Key Points
The counts of tissue mast cells were inversely associated with clinical severity of lower GIT GVHD.
The counts of tissue mast cells were inversely associated with nonrelapse mortality.
Abstract
The functions of mast cells in human graft-versus-host disease (GVHD) are unknown. We studied 56 patients who had an allogeneic hematopoietic cell transplantation (alloHCT) with a biopsy for diagnosis of gastrointestinal tract (GIT) GVHD before any treatment (including steroids): 35 with GIT GVHD, 21 HCT recipients whose biopsies did not confirm GVHD, and 9 with a new diagnosis of inflammatory bowel disease (IBD) as a comparison. The median number of mast cells (mean of CD117+ cells, counted in 3 selected spots under 40× magnification) was similar between patients with GVHD (59 cells) and those without GVHD (60 cells). However, the median number of mast cells was significantly associated with maximum clinical stage of GIT GVHD; the lowest counts of mast cells were observed in the highest clinical stage of GIT GVHD (stage 1, 80; stage 2, 69; stage 3, 54; stage 4, 26; P = .01). Moreover, every decrease by 10 mast cells was associated with increased nonrelapse mortality through 1 year (hazard ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05). AlloHCT recipients all had significantly fewer mast cells, even those without GVHD compared with those with IBD (median, 59 vs 119; P < .01). The median number of GIT mast cells was also significantly lower in patients who received myeloablative conditioning (61.5 cells) than in those who received reduced intensity conditioning (78 cells) in the entire study population (P = .02). We conclude that GIT mast cells are depleted in all alloHCT patients, more prominently in those receiving myeloablative conditioning and those with severe GIT GVHD. Our novel findings warrant further investigation into the biological effects of mast cells in GIT GVHD.
Introduction
Acute graft-versus-host disease (aGVHD) is one of the most important complications of allogeneic hematopoietic cell transplantation (alloHCT). It affects primarily the skin, liver, and upper or lower gastrointestinal tract (GIT)1-3 and is associated with increased mortality.1,2 Histopathologic examination of organs involved in aGVHD shows significant immune-mediated inflammatory changes.2
Mature mast cells in tissues are long-lived cells present throughout the body and within blood vessels and nerves in vascularized human tissues.4 Large numbers of mast cells can also be found near body surfaces in the skin, airways, and GIT.5 Mast cells are derived from bone marrow and particularly depend upon stem cell factor and KIT receptors for their survival.5-7 Although the role of mast cells in human physiology is not thoroughly understood, it is well-established that mast cells are a component of the innate immune system.8 Mast cells, along with dendritic cells and monocytes, are involved in the innate immune system that interacts with environmental antigens and allergens, invading pathogens, or environmentally derived toxins.9 Mast cells participate in pathologic allergic and hypersensitivity reactions, including allergic rhinitis and asthma.10-12 In those hypersensitivity disorders, mast cells contribute to the destructive and proinflammatory effects of these conditions.13-15 However, masts cells are not only proinflammatory, they can also decrease inflammation through a regulatory function.16
Given that mast cells are a key player in inflammation and are present in organs affected by aGVHD, mast cells in GVHD were evaluated decades ago in animal studies. Although these studies supported involvement of mast cells in GVHD pathogenesis,17-22 studies in humans are rare.23 We evaluated GIT mast cells in biopsies from patients with GIT GVHD compared with patients with no GIT GVHD and with patients with inflammatory bowel disease (IBD).
Patients and methods
Patients
Adult patients who received an alloHCT were included in this retrospective study. Patients who developed diarrhea after alloHCT and underwent sigmoidoscopic biopsy for evaluation of lower GIT GVHD were included, whereas patients who had received any corticosteroids within 3 days before biopsy were excluded. All patients included in the study provided consent for data collection and were treated according to protocols approved by the University of Minnesota Institutional Review Board.
Data on pretransplantation comorbidities were collected prospectively and confirmed retrospectively for all patients using the HCT-specific comorbidity index24 and were categorized as low risk (score 0), intermediate risk (score 1-2), and high risk (score ≥3). The pathologist (K.A.) who assessed mast cell staining on the biopsies was unaware of the patients’ histopathologic and clinical diagnosis, treatment, and outcome before pathologic review.
Pathologic examination
Immunohistochemistry and slide evaluation.
In this study, we selected CD117 for immunohistochemistry (IHC) for several reasons. First, CD117 is a highly specific marker for counting mast cells in GIT biopsies. Other CD117+ cells would be myeloid precursors, but those cells are absent in GIT mucosa unless there is involvement of the GIT by myeloproliferative disorder and some mesenchymal cells of the GIT (eg, for interstitial cells of Cajal, staining is lighter and cell morphology is quite different from mast cells). CD117 is also used clinically to evaluate mast cells disorders. Second, it has crisp staining without any significant background, and other cells that can be positive for CD117 are rare. Third, we have considerable experience in this area, and the CD117 IHC assay has robust validity in our laboratory. Although stain for CD117 is a good morphologic stain, it cannot differentiate whether masts cells are activated or inactivated. CD117 IHC was performed on 5-μm-thick paraffin embedded sections. The antibody used for staining was a ready-to-use prediluted rabbit monoclonal antibody, clone Y145 (Biocare Medical). The sections were pretreated with Ultra Cell conditioning 1 for 32 minutes and incubated with antibody for 16 minutes followed by treatment with the amplification kit for 4 minutes. The final staining was performed on the Benchmark ULTRA platform using the Ultraview detection kit (Roche Diagnostics, Indianapolis, IN). GIT stromal tumor sections were used as a positive control.
The quantitative histologic evaluation involved initial scanning at low-power magnification (4× objective), and 3 selected areas with the highest concentration of CD117+ mast cells were identified. Only positive cells within the lamina propria of GIT mucosa were counted. The CD117 counts are the mean of 3 individual high-power (40×) fields with the highest concentration of CD117+ cells selected after microscopic examination scanning the entire biopsy tissue. Colonic samples from patients with treatment-naïve, newly diagnosed IBD were also examined for mast cells as a comparison group.
Transplantation and supportive care.
Most patients received umbilical cord blood (UCB) or sibling grafts. UCB grafts were matched at 4/6 HLA-A, -B (antigen level), and -DRB1 (allele level) to the recipient; patients receiving 2 UCB units were similarly matched to each other.25
Patients who underwent a reduced intensity conditioning (RIC) regimen generally received cyclophosphamide (50 mg/kg IV on day −6), fludarabine (30-40 mg/m2 IV once per day from days −6 through −2), and total body irradiation (TBI; 200 cGy on day −1) or fludarabine (30 mg/m2 IV once per day from days −6 through −2) and busulfan (3.2 mg/kg IV once per day on days −5 and −4). Equine antithymocyte globulin (15 mg/kg IV once every 12 hours for 6 doses) was added for a subgroup of patients (n = 23) who had received no multidrug chemotherapy within 3 months of alloHCT. Myeloablative conditioning (MAC) most often included cyclophosphamide (60 mg/kg IV once per day for 2 days) and 1320 cGy TBI divided into 8 fractions. The remaining group received busulfan (12.8 mg/kg), fludarabine, and melphalan (n = 4) or busulfan-cyclophosphamide (n = 3). In MAC regimens, each TBI dose and busulfan dose was higher than the myeloablative doses indicated by conditioning intensity working definitions from the Center for International Blood and Marrow Transplant Research (eg, TBI ≥800 cGy and busulfan >8 mg/kg).26
GVHD prophylaxis for UCB and RIC HCT consisted of cyclosporine and mycophenolate mofetil or sirolimus plus mycophenolate mofetil. Mycophenolate mofetil was discontinued on day +30. During the remainder of the study, cyclosporine was used (most often with short-course methotrexate; n = 27). Filgrastim was administered to all patients from day +1 until the absolute neutrophil count was more than 2.5 × 109/L for 2 days. Institutional standard antimicrobial prophylaxis using fungal, bacterial, and viral agents was administered to all patients.
Statistical methods.
The primary objective of this observational study was to study the association between the number of mast cells in GIT aGVHD and 1-year nonrelapse mortality (NRM). The clinical data and patient and transplant characteristics were prospectively collected but retrospectively reviewed. GIT aGVHD was defined as having 4 or 5 conventional clinical stages. One-year NRM and relapse were assessed by using Fine and Gray regression,27 and overall survival (OS) was assessed by using Cox regression when measuring their association with the number of mast cells, treating the number of mast cells as a continuous measure. OS and NRM by quartiles of mast cells were estimated by Kaplan-Meier curves and cumulative incidence, respectively. The distribution of mast cells was compared across patient characteristics using the general Wilcoxon or Kruskal-Wallis test. The Jonkheere-Terpstra test for trend was used to evaluate the stage of GIT GVHD against the number of mast cells.28 Given the number of comparisons, the false-discovery rate was used to adjust for the type I error in these comparisons. P values were 2-sided. SAS 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Patient characteristics
Fifty-six patients were included in the study (Table 1). The median age was 56 years, with most patients (70%) undergoing alloHCT for treatment of acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome. In most cases, transplant grafts were from UCB (55%), and some transplant donors were matched siblings (36%). Combined cyclosporine and mycophenolate mofetil was the most commonly used prophylaxis for GVHD (63%).
Patient characteristics
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| Total no. of patients | 56 | |||
| Male sex | 28 | 50 | ||
| Age, y | 53 | 2-68 | ||
| Donor type | ||||
| Matched sibling | 20 | 36 | ||
| Haploidentical | 1 | 2 | ||
| Matched unrelated donor | 4 | 7 | ||
| Single-unit umbilical cord blood | 8 | 14 | ||
| Double-unit umbilical cord blood | 23 | 41 | ||
| Diagnosis | ||||
| AA | 1 | 2 | ||
| ALL/AML/MDS | 39 | 70 | ||
| CML | 2 | 4 | ||
| NHL/HL | 8 | 14 | ||
| MPN/MM | 6 | 11 | ||
| Disease risk index | ||||
| Low | 11 | 20 | ||
| Intermediate | 34 | 61 | ||
| High | 7 | 13 | ||
| Very high | 3 | 5 | ||
| Nonmalignant | 1 | 2 | ||
| Conditioning | ||||
| MAC/TBI | 25 | 45 | ||
| MAC/no TBI | 4 | 7 | ||
| RIC | 27 | 48 | ||
| GVHD prophylaxis | ||||
| Cyclosporine A/methotrexate | 10 | 18 | ||
| Cyclosporine A/mycophenolate mofetil | 35 | 63 | ||
| Sirolimus/mycophenolate mofetil | 7 | 13 | ||
| Other | 4 | 7 | ||
| Karnofsky score | ||||
| ≤80 | 11 | 20 | ||
| >80 | 45 | 80 | ||
| HCT-specific comorbidity index | ||||
| Low risk | 16 | 29 | ||
| Intermediate risk | 19 | 34 | ||
| High risk | 20 | 36 | ||
| Missing | 1 | 2 | ||
| Total nucleated cell count × 108/kg | 0.8 | 0.2-18.6 | ||
| CD34 × 106/kg | 1.4 | 0.02-21.8 | ||
| CD3 × 108/kg | 0.1 | 0.02-0.9 |
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| Total no. of patients | 56 | |||
| Male sex | 28 | 50 | ||
| Age, y | 53 | 2-68 | ||
| Donor type | ||||
| Matched sibling | 20 | 36 | ||
| Haploidentical | 1 | 2 | ||
| Matched unrelated donor | 4 | 7 | ||
| Single-unit umbilical cord blood | 8 | 14 | ||
| Double-unit umbilical cord blood | 23 | 41 | ||
| Diagnosis | ||||
| AA | 1 | 2 | ||
| ALL/AML/MDS | 39 | 70 | ||
| CML | 2 | 4 | ||
| NHL/HL | 8 | 14 | ||
| MPN/MM | 6 | 11 | ||
| Disease risk index | ||||
| Low | 11 | 20 | ||
| Intermediate | 34 | 61 | ||
| High | 7 | 13 | ||
| Very high | 3 | 5 | ||
| Nonmalignant | 1 | 2 | ||
| Conditioning | ||||
| MAC/TBI | 25 | 45 | ||
| MAC/no TBI | 4 | 7 | ||
| RIC | 27 | 48 | ||
| GVHD prophylaxis | ||||
| Cyclosporine A/methotrexate | 10 | 18 | ||
| Cyclosporine A/mycophenolate mofetil | 35 | 63 | ||
| Sirolimus/mycophenolate mofetil | 7 | 13 | ||
| Other | 4 | 7 | ||
| Karnofsky score | ||||
| ≤80 | 11 | 20 | ||
| >80 | 45 | 80 | ||
| HCT-specific comorbidity index | ||||
| Low risk | 16 | 29 | ||
| Intermediate risk | 19 | 34 | ||
| High risk | 20 | 36 | ||
| Missing | 1 | 2 | ||
| Total nucleated cell count × 108/kg | 0.8 | 0.2-18.6 | ||
| CD34 × 106/kg | 1.4 | 0.02-21.8 | ||
| CD3 × 108/kg | 0.1 | 0.02-0.9 |
AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM multiple myeloma; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma.
The median number of GIT mast cells in these 56 patients was 59 per high-power field (range, 3-142 GIT mast cells; Table 2). Mast cell numbers were not associated with patient age, sex, donor type, disease type, disease risk index (DRI), or HCT-specific comorbidity index (Table 3). However, conditioning intensity and myeloablative TBI were significantly associated with mast cell numbers. Patients receiving RIC had higher mast cell numbers than those who received MAC (especially MAC with TBI) (Table 3). GIT GVHD stage was not significantly different between patients who received RIC or MAC HCTs (MAC: stage 1, 20%; stage 2, 20%; stage 3, 53%; and stage 4, 7%; RIC: stage 1, 10%; stage 2, 40%; stage 3, 40%; and stage 4, 10%; P = .54). CD3 and CD34+ counts and total nucleated cell counts were not correlated with mast cell counts and were not associated with GIT aGVHD.
Correlation of mast cells with clinical characteristics
| Factor . | No. . | Median . | Range . | P . |
|---|---|---|---|---|
| Total no. of mast cells | 56 | 59 | 3-142 | |
| Sex | NS | |||
| Male | 28 | 55.5 | 15-142 | |
| Female | 28 | 60 | 3-118 | |
| Age, y | NS | |||
| <18 | 4 | 40.5 | 3-100 | |
| ≥18 | 52 | 59.5 | 13-142 | |
| Donor type | NS | |||
| Matched sibling/haploidentical | 20/1 | 59 | 13-142 | |
| Matched unrelated donor | 4 | 66 | 26-100 | |
| Single-unit umbilical cord blood | 8 | 51 | 23-126 | |
| Double-unit umbilical cord blood | 23 | 60 | 3-118 | |
| Diagnosis | NS | |||
| AA | 1 | 26 | ||
| ALL/AML/MDS/MPN | 39 | 60 | 3-142 | |
| CML | 2 | 55 | 51-59 | |
| NHL/HL | 8 | 53.5 | 25-78 | |
| MM | 6 | 75.5 | 47-126 | |
| Disease risk index | NS | |||
| Low | 11 | 50 | 3-78 | |
| Intermediate | 34 | 67.5 | 13-142 | |
| High/very high | 10 | 58 | 32-91 | |
| Nonmalignant | 1 | 26 | ||
| Conditioning | <.01* | |||
| MAC | 29 | 47 | 3-100 | |
| RIC | 27 | 78 | 32-142 | |
| GVHD prophylaxis | NS | |||
| Cyclosporine A/methotrexate | 10 | 59 | 25-100 | |
| Cyclosporine A/mycophenolate mofetil | 35 | 59 | 3-142 | |
| Sirolimus/mycophenolate mofetil | 7 | 60 | 32-126 | |
| Other | 4 | 50 | 23-91 | |
| Karnofsky score | NS | |||
| ≤80 | 11 | 50 | 23-129 | |
| >80 | 45 | 59 | 3-142 | |
| HCT-specific comorbidity index | NS | |||
| Low risk | 16 | 46.5 | 3-126 | |
| Intermediate risk | 19 | 67 | 13-142 | |
| High risk | 20 | 59.5 | 26-118 | |
| Missing | 1 | — |
| Factor . | No. . | Median . | Range . | P . |
|---|---|---|---|---|
| Total no. of mast cells | 56 | 59 | 3-142 | |
| Sex | NS | |||
| Male | 28 | 55.5 | 15-142 | |
| Female | 28 | 60 | 3-118 | |
| Age, y | NS | |||
| <18 | 4 | 40.5 | 3-100 | |
| ≥18 | 52 | 59.5 | 13-142 | |
| Donor type | NS | |||
| Matched sibling/haploidentical | 20/1 | 59 | 13-142 | |
| Matched unrelated donor | 4 | 66 | 26-100 | |
| Single-unit umbilical cord blood | 8 | 51 | 23-126 | |
| Double-unit umbilical cord blood | 23 | 60 | 3-118 | |
| Diagnosis | NS | |||
| AA | 1 | 26 | ||
| ALL/AML/MDS/MPN | 39 | 60 | 3-142 | |
| CML | 2 | 55 | 51-59 | |
| NHL/HL | 8 | 53.5 | 25-78 | |
| MM | 6 | 75.5 | 47-126 | |
| Disease risk index | NS | |||
| Low | 11 | 50 | 3-78 | |
| Intermediate | 34 | 67.5 | 13-142 | |
| High/very high | 10 | 58 | 32-91 | |
| Nonmalignant | 1 | 26 | ||
| Conditioning | <.01* | |||
| MAC | 29 | 47 | 3-100 | |
| RIC | 27 | 78 | 32-142 | |
| GVHD prophylaxis | NS | |||
| Cyclosporine A/methotrexate | 10 | 59 | 25-100 | |
| Cyclosporine A/mycophenolate mofetil | 35 | 59 | 3-142 | |
| Sirolimus/mycophenolate mofetil | 7 | 60 | 32-126 | |
| Other | 4 | 50 | 23-91 | |
| Karnofsky score | NS | |||
| ≤80 | 11 | 50 | 23-129 | |
| >80 | 45 | 59 | 3-142 | |
| HCT-specific comorbidity index | NS | |||
| Low risk | 16 | 46.5 | 3-126 | |
| Intermediate risk | 19 | 67 | 13-142 | |
| High risk | 20 | 59.5 | 26-118 | |
| Missing | 1 | — |
NS, not significant.
False discovery rate, 0.02.
GVHD characteristics
The median time to onset of aGVHD was 37 days (range, 15-180 days). Of 56 patients, 35 (62%; 95% confidence interval [CI], 49%-75%) were diagnosed with GIT GVHD, confirmed histopathologically, and treated for GVHD. Of the 35 patients with lower maximal stage GIT GVHD, 5 (14%) had stage 1, 11 (31%) had stage 2, 16 (46%) had stage 3, and 3 (9%) had stage 4 (Table 2). Maximum clinical grading for these patients was grade 2 for 6 patients (17%), grade 3 for 25 patients (71%), and grade 4 for 4 patients (11%).
Mast cells and GVHD
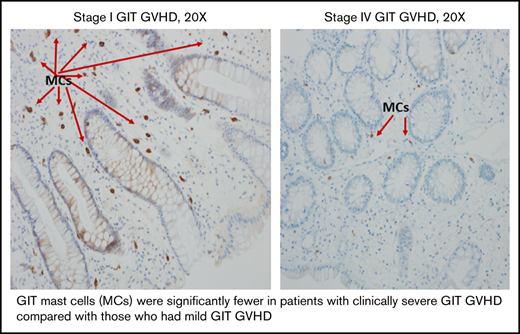
Mast cell numbers were similar in alloHCT patients with GIT GVHD (median, 59; range, 3-142) and in those without GIT GVHD (median, 60; range, 16-129; P = .92; Table 2). However, in patients with GVHD, fewer mast cells were observed with increasing clinical GIT GVHD severity (P = .01; Table 2; Figure 1A-B). There was no association between time of onset for aGVHD and the number of mast cells. All patients who received an alloHCT, regardless of GVHD, had significantly fewer GIT mast cells compared with those who had IBD (Table 2; Figure 1C).
Mast cells in patients with clinical GIT GVHD and IBD. Stage 1 (A) and stage 4 (B) GIT GVHD. (C) Increased mast cells in a patient with IBD.
Mast cells in patients with clinical GIT GVHD and IBD. Stage 1 (A) and stage 4 (B) GIT GVHD. (C) Increased mast cells in a patient with IBD.
Mast cells and NRM
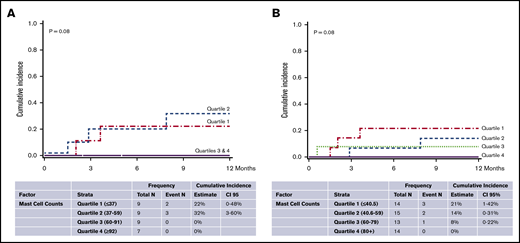
We evaluated the effect of mast cell counts on NRM in patients with GVHD as well as in the entire cohort of alloHCT patients (ie, for both GVHD positive and negative patients). In each analysis, higher quartiles of mast cell counts were associated with low NRM (Figure 2A-B) compared with lower quartiles. Moreover, in all of the patients who had received alloHCT, for every 10-unit increase in mast cell count, there was an associated 23% decrease in 1-year NRM (hazard ratio [HR], 0.77; 95% CI, 0.59-1.00; P = .05) when using a Fine and Gray regression model that treated mast cells as a continuous variable. In all alloHCT patients, neither OS at 2 years (HR, 0.94; 95% CI, 0.88-1.09; P = .44) nor relapse at 2 years (HR, 1.05; 95% CI, 0.92-1.19; P = .48) was associated with the number of GIT mast cells.
NRM by quartiles of mast cell counts. In patients with GVHD (A) and all patients with alloHCT (B).
NRM by quartiles of mast cell counts. In patients with GVHD (A) and all patients with alloHCT (B).
Discussion
In this study, we showed that fewer GIT mast cells were associated with increased severity of GIT aGVHD and higher NRM. We carefully planned several steps into our analysis procedures to ensure data quality. First, to optimize mast cell counting in the tissues, we used CD117 immunostaining to detect mast cells, which is superior to staining intracellular granules. This helps avoid the problem of underestimating the mast cell numbers because of degranulated mast cells.29 To eliminate treatment effect, we included only tissues from biopsies obtained before any steroid treatment.
These results may suggest that GIT mast cells are associated with less severe GIT GVHD (ie, they are more protective or immunomodulatory rather than inflammatory) or that the tissue damage caused by GVHD was associated with depletion of mast cells. Consistent with our study in humans, a murine GVHD model demonstrated that lack of mast cells was associated with more severe GVHD and thus inferior survival in C57BL/6-KitW-sh/W-sh mice compared with C57BL/6 wild-type (WT) recipients (13 vs 60 days; P < .0001).22 In mast cell–deficient mice, there were increased numbers of T cells in lymph nodes, liver, and the GIT, but the number and function of regulatory T cells (Tregs) was not decreased. Moreover, survival of C57BL/6-KitW-sh/W-sh mice with GVHD was significantly improved after engrafting with bone marrow–derived cultured mast cells from WT C57BL/6 mice. In another animal study, mast cells improved the tolerance and survival of allogeneic skin grafts in mice.30
Once mast cells are stimulated by specific proinflammatory, tissue-destructive peptides (endothelin-1, neurotensin, or snake venom), they can mitigate peptide-induced tissue damage by cleaving (destroying) these peptides.31,32 Tregs are another way that mast cells can suppress inflammatory reactions30 because they have crucial suppressive functions in self-tolerance, graft tolerance, and rejection.33-37 Mast cells stimulate Tregs by producing interleukin-10 (IL-10).38,39 In a murine study, the reduced GVHD from mast cells was a result of decreasing conventional T-cell proliferation via a mechanism involving IL-10,22 and the effect was independent of Tregs.
AlloHCT recipients had fewer GIT mast cells than patients with IBD. Others have reported that patients with IBD have more mast cells in their GIT than healthy controls do.29,40,41 Moreover, activity of these mast cells has been increased in IBD or experimental colitis.41-44 This leads to an interesting hypothesis that mast cells can be protective in acute gut GVHD (an alloreactive disorder) but can promote gut injury in an autoimmune disorder. However, these findings may result from the fact that biopsies obtained in different settings of inflammation show differing numbers and functions of tissue-resident mast cells. Indeed, when inflammation subsides, mast cells have more prominent immunoregulatory functions, such as preventing excessive tissue damage and the development of chronic inflammation.45,46 He et al47 showed that mast cells played a significant role in tissue healing via IL-33/ST2 signaling in experimental colitis induced in mice.
Interestingly, our study also indicated that the intensity of the conditioning regimen might influence mast cell numbers because RIC was associated with a higher number of mast cells compared with MAC. Because MAC regimens48 are associated with increased aGVHD, this mast cell depletion could be a contributing factor.49,50
One other human study evaluated cutaneous mast cells in patients with skin GVHD.23 Mast cell density was not associated with the grade of acute skin GVHD, but increased Tregs were found in patients with higher-grade skin GVHD. Moreover, mast cells may have a different role in chronic GVHD (cGVHD). In a murin skin cGVHD model, WT mice (C57BL/6J) had significantly more severe cGVHD than mast cell–deficient (B6.Cg-KitW-sh) mice.51 This murine clinical scoring correlated with a significant increase in skin pathology, collagen deposition, and expression of profibrotic genes in WT mice compared with mast cell–deficient mice.
In conclusion, although our study suggests that tissue mast cell numbers in the GIT may be associated with the severity of GVHD and NRM, the small number of patients and not having tissue samples from different time points limit our findings and interpretations. Additional larger studies are needed to better understand the role of mast cells in GVHD, which is likely very complex and may depend on the phase of GVHD (acute vs chronic) or the organ involved (skin vs gut). These studies, however, are warranted because of their potential to lead to new treatment options for patients with GVHD.
Data may be obtained by sending an e-mail request to Celalettin Ustun at celalettin_ustun@rush.edu.
Authorship
Contribution: C.U. conceived the study hypothesis and collected and analyzed data; K.A. evaluated pathologic samples and collected data; T.E.D. collected and analyzed data; F.K.K. collected data; D.J.W., S.G.H., and B.R.B. analyzed data; and H.D.Y., S.N., and C.G.B., along with all of the other authors, helped search the literature and helped write and edit the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Celalettin Ustun, Division of Hematology Oncology and Cellular Therapy, Department of Medicine, Rush University, 1725 W Harrison St, Suite 39, Chicago, IL 60612; e-mail: celalettin_ustun@rush.edu.