Key Points
Standard chemotherapy can still be used for new diagnosis of acute lymphoblastic leukemia in patients with SARS-CoV-2.
Corticosteroid can be given safely to patients with SARS-CoV-2 presenting with acute respiratory distress syndrome and ALL.
Introduction
Although recommendations are emerging for the general management of oncology patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1,2 there is little experience in patients with newly diagnosed acute lymphoblastic leukemia (ALL). Providers may have concern about initiating multiagent chemotherapy in patients with SARS-CoV-2, particularly corticosteroids, which are an essential part of induction regimens, but raise the theoretical possibility of delayed viral clearance. We describe our experience of successfully initiating therapy for an adolescent diagnosed with ALL, while managing severe SARS-CoV-2 infection marked by respiratory failure, systemic inflammation, and autoimmune hemolytic anemia (AIHA).
Case description
An 18-year-old adolescent male presented to our emergency room with pallor, having experienced cough and fever for 1 week. He tested positive for SARS-CoV-2 by polymerase chain reaction. He had multiple known exposures: his father had recently died of SARS-CoV-2 and his mother and siblings were symptomatic at home. Initial laboratory evaluation was significant for the following counts: white blood cells (WBCs), 105 × 103/µL with 95% blasts; hemoglobin (Hgb), 3.7 g/dL; and platelets, 30 × 103/µL. Flow cytometry of peripheral blood confirmed the diagnosis of B-cell ALL.
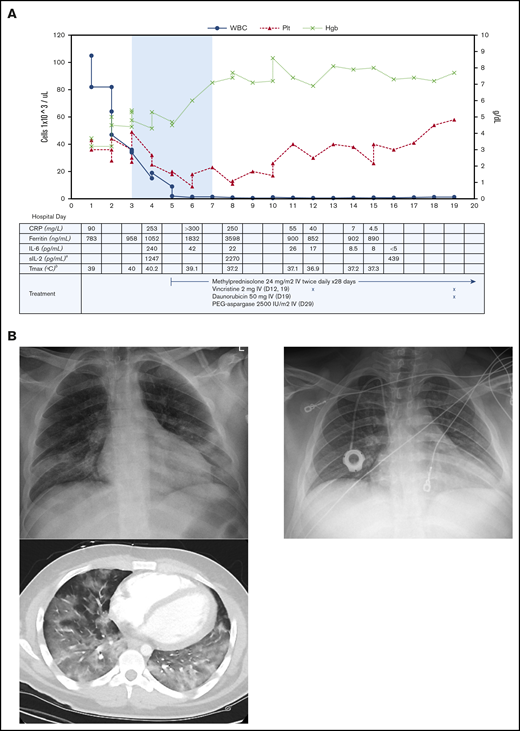
He had low-grade fever, normal oxygen saturation, and no respiratory distress at time of presentation, with the remainder of his laboratory results notable for hyperuricemia and mildly elevated lactate dehydrogenase. He received supportive care with hyperhydration and allopurinol, given his high risk for tumor lysis syndrome, and broad-spectrum antibiotics (piperacillin/tazobactam). Shortly after admission, he developed persistent high fevers and intermittent hypoxia with sudden respiratory decompensation requiring mechanical ventilation and hypotension requiring vasopressor support on hospital day 3 (HD 3). A chest radiograph showed bibasilar opacities consistent with moderate acute respiratory distress syndrome (ARDS).3 In the setting of worsening SARS-CoV-2 disease, his WBC count downtrended, with hydration but no specific antileukemic therapy, to 8 × 103/µL; in addition, he developed transfusion-resistant anemia and thrombocytopenia and had a mild increase in bilirubin. He was found to have AIHA with positive immunoglobulin G and C3 polyspecific direct antiglobulins, but a negative eluate and no alloantibodies. He also had rising ferritin and elevated interleukin 6 (IL-6) and soluble IL-2 receptor (sIL2R) levels (Figure 1), which raised concern for a hemophagocytic lymphohistiocytosis (HLH) vs a SARS-CoV-2–related cytokine storm.
Patient’s clinical and radiographic findings. (A) Cell counts and inflammatory markers throughout the patient’s hospital stay, from day of presentation to discharge. Top, The shaded area indicates time requiring mechanical ventilation. Left y-axis, WBC and platelet (Plt) counts in cells ×103 per microliter. Right y-axis, Hgb in grams per deciliter. Bottom, Patient’s antileukemic treatment included methylprednisolone, vincristine, and daunorubicin; timing staggered per clinical discretion. Systemic steroid therapy was started on HD 5, for a total course of 28 days. Vincristine was given on HD 12 and continued weekly for 4 doses. Daunorubicin was given on HD 19 and continued weekly for 4 doses. Treatment continued in the outpatient setting after hospital discharge, and additionally included 1 dose of polyethylene glycol (PEG)–aspargase. aIL-1β, IL-4, IL-5, IL-10, IL-12, IL-13, IL-17, interferon γ, and tumor necrosis factor α also tested without elevation in levels. bMaximum temperature (Tmax) recorded that calendar day. Normal reference values per reporting laboratory: C-reactive protein (CRP), 0-10 mg/L; ferritin, 30-400 ng/mL; IL-6, <5 pg/mL; sIL-2, <1033 pg/mL. (B) Chest radiograph and computed tomography scan at time of presentation (left), showing patchy consolidation and characteristic ground-glass pulmonary infiltrates throughout the lungs, predominant in the lower lobes. Chest radiograph 2 months later (right), showing resolution of consolidation.
Patient’s clinical and radiographic findings. (A) Cell counts and inflammatory markers throughout the patient’s hospital stay, from day of presentation to discharge. Top, The shaded area indicates time requiring mechanical ventilation. Left y-axis, WBC and platelet (Plt) counts in cells ×103 per microliter. Right y-axis, Hgb in grams per deciliter. Bottom, Patient’s antileukemic treatment included methylprednisolone, vincristine, and daunorubicin; timing staggered per clinical discretion. Systemic steroid therapy was started on HD 5, for a total course of 28 days. Vincristine was given on HD 12 and continued weekly for 4 doses. Daunorubicin was given on HD 19 and continued weekly for 4 doses. Treatment continued in the outpatient setting after hospital discharge, and additionally included 1 dose of polyethylene glycol (PEG)–aspargase. aIL-1β, IL-4, IL-5, IL-10, IL-12, IL-13, IL-17, interferon γ, and tumor necrosis factor α also tested without elevation in levels. bMaximum temperature (Tmax) recorded that calendar day. Normal reference values per reporting laboratory: C-reactive protein (CRP), 0-10 mg/L; ferritin, 30-400 ng/mL; IL-6, <5 pg/mL; sIL-2, <1033 pg/mL. (B) Chest radiograph and computed tomography scan at time of presentation (left), showing patchy consolidation and characteristic ground-glass pulmonary infiltrates throughout the lungs, predominant in the lower lobes. Chest radiograph 2 months later (right), showing resolution of consolidation.
Due to the severity of his condition, antileukemic therapy was initiated with methylprednisolone alone on HD 5. His clinical condition steadily improved thereafter. He was extubated on HD 7, showed resolution of fevers by HD 8, and his inflammatory markers decreased (Figure 1). His response to transfusions improved, and Hgb and platelet count stabilized. Induction chemotherapy for ALL was then continued with vincristine on HD 11 and daunorubicin on HD 18. Polyethylene glycol (PEG)-asparaginase was held due to an elevated lipase level of 179 U/L (1.9× upper limit of normal), thought to be due to SARS-CoV-2 infection. He tolerated the chemotherapy well and was discharged home on HD 18. He received further chemotherapy (vincristine, daunorubicin) per the standard induction protocol.4 PEG-asparaginase was given 10 days after hospital discharge once his lipase level returned to normal. His end-of-induction bone marrow showed complete remission with minimal residual disease of 2.7% by flow cytometry. JAK1 mutations (p.Arg724Cys, p.Arg724His), JAK2 mutations (p.Arg938Gln, p.Arg867Gln), loss of IKZF1, PAX5, and CDKN2A/2B, and cytokine receptor–like factor 2 rearrangement were found, consistent with Philadelphia chromosome–like ALL. He has subsequently been enrolled in a phase 2 study of ruxolitinib with chemotherapy (clinicaltrials.gov NCT02723994).
Methods
We collected the patient’s medical records including clinical course, laboratory parameters, treatment record, and outcome.
Results and discussion
Patients receiving myelosuppressive cancer therapy certainly have a theoretically increased risk for SARS-CoV-2 and more severe disease, although currently reported data paint a mixed picture.5 Reviews of pediatric oncology centers found a relatively low overall number of infected patients, with mild infection most common.6,7 Whether treatments should be altered in pediatric cancer patients remains unclear, although emerging guidelines suggest postponing high-intensity treatments.1,2
In this case, intensive remission induction chemotherapy was initially delayed due to concern for potential worsening of SARS-CoV-2 disease by exacerbating the patient’s already immunocompromised state in the setting of ALL. However, the patient developed progressively worsening symptoms, laboratory markers suggestive of cytokine storm, and rapid clinical deterioration to respiratory failure and shock. Restoration of this patient’s immune function was not expected without initiation of cytotoxic chemotherapy. He additionally showed evidence of immune dysregulation with persistent fevers, pancytopenia, and elevated serum ferritin, IL-6, and sIL2R. Notably, as the patient’s clinical condition deteriorated, his high initial WBC count decreased drastically with hyperhydration alone. This finding was concurrent with the development of transfusion-unresponsive AIHA and thrombocytopenia. This constellation of findings could be attributed not only to SARS-CoV-2 infection but also to possible secondary HLH from untreated acute leukemia.8,9 Additionally, secondary HLH may have contributed to SARS-CoV-2–induced lung inury.10 Reports from China at the onset of the SARS-CoV-2 pandemic reported that patients with more severe disease had higher ferritin, sIL2R, IL-6, IL-8, IL-10, and tumor necrosis factor α (TNF-α).11 Although lymphopenia is well described with SARS-CoV-2 infection,12,13 this patient’s case suggests that the hyperinflammatory response seen in severe SARS-CoV-2 infection may involve immune dysregulation leading to consumptive thrombocytopenia and AIHA, which should be further explored among patients infected with SARS-CoV-2. AIHA in this patient gave concern for breakdown of immunologic tolerance.14 As there was evidence of both impaired and dysregulated immune function with evolving ARDS and shock, the decision was made to initiate corticosteroid therapy.
Significant systemic inflammation with elevated sIL2R and IL-6 levels, as seen in our patient, has led to the investigation of targeted cytokine blockade for patients with severe SARS-CoV-2 disease, with particular interest in targeting IL6 with tocilizumab.15-17 This was considered in our patient: however, in light of concurrent ALL and concern for possible secondary HLH, the decision was made to initiate therapy with corticosteroids and reserve tocilizumab as second-line therapy. The benefit to mortality with steroid use in patients with severe SARS-CoV-2 disease was unclear, with mixed outcomes in observational studies at the time of our patient’s treatment.18-21 Current recommendations for the management of severe SARS-CoV-2 infection do not support routine systemic corticosteroid use outside of a clinical trial, but do allow for clinical discretion with regard to moderate ARDS.3,22-25 In this patient’s case, however, corticosteroids were an integral part of his antileukemic therapy and management of AIHA and HLH, and had anti-inflammatory potential; thus, benefits were determined to outweigh the risks. Our patient’s clinical condition and inflammatory marker elevation rapidly improved with steroid treatment, allowing him to continue standard ALL treatment. This suggests that in the setting of active SARS-CoV-2 infection in leukemia, systemic corticosteroids can be safely given without delay as a bridge to more myelosuppressive therapy.
Apart from supportive care and steroids, the patient did not receive any other SARS-CoV-2–directed therapy. He showed remarkable improvement upon initiation of steroids with resolution of fevers, decrease in inflammatory markers and D-dimer, stabilization of transfusion requirements, and rapid respiratory improvement with extubation possible within 3 days. Given his rapid recovery, additional antileukemic therapy with a modified 4-drug combination4 was given, first with addition of vincristine and subsequently daunorubicin and PEG-asparaginase once the patient showed further clinical recovery from his SARS-CoV-2 infection. He tolerated the remainder of induction and achieved complete remission. We would thus recommend a similar stepwise approach in initiating systemic chemotherapy in patients with newly diagnosed ALL complicated by SARS-CoV-2 infection, utilizing steroids along with nonmyelosuppressive chemotherapy upfront, with addition of more toxic chemotherapy once the critical window of clinical deterioration from SARS-CoV-2 passes.
The authors are available to share data via e-mails to the corresponding author, Lia Phillips, at lip9085@nyp.org.
Acknowledgment
No external funding was secured for this study.
Authorship
Contribution: All authors were involved in the clinical care of this patient and contributed toward completion of this manuscript.
Conflict-of-interest disclosure: N.H. is a consultant for Novartis and Incyte. The remaining authors declare no competing financial interests.
Correspondence: Lia Phillips, Columbia University Medical Center, Herbert Irving Pavilion, 161 Fort Washington Ave, New York, NY 10032; e-mail: lip9085@nyp.org; and Nobuko Hijiya, Columbia University Medical Center, Herbert Irving Pavilion, 161 Fort Washington Ave, New York, NY 10032; e-mail: nh2636@cumc.columbia.edu.