Key Points
Blood group O is associated with a decreased risk for contracting SARS-CoV-2 infection.
Abstract
Identification of risk factors for contracting and developing serious illness following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is of paramount interest. Here, we performed a retrospective cohort analysis of all Danish individuals tested for SARS-CoV-2 between 27 February 2020 and 30 July 2020, with a known ABO and RhD blood group, to determine the influence of common blood groups on virus susceptibility. Distribution of blood groups was compared with data from nontested individuals. Participants (29% of whom were male) included 473 654 individuals tested for SARS-CoV-2 using real-time polymerase chain reaction (7422 positive and 466 232 negative) and 2 204 742 nontested individuals, accounting for ∼38% of the total Danish population. Hospitalization and death from COVID-19, age, cardiovascular comorbidities, and job status were also collected for confirmed infected cases. ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval [CI], 37.30-39.50) of the patients belonging to blood group O compared with 41.70% (95% CI, 41.60-41.80) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19. This study identifies ABO blood group as a risk factor for SARS-CoV-2 infection but not for hospitalization or death from COVID-19.
Introduction
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) has spread rapidly since its emergence in December 2019, causing a pandemic with 18 093 891 people infected globally (as of 3 August 2020). In severe cases, individuals develop a number of symptoms, including acute respiratory illness, collectively known as coronavirus disease 2019 (COVID-19). Development of COVID-19 is associated with age, sex, and comorbidities, such as cardiovascular diseases,1 although severe disease is not limited to these risk groups. Recent reports have suggested that ABO blood groups might play a role in the infection,2-6 with a lower than expected prevalence of blood group O individuals among patients. ABO blood groups are increasingly recognized to influence susceptibility to certain viruses, including SARS-CoV-17 and norovirus.8,9 Blood group A and B glycosyltransferases also affect glycosylation in a large number of cell types, including epithelial cells in the respiratory tract10 and shed viral particles.11,12 A, B, and AB individuals are also at increased risk for thrombosis and cardiovascular diseases,13 which are important comorbidities among hospitalized COVID-19 patients,14 possibly mediated by glycosylation of proteins involved in hemostasis.15 To obtain valid estimates of relative risk (RR), representativeness of the reference material is important. Although blood group frequencies and susceptibility for SARS-CoV-2 can vary substantially among ethnic groups, Denmark is a relatively ethnically homogenous society with free access to health care services. Here, we take of advantage of this, as well as the Danish centralized registries, to compare the blood groups of 473 654 individuals tested for SARS-CoV-2 and stratified for severity, measured as hospitalization or death from COVID-19.
Methods
Study design, variables, and oversight
A retrospective cohort analysis of all individuals with registered ABO and RhD blood groups in Denmark was performed. Data were extracted from electronic health records capturing all Danish patients, the Danish Microbiology Database, the Danish Civil Registration System, Danish Authorizations Register for health care workers, the Danish Patient Safety Authorization Register, and the Danish National Patient Register in a pseudo-anonymized form.16 Information concerning ABO and RhD blood groups, SARS-CoV-2 test results, and hospitalization and/or death from COVID-19 was collected, as well as demographic information (sex, age, authorization as health care personnel) and cardiovascular comorbidities. Death from COVID-19 was defined as death within 60 days following diagnosis. Cardiovascular comorbidities were defined by the 10th revision of the International Statistical Classification of Diseases and Related Health Problems codes for acute myocardial infarction (I-21), heart failure (I-50), and cerebral hemorrhage or infarction (I-60-64). Cases 60 years of age or older were grouped as “older.”
According to Danish law, studies based entirely on registry data do not require approval from an ethics review board.17 The study was registered at the University of Southern Denmark’s data inventory (record no. 10/960).
Study population
From 27 February 2020 to 30 July 2020, 841 327 individuals were tested by real-time polymerase chain reaction for SARS-CoV-2 infection in Denmark. Eligibility was determined by whether ABO and RhD blood group information was available, which was the case for 473 654 individuals (56%). A reference group with relevant blood group information from 2 204 742 nontested individuals was included (alive on 31 January 2020).
Supplemental Figure 1 outlines the testing strategy in Denmark during the spring of 2020, which is explained in detail by Pottegård et al.16 As a result of the strategy initially followed in Denmark, a large number of health care workers are among the tested individuals. Twenty-nine percent of this population was male, and 71% was female. The majority of patients (74%) in this study had mild disease and were not hospitalized.
Study outcomes
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. Secondary outcomes were hospitalization and death from COVID-19.
Statistical methods
We report proportions for binary test results and occurrence of clinical outcomes with accompanying 95% exact confidence intervals (CIs). Equality of proportions between groups was tested using a likelihood ratio χ2 test and evaluated at a 5% significance level. Because we had an a priori hypothesis about specific blood groups affecting the risk of infection, we computed RRs for contracting SARS-CoV-2 for each blood group. In a sensitivity analysis, we modeled the potential impact that overrepresentation of immigrants of nonwestern origin in the patient population could have on the estimated risk ratios. All analyses were done in Stata 16.
Results
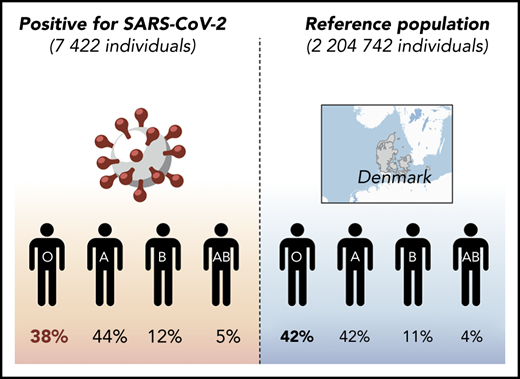
Of 841 327 people tested for SARS-CoV-2 in Denmark, ABO and RhD blood groups could be identified for 473 654 individuals (supplemental Figure 2). ABO and RhD data from 2 204 742 individuals not tested for SARS-CoV-2 were used as a reference, corresponding to ∼38% of the entire Danish population.
Supplemental Figure 3 shows the demographic characteristics of the tested population, stratified by sex and age. Of 473 654 individuals tested, 7422 were positive for SARS-CoV-2, and 466 232 were negative. In the population that tested negative for SARS-CoV-2, 32.0% were men, and the median age was 50 years (interquartile range, 36-64). Among positive cases, 32.9% were men, and the median age was 52 years (interquartile range, 40-67).
The distribution of ABO groups among those tested and the 2 204 742 nontested reference individuals is shown in Table 1. There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-2− individuals and the reference population (P < .001). Among the SARS-CoV-2+ individuals, considerably fewer group O individuals were found (P < .001); conversely, more A, B, and AB individuals were noted (P < .001, P = .011, and P = .091, respectively). When blood group O was excluded, no significant difference was seen among A, B, and AB (P = .30). No difference in the RhD group was found between positively tested cases and the reference population (P = .15). We classified individuals after risk factors associated with COVID-19, including age ≥60 years, cardiovascular comorbidity, and job in the health care sector. No difference in the distribution of ABO groups was found when infected individuals with a given risk factor were compared with those without (all P > .1; supplemental Table 1). Together, these data indicate that the RRs for contracting SARS-CoV-2 are 0.87 (95% CI, 0.83-0.91), 1.09 (95% CI, 1.04-1.14), 1.06 (95% CI, 0.99-1.14), and 1.15 (95% CI, 1.03-1.27) for O, A, B, and AB individuals, respectively.
Distribution of ABO blood groups among individuals tested and not tested for SARS-CoV-2 in Denmark
| Blood group . | SARS-CoV-2 tested, n (%) . | Reference population, n (%) . | P, SARS-CoV-2+ vs reference population . | RR (95% CI), positive individuals vs reference population . | |
|---|---|---|---|---|---|
| Positive . | Negative . | ||||
| O | 2851 (38.41) | 193 401 (41.48) | 919 303 (41.69) | <.001 | 0.87 (0.82-0.91) |
| A | 3296 (44.41) | 199 211 (42.73) | 934 421 (42.39) | <.001 | 1.09 (1.02-1.13) |
| B | 897 (12.09) | 52 838 (11.33) | 252 559 (11.46) | .091 | 1.06 (1.03-1.19) |
| AB | 378 (5.09) | 20 782 (4.46) | 98 459 (4.47) | .011 | 1.15 (1.05-1.31) |
| Total, n | 7422 | 466 232 | 2 204 742 | ||
| Blood group . | SARS-CoV-2 tested, n (%) . | Reference population, n (%) . | P, SARS-CoV-2+ vs reference population . | RR (95% CI), positive individuals vs reference population . | |
|---|---|---|---|---|---|
| Positive . | Negative . | ||||
| O | 2851 (38.41) | 193 401 (41.48) | 919 303 (41.69) | <.001 | 0.87 (0.82-0.91) |
| A | 3296 (44.41) | 199 211 (42.73) | 934 421 (42.39) | <.001 | 1.09 (1.02-1.13) |
| B | 897 (12.09) | 52 838 (11.33) | 252 559 (11.46) | .091 | 1.06 (1.03-1.19) |
| AB | 378 (5.09) | 20 782 (4.46) | 98 459 (4.47) | .011 | 1.15 (1.05-1.31) |
| Total, n | 7422 | 466 232 | 2 204 742 | ||
There was no difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients vs hospitalized patients or for deceased patients vs living patients (Table 2).
Distribution of ABO blood groups and clinical severity among individuals infected with SARS-CoV-2 in Denmark
| Blood group . | Confirmed SARS-CoV-2 infected, n (%) . | P, hospitalized vs nonhospitalized . | P, deceased vs alive . | |||
|---|---|---|---|---|---|---|
| Hospitalized . | Nonhospitalized . | Deceased . | Alive . | |||
| O | 721 (36.96) | 2130 (38.93) | 201 (36.55) | 2650 (38.56) | .12 | .35 |
| A | 896 (45.93) | 2400 (43.87) | 259 (47.09) | 3037 (44.19) | .12 | .19 |
| B | 233 (11.94) | 664 (12.14) | 64 (11.64) | 833 (12.12) | .82 | .74 |
| AB | 101 (5.18) | 277 (5.06) | 26 (4.73) | 352 (5.12) | .84 | .68 |
| Total, n | 1951 | 5471 | 550 | 6872 | ||
| 7422 | 7422 | |||||
| Blood group . | Confirmed SARS-CoV-2 infected, n (%) . | P, hospitalized vs nonhospitalized . | P, deceased vs alive . | |||
|---|---|---|---|---|---|---|
| Hospitalized . | Nonhospitalized . | Deceased . | Alive . | |||
| O | 721 (36.96) | 2130 (38.93) | 201 (36.55) | 2650 (38.56) | .12 | .35 |
| A | 896 (45.93) | 2400 (43.87) | 259 (47.09) | 3037 (44.19) | .12 | .19 |
| B | 233 (11.94) | 664 (12.14) | 64 (11.64) | 833 (12.12) | .82 | .74 |
| AB | 101 (5.18) | 277 (5.06) | 26 (4.73) | 352 (5.12) | .84 | .68 |
| Total, n | 1951 | 5471 | 550 | 6872 | ||
| 7422 | 7422 | |||||
Immigrants from nonwestern countries and their first-generation descendants are overrepresented among Danish COVID-19 patients (constituting 18% of cases but only 9% of the population),18 which could confound the ABO distribution among SARS-CoV-2+ individuals. Therefore, we performed a sensitivity analysis by calculating a weighted reference ABO distribution, taking into account the number of COVID-19 cases among immigrants from the nations constituting the largest sources of nonwestern immigration in Denmark (Pakistan, Morocco, Somalia, Turkey, and Iraq) and reference ABO distributions in their respective country of origin. Using this weighted ABO distribution as an alternative reference population, we reanalyzed and found an adjusted RR for blood group O individuals of 0.88 (supplemental Table 2), which was very similar to our initial result (RR, 0.87; Table 1).
Discussion
Here, we demonstrate that blood group O is significantly associated with reduced susceptibility to SARS-CoV-2 infection. Additionally, ABO blood groups were not associated with rates of hospitalization or death following infection.
Our study has several limitations. ABO blood group information was only available for 62% of all tested individuals, and only doctors and nurses were identified as health care personnel. The sex of the tested population was skewed, with females accounting for 71% of those who screened negative and 67% of those who screened positive. However, blood groups are generally independent of sex. More importantly, however, is the fact that blood group distributions vary among ethnic subgroups with different susceptibility for infection. Indeed, a higher than expected contribution of immigrants from nonwestern countries is noted among Danish COVID-19 patients,18 but a sensitivity analysis indicated this is not a major bias and, thus, is unlikely to affect the overall conclusion.
The data presented here are consistent with other studies reporting primarily on hospitalized patients2-6 and those exploring SARS-CoV-1 susceptibility.7 Several hypotheses could explain these results. In vitro studies have indicated that SARS-CoV-1 particles can be glycosylated by the A variant of the ABO glycosyltransferases, allowing anti-A antibodies to neutralize the virus.11 Although not studied, it is likely to be the case for the B variant, as well, and, therefore, for anti-B antibodies. Because anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group,19 this might explain the relative protection of blood group O individuals. In this context, it is interesting that the highest RR for infection was found for blood group AB lacking both antibodies. However, the RR of group AB did not differ significantly from that of group A or B; because of the paucity of blood group AB, it would require a considerably larger number of patients to clarify this. Alternatively, the effect could be indirect, as a result of the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals15 and who are also more prone to arterial and venous thrombosis,20 which are important comorbidities for COVID-19. Importantly, the majority of patients reported here were not hospitalized and, therefore, were less likely to have such comorbidities.
Our findings of similar relative protection by blood group O in young individuals, in health care personnel, and in individuals without a registered cardiovascular diagnosis suggest that associations between non-O blood groups and comorbidities do not explain the apparent protection enjoyed by group O individuals, in line with findings reported by Zietz and Tatonetti.3 Moreover, our data do not indicate an association between blood group and progression to hospitalization or death from COVID-19. This is consistent with data from Ellinghaus et al,6 who did not find any differences in the usage of oxygen or ventilation between ABO blood groups in hospitalized patients. Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (eg, by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19). Furthermore, it should be emphasized that the 2 hypotheses do not exclude each other.
Data sharing requests should be sent to Torben Barington (torben.barington@rsyd.dk).
Acknowledgments
The authors thank Ulrik Sprogøe (Department of Clinical Immunology, Odense University Hospital) for helpful input and discussion, as well as the Departments of Clinical Immunology and Departments of Clinical Microbiology throughout Denmark for contributing data to this study.
Authorship
Contribution: M.B.B., A.P., H.S., and T.B. designed and performed research; M.B.B., A.P., T.M.H., K.H., R.L., M.B.H., K.T., B.A., and B.K.M. collected data; and all authors analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: H.S. has received personal fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Torben Barington, Odense University Hospital, J.B. Winsløws Vej 4, 5000 Odense, Denmark; e-mail: torben.barington@rsyd.dk.
References
Author notes
The full-text version of this article contains a data supplement.