4 levels, IBL-202 and venetoclax as single agents significantly (P < .05) reduced the number of migratory CLL cells by 1.64- ± 0.38-fold and 2.69- ± 0.54-fold respectively. Combining the drugs had a significantly (P < .01) greater effect than either drug alone, inducing a 5.13- ± 0.19-fold decrease in the number of migratory CLL cells relative to SDF-1α control cultures.
Discussion
Ibrutinib, idelalisib, and venetoclax target key components of prosurvival signaling pathways and interfere with the interaction of CLL cells with accessory cells that comprise the TME.19,53 However, toxicity16 and drug-resistant disease20 remain challenges in the clinical management of CLL. Identifying rational combinations of drugs or novel treatment strategies remains an important area of research in CLL.
Emerging evidence suggests that PI3K plays a central role in regulating multiple signaling pathways downstream of BCR, including prosurvival signaling mediated via BTK and the Bcl-2 family of proteins.26,54 We previously demonstrated that dual inhibition of PIM kinases and PI3Ks36 MEK1/2 and Bcl-229 or MEK1/2 and AKT49 is effective under in vitro conditions mimicking the TME. Given the results of a recent clinical trial demonstrating activity of venetoclax in CLL patients with deletions of TP5323 and our data on the in vitro efficacy of IBL-202,36 we investigated the possibility of combining IBL-202 with venetoclax for high-risk CLL disease.
We demonstrated strong synergy between IBL-202 and venetoclax against primary CLL and OSU-CLL cells, irrespective of TP53 aberrations, evident under in vitro conditions that mimic the TME. We know from the literature,55 data in the current study (Figure 2A-C), and previous studies36 that coculturing CLL cells with CD40L fibroblasts stimulates signaling via many of the same pathways downstream of BCR and upregulates the expression of prosurvival members of the Bcl-2 protein family. Furthermore, the interaction of CLL cells with stromal cells and the upregulation of Bcl-2 family proteins significantly reduce the sensitivity of CLL cells to BH3 mimetics.41
The mechanisms of the synergy between IBL-202 and venetoclax include attenuation of AKT, BAD, and NF-kB phosphorylation, downregulation of Bcl-xL levels, and increase in the Noxa/Mcl-1 ratio (Figure 2). Expression of Mcl-1 and Bcl-xL is known to reduce the sensitivity of CLL cells to venetoclax,26 and in certain cancers, venetoclax may even potentiate the prosurvival functions of Mcl-1 through inhibition of its binding partner Noxa.56 In the current study, we also observed an attenuation of Noxa expression in CLL cells treated with venetoclax, which we suggest may have significant implications for any treatment strategy in which venetoclax is combined with a drug that acts by inhibiting Mcl-1. The data presented here (Figure 2) and in our previous study36 suggest that IBL-202 significantly increases the Noxa/Mcl-1 ratio and at least partially mitigates the inhibitory effects of venetoclax on Noxa. In light of these observations, the effects of IBL-202 on Bcl-xL may be particularly important in mediating the synergy between the 2 drugs, supporting the assertion that Bcl-xL level is an important determinant of sensitivity to venetoclax.57
Synergy between PI3K inhibitors and venetoclax,26,58 PIM kinase inhibitors and ABT-737,59 and idelalisib and the PIM inhibitor pPIMi36 supports the rationale for investigating IBL-202 in combination with venetoclax. Crosstalk and overlap between the PI3K and PIM kinase pathways means it is difficult to delineate the effects of IBL-202 on the 2 pathways. Although the effects of IBL-202 on AKT phosphorylation are likely due to inhibition of the PI3Ks, evidence from several studies in other cancers34,60-64 suggests that inhibition of the PIM kinases may also reduce Mcl-1 levels and NF-κB and BAD phosphorylation. Furthermore, the increased Noxa levels observed here (Figure 2C) and in our previous study36 could be due to inhibition of either kinase family. Because PIM-1 is essential for Myc-dependent transcription and tumorigenesis,65 and c-Myc is downregulated by PIM inhibition,63 the downregulation of c-Myc we observed here (Figure 2B) supports the notion that inhibition of the PIM kinases is crucial in the effects of IBL-202 on CLL cells and its synergy with venetoclax. Ultimately, the changes in levels of the Bcl-2 family proteins we observed represent a coordinated proapoptotic shift in the balance of these proteins. For example, the decrease in phosphorylation of BAD in response to IBL-202 enables its binding to and inhibition of Bcl-xL,64 whereas increased expression of Noxa suggests inhibition of Mcl-1 activity. Although Bcl-xL is an attractive drug target in CLL because of its role in mediating the interaction between CLL cells and stromal cells,41 studies of navitoclax, a predecessor to venetoclax, suggest that thrombocytopenia resulting from inhibition of Bcl-xL in platelets is a dose-limiting toxicity.66 It is conceivable that despite its effects on Bcl-xL, the effects of IBL-202 on Mcl-1 and NF-κB36 and its synergy with venetoclax may widen the therapeutic window within which this toxicity might be manageable.
Despite the advances in therapy, deletions or mutations of TP53 remain strongly associated with poor clinical outcomes and treatment response rates in CLL.67,68 To investigate the efficacy of combining IBL-202 and venetoclax in the context of TP53 deficiency, we studied a panel of samples from patients with TP53 lesions and generated a TP53 KO OSU-CLL cell line using CRISPR-Cas9 technology. TP53 deletion reduced the sensitivity of OSU-CLL cells to venetoclax, which seems to contradict evidence suggesting the cytotoxic effects of venetoclax are p53 independent,69 whereas the reduced sensitivity of OSU-CLL-TP53ko cells to IBL-202 is consistent with previous studies of PI3K inhibitors.70 In contrast, there was no significant difference in the sensitivity of CLL cells derived from patients with TP53 dysfunction to either IBL-202 or venetoclax. This may be related to the differences in the proliferative capacity of the OSU-CLL and primary CLL cells; peripheral blood–derived CLL cells are predominantly quiescent and arrested in G0/G1, regardless of their TP53 status. The reduction in the proliferation of OSU-CLL-TP53ko cells (Figure 3D) compared with OSU-CLL cells may seem counterintuitive given the known functions of TP53; however, unlike mutations of TP53, which are often associated with a gain of function,71 TP53 KO may confer a growth disadvantage and, in the context of the current study, may alter sensitivity to IBL-202 and venetoclax. Despite the decreased sensitivity to both drugs, strong synergy was apparent between IBL-202 and venetoclax against OSU-CLL-TP53ko cells (Figure 3B) and primary CLL cells from patients with p53 lesions (Figure 1A). A recent study from our group demonstrated synergy between IBL-202 and venetoclax against diffuse large B-cell lymphoma lines,72 suggesting that the drug combination may also have potential in the treatment of patients who experience a transformation of their CLL to a high-grade lymphoma. Collectively, these data suggest that IBL-202 in combination with venetoclax may be highly effective at targeting CLL cells both in the peripheral circulation and within lymph nodes and marrow and may be effective for high-risk patients with TP53 lesions or aggressive disease transformation.
Clinical trials combining venetoclax with ibrutinib have shown promising results.73-75 However, it is conceivable that poor response or relapse of patients on these regimens may be related to upregulation of Mcl-1.40,76 Our data demonstrate that ibrutinib, at a clinically relevant dose, did not decrease levels of Bcl-xL, increase levels of cleaved PARP, or increase the Noxa/Mcl-1 ratio (Figure 2D). Because a significant proportion of patients develop resistance to ibrutinib resulting from mutations in the BTK or PLC-γ2 genes,77 accompanied by increased expression of Mcl-1 and Bcl-xL,78 it is conceivable that the distinct mechanisms of IBL-202, as a single agent or in combination with venetoclax, may benefit these patients.
Interfering with the mechanisms that retain CLL cells within the lymph nodes can sensitize the leukemic cells to other therapies, including rituximab, fludarabine, ibrutinib, and bendamustine,79 by liberating CLL cells from sanctuary sites. The effects of IBL-202 in combination with venetoclax on CLL cell-cycle progression, proliferation, and migratory capacity in vitro were investigated to explore whether these drugs may limit certain functions of the TME. IBL-202 and venetoclax as single agents downregulated expression of CD49d and CXCR4 and reduced the capacity of CLL cells to migrate toward SDF-1α, consistent with our study of IBL-202.36 IBL-202 and venetoclax in combination had a significant, greater-than-additive effect on expression of these antigens and on the migratory capacity of CLL cells. Our data suggest that, as with ibrutinib,80,81 the drug combination may be effective at interfering with the homing and retention of CLL cells within the lymph nodes.
Collectively, the study suggests that IBL-202 and venetoclax are synergistic through inhibition of key signaling pathways that mediate the pathogenic and prosurvival effects of the TME. The drug combination also has the potential to interfere with the homing of CLL cells to lymph nodes and with the interaction of CLL cells with the stroma comprising the TME. The data support the proposal that IBL-202 and venetoclax in combination may be a highly effective treatment option, especially for CLL patients with poor-risk disease.
Send data sharing requests via e-mail to the corresponding author, O. Giles Best (giles.best@flinders.edu.au).
Acknowledgment
This study was supported by funding from the CLL Australian Research Consortium.
Authorship
Contribution: O.G.B. and Y.S. designed experiments; Y.S., K.C., N.F., and O.G.B. performed experimental work; Y.S. and O.G.B. analyzed the data; and Y.S., O.G.B., M. O’Neill, M. O’Dwyer, S.P.M., and R.I.C. contributed to preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: O. Giles Best, Department of Molecular Medicine and Genetics, Flinders Health and Medical Research Institute, Flinders University, Adelaide, SA 5042, Australia; e-mail: giles.best@flinders.edu.au.
References
Author notes
The full-text version of this article contains a data supplement.