TO THE EDITOR:
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive bone marrow malignancy that accounts for ∼10% to 15% of pediatric acute lymphoblastic leukemia (ALL) and 20% of adult ALL.1,2 Despite improvements in frontline treatments, outcomes in primary refractory (PR) or relapsed T-ALL remain dismal, and novel therapies are urgently needed.3-5 In relapsed B-cell ALL, chimeric antigen receptor (CAR) T cells targeting CD19 have shown promising results and have recently been approved by the US Food and Drug Administration. In contrast, identifying suitable CAR T-cell targets against T-ALL has been far more challenging. Because CAR T cells share antigens with malignant T cells, mutual killing of CAR T cells, termed “fratricide,” can occur, preventing the generation, expansion, and persistence of CAR T cells.6 Furthermore, unlike B-cell aplasia, which is largely manageable, prolonged and profound T-cell aplasia is intolerable and can result in life-threatening opportunistic infections.7 Therefore, the ideal target would be one that is highly expressed on the malignant cells, including refractory disease, maintained at relapse, and absent on normal T cells.
Sánchez-Martínez et al chose to focus on CD1a, a surface antigen present on the cortical subtype of T-ALL but absent on developing and mature T cells.8 The fratricide-resistant CD1a CAR showed excellent CD1a-specific in vivo killing of cortical T-ALL in a mouse model, and the investigators now plan to move toward human trials in relapsed/refractory (R/R) T-ALL. Although CD1a+ cortical T-ALL is a common immunophenotype in newly diagnosed T-ALL, several studies suggest that this subgroup of patients have a good prognosis, as evidenced by higher complete remission rates and lower relapse rates.3,9-11 With a lack of comprehensive immunophenotypic data for T-ALL in the relapse setting, it is difficult to ascertain whether CD1a-targeted approaches are likely to be tractable in terms of clinical trial recruitment and patient need. To address this, we investigated the immunophenotype of all new and relapsed cases of pediatric and adult T-ALL diagnosed between 2011 and 2019 at 2 major hemato-oncology centers: University College London Hospital and Great Ormond Street Hospital for Children, London. In addition, to permit particular focus on the R/R group, we supplemented the cohort with a group of R/R pediatric T-ALL cases from the Blood Cancer UK Childhood Leukaemia CellBank.
Immunophenotyping was performed using standard diagnostic protocols on bone marrow, peripheral blood, cerebrospinal fluid, or pleural fluid. Further details are provided in supplemental Methods. Although the eligibility criteria regarding antigen positivity level vary considerably in current CAR T-cell trials, in clinical practice we would not use CAR T-cell therapy in the presence of a high proportion of antigen-negative cells, given that antigen-negative immune escape remains a major issue after CAR T-cell therapy. For this reason, we have deviated from the standard definition of antigen positivity, as per the Clinical and Laboratory Standards Institute/International Clinical Cytometry Society definition of ≥20% of cells above the background12,13 and have used a more stringent definition of antigen positivity of ≥80% of cells above the background, unless otherwise mentioned. Representative plots of CD1a expression are shown in supplemental Figure 1.
One hundred and twelve patients had immunophenotyping results available at presentation. The median age at diagnosis was 10.5 years. Seventy-nine (65%) were pediatric patients (<18 years old), and 33 (35%) were adult (≥18 years). The presentation samples included 22 patients who were subsequently refractory to induction chemotherapy. In addition, there were 28 cases of relapsed disease. Further details are provided in supplemental Table 1.
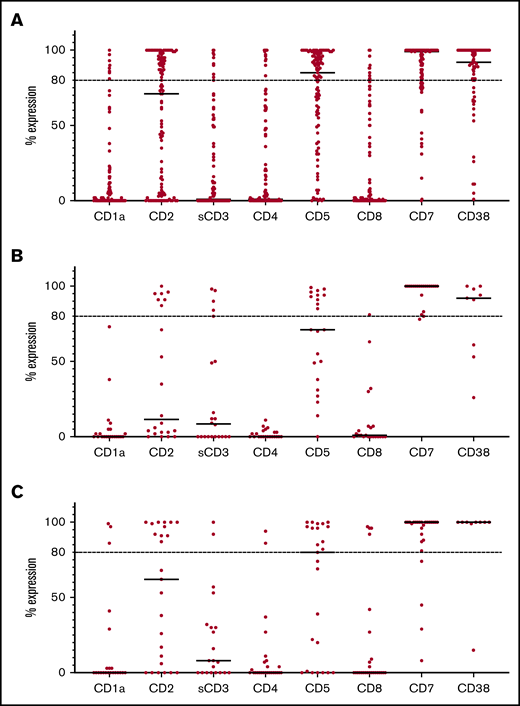
CD1a expression levels were available for 92% of cases at presentation and for 79% of cases at relapse. Although CD1a was partially expressed at ≥10% in 25% of samples, only 8 of the 103 cases (8%) had CD1a expression >80% (Figure 1A). Importantly, all cases with high expression remain in complete remission. Of the 22 patients with PR disease, none had CD1a expression at presentation (Figure 1B). Six of the PR cases also had flow cytometry performed on the postchemotherapy refractory population, which remained CD1a negative in all cases. In the relapse cohort, only 3 of 28 cases expressed ≥80% CD1a positivity (Figure 1C).
Antigen expression in T-ALL. (A) Percentage of antigen expression by flow cytometry in all cases of T-ALL. (B) Percentage of antigen expression by flow cytometry in PR cases. (C) Percentage of antigen expression by flow cytometry at relapse. Horizontal bars indicate median expression. Horizontal dotted lines indicate expression >80%.
Antigen expression in T-ALL. (A) Percentage of antigen expression by flow cytometry in all cases of T-ALL. (B) Percentage of antigen expression by flow cytometry in PR cases. (C) Percentage of antigen expression by flow cytometry at relapse. Horizontal bars indicate median expression. Horizontal dotted lines indicate expression >80%.
In view of the low expression of CD1a in R/R disease, we went on to explore other potential target antigens. As shown in Figure 1A, CD7 and CD38 had the most consistent expression at presentation, with high expression in 80% (89/111) and 75% (49/65) of cases, respectively. CD2 and CD5 showed moderate positivity, with 44% (48/109) and 57% (58/101) of cases showing high expression, respectively. Other antigens, including soluble CD3 (10/92; 11%), CD4 (12/110; 11%), and CD8 (11/110; 10%), were absent in the majority of cases at presentation.
The PR group showed a similar pattern of expression, with CD7 (95%; 21/22) and CD38 (67%; 6/9) being the most consistently expressed antigens (Figure 1B). Other antigens showed low or negative expression, including CD2 (32%), CD5 (45%), CD3 (23%), CD4 (0%), and CD8 (5%). Importantly, almost half of the refractory samples (41%; 9/22) had an immunophenotype consistent with the early thymic precursor subtype, which is known to be associated with an increased risk for refractory disease.14
Results were similar at relapse (Figure 1C), with CD7 expression present in 85% (22/26) of cases. Persistent high levels of CD38 expression were also found in 89% (8/9) of relapsed cases. CD2 and CD5 expression was present in 44% (11/25) and 52% (13/25) of cases, with other antigens largely negative, including CD3 (10%; 2/21), CD4 (8%, 2/26) and CD8 (15%; 4/26).
Overall, our results show that, although a CAR T-cell targeting CD1a could benefit patients with cortical T-ALL, these patients generally have an excellent outcome; there were no relapses in patients with CD1a expression at presentation. The PR group, which would also be a key indication for CAR T-cell therapy, showed CD1a negativity in all patients, although we acknowledge that CD1a negativity after chemotherapy could only be confirmed in 6 samples, and further investigation of the postchemotherapy immunophenotype in refractory disease is, therefore, required. Despite this, in our view, a CAR T-cell trial targeting CD1a will face major recruitment challenges for R/R T-ALL, as exemplified by the fact that, between our 2 institutions and over a 9-year period, only 3 relapse cases showed high CD1a expression. Thus, such trials are likely to require multi-institutional and international recruitment networks to be viable.
Instead, our results identify CD7 and CD38 as the most consistently expressed antigens at presentation and relapse, making them strong candidate targets for CAR T-cell therapy. Given the universal expression of CD7 on normal T cells, several groups have already explored genomic editing to ablate CD7 expression on CAR T cells to avoid fratricide and allow persistent CAR expansion.15-19 However, this will not overcome profound T-cell aplasia in patients, likely necessitating subsequent bone marrow transplant, a highly intensive therapy.
In contrast, CD38 is only expressed on a small proportion of normal T cells, potentially minimizing T-cell aplasia and allowing a more leukemia-specific effect.20 Although it is expressed on other hematological lineages, including activated B cells and some myeloid cells,21 as well as smooth muscle and pancreatic islet cells,22 the anti-CD38 monoclonal antibody daratumumab is safely used in multiple myeloma and has already shown promise in relapsed/refractory T-ALL,23-25 which is consistent with the persistent CD38 expression that we demonstrate in relapsed disease. CD38 CARs have also been studied in multiple myeloma,26 and our data suggest that this approach could be considered for relapsed T-ALL; however, careful monitoring for off-target toxicities is required.
Given that CAR T-cell approaches will primarily be used in the R/R setting, further studies exploring the cell surface antigen profile in this setting are clearly required. Measurement of antigen expression to determine eligibility for CAR T-cell therapies should use the brightest and most stable fluorochrome, ideally phycoerythrin. Furthermore, it is important that standardized panels are used to facilitate reproducibility across centers, as will become standard practice in the United Kingdom within the forthcoming ALLTogether1 trial.
Data sharing requests should be sent to David O’Connor (david.o’connor@ucl.ac.uk).
Acknowledgments:
The authors thank the Blood Cancer UK Childhood Leukaemia CellBank for provision of primary patient samples.
This work was supported by the National Institute for Health Research University College London Hospitals and Great Ormond Street Hospital Biomedical Research Centres. D.O. and F.P. are funded by a Cancer Research UK Clinician Scientist Fellowship.
Contribution: S.L., M.R.M., and D.O. wrote the first and subsequent drafts of the manuscript; and all authors contributed to the acquisition or analysis of the data, revised the manuscript critically, approved the final version for publication, and agreed to be accountable for the results published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David O’Connor, Department of Haematology, UCL Cancer Institute, Huntley St, London WC1E 6DD, United Kingdom; e-mail; david.o’connor@ucl.ac.uk; and Marc R. Mansour, UCL Cancer Institute, Huntley St, London WC1E 6DD, United Kingdom; e-mail; m.mansour@ucl.ac.uk.
References
Author notes
The full-text version of this article contains a data supplement.