Key Points
Lenalidomide maintenance increases the depth of response in myeloma, promoting the achievement of MRD negativity with a survival benefit.
The monitoring of MRD kinetics identifies patients with different prognoses and may help in their clinical management.
Abstract
Lenalidomide is an immunomodulatory drug approved for maintenance treatment in newly diagnosed multiple myeloma, and it has been shown to improve progression-free survival (PFS) and, in several studies, overall survival. Nevertheless, the impact of prolonged treatment with lenalidomide on the kinetics of minimal residual disease (MRD) and its prognostic impact have not been studied in depth. To obtain better knowledge in this regard, we retrospectively analyzed 139 patients who received lenalidomide maintenance in real-world clinical practice and whose MRD levels were observed during the treatment period by multiparametric flow cytometry or next-generation sequencing with a sensitivity of at least 10−4. Lenalidomide maintenance correlated with an increased depth of the disease response, with 38.1% of patients achieving maximal response during maintenance. Moreover, 34.3% of patients who were MRD positive after induction treatment achieved MRD-negative status during maintenance and ultimately had improved PFS. Sequential MRD assessments identified patients with progressively decreasing MRD levels who also had better PFS outcomes, compared with patients not showing a decreasing pattern of MRD. These results support the role of maintenance therapy, not only to sustain, but also to increase the depth of disease response with a PFS benefit. In addition, MRD monitoring during maintenance identifies patients with better prognosis and may help in their clinical management.
Introduction
Maintenance therapy has been considered to be a key component in the treatment of multiple myeloma (MM) for at least a decade.1-4 However, despite its proven benefits, it has only very recently been approved by all regulatory agencies. The ideal maintenance should be convenient (therefore oral) and well tolerated and, for these reasons, most treatments for MM have been unsuitable for prolonged maintenance.
The underlying concept of maintenance is to control the disease by both direct tumoricidal activity against malignant plasma cells and enhancement of the immune response. Lenalidomide is an immunomodulatory drug approved as maintenance treatment in patients with newly diagnosed MM. In large randomized phase 3 clinical trials, lenalidomide maintenance has demonstrated an improvement in progression-free survival (PFS) and overall survival (OS) in both elderly and younger patients5-8 ; however, several concerns have been raised including second primary neoplasms (SPNs). Nonetheless, an overall benefit has been confirmed along with a positive impact on quality of life and use of health resources.9-11
A recent study has described the pattern of molecular evolution at relapse after lenalidomide maintenance.12 Also, the positive effect of lenalidomide maintenance therapy on the proportion of patients achieving minimal residual disease (MRD)–negative status has recently been reported,13 with a related impact on survival. Some questions remain unanswered, such as the ideal length of the maintenance treatment, and there is a paucity of data on the effect of lenalidomide maintenance therapy on the depth of response, including all response categories. Accordingly, a more detailed exploration of the kinetics of MRD and its prognostic implications is needed.
This retrospective study is focused on the clinical impact of the real-world use of lenalidomide maintenance, particularly in terms of PFS and evolution of MRD status.
Methods
Patients
We performed a retrospective analysis on 139 patients with newly diagnosed MM from 3 health centers who had available MRD data: University of California San Francisco (UCSF; n = 75; San Francisco, CA); Hospital Universitario 12 de Octubre (H12O; n = 48; Madrid, Spain); and Hospital Universitario Virgen de las Nieves (HVN; n = 16; Granada, Spain). The study was approved by the UCSF and H12O Institutional Review Boards (no. 15-17721). Patients included in the study received lenalidomide maintenance treatment during first-line therapy from 2010 through 2018. We gathered all available data regarding clinical and biological parameters, induction treatment, response monitoring, and adverse events (AEs).
Baseline features of patients were used to characterize the disease at the beginning of the specific period (Table 1). When available, cytogenetic data based on fluorescence in situ hybridization and karyotype were collected, and cytogenetic risk was classified according to International Myeloma Working Group (IMWG) consensus updates.14,15 Disease assessment by imaging was conducted in some patients (n = 119) with 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT), according to previously described procedures.16 The induction schemes before maintenance were heterogeneous (Table 1), and 83.5% of the patients (n = 116) received ASCT. Maintenance treatment was performed with oral lenalidomide once daily on days 1 to 21 of repeated 28-day cycles. The starting dose was 10 to 15 mg/d, which was subsequently adjusted individually according to tolerability. In addition, 43.2% of patients received concomitant treatment with dexamethasone during maintenance, usually at a dose of 40 mg/wk (20 mg for patients >75 years of age) with later adjustments according to toxicity.
Baseline patient characteristics and summary of first-line treatments
| . | Total . |
|---|---|
| Characteristics | |
| Male/female, n (%) | 73 (52.5)/66 (47.5) |
| Mean age (SD), y | 59.5 (9.5) |
| Myeloma type IgG/IgA/light chains/other, n (%) | 83 (59.7)/30 (21.6)/24 (17.3)/2 (1.4) |
| Serum creatinine ≥2 mg/dL, n (%) | 25 (18.0) |
| SR/HR cytogenetics, n (%) | 50 (63.3)/29 (36.7) |
| ISS I/II/III, n (%) | 43 (33.3)/39 (30.2)/47 (36.4) |
| Positive PET-CT, n (%) | 15 (10.8) |
| First line treatment, n (%) | |
| VD, VMP, VCD, VBP | 34 (24.5) |
| VTD | 23 (16.5) |
| VRD | 60 (43.2) |
| KRD | 5 (3.6) |
| RD, MPR, RCD | 8 (5.8) |
| Chemotherapy-based regimens | 9 (6.5) |
| ASCT | 116 (83.5) |
| Consolidation | 14 (10.1) |
| . | Total . |
|---|---|
| Characteristics | |
| Male/female, n (%) | 73 (52.5)/66 (47.5) |
| Mean age (SD), y | 59.5 (9.5) |
| Myeloma type IgG/IgA/light chains/other, n (%) | 83 (59.7)/30 (21.6)/24 (17.3)/2 (1.4) |
| Serum creatinine ≥2 mg/dL, n (%) | 25 (18.0) |
| SR/HR cytogenetics, n (%) | 50 (63.3)/29 (36.7) |
| ISS I/II/III, n (%) | 43 (33.3)/39 (30.2)/47 (36.4) |
| Positive PET-CT, n (%) | 15 (10.8) |
| First line treatment, n (%) | |
| VD, VMP, VCD, VBP | 34 (24.5) |
| VTD | 23 (16.5) |
| VRD | 60 (43.2) |
| KRD | 5 (3.6) |
| RD, MPR, RCD | 8 (5.8) |
| Chemotherapy-based regimens | 9 (6.5) |
| ASCT | 116 (83.5) |
| Consolidation | 14 (10.1) |
ASCT, autologous stem cell transplant; B, bendamustine; C, cyclophosphamide; D, dexamethasone; HR, high risk; Ig, immunoglobulin; K, carfilzomib; M, melphalan; P, prednisone; R, lenalidomide; T, thalidomide; V, bortezomib; ISS, International Staging System; SD, standard deviation.
MRD assessment
MRD in patients from H12O and HVN was followed up by multiparametric flow cytometry (MFC). Fresh bone marrow samples were collected for MRD analysis before treatment began, and at various time points during treatment and subsequent follow-up periods. Erythrocyte-lysed whole bone marrow samples were immunophenotyped, acquiring 1 to 2 × 106 events and using a second-generation 4-color antibody combination, as previously reported.17,18 Data acquisition was performed with FACSCalibur and FACSCanto II flow cytometers (Becton-Dickinson, San Jose, CA) and analyzed with Infinicyt software (Cytognos, Salamanca, Spain). These procedures, performed according to standards used by the Spanish Myeloma Group, were homogeneous between the 2 hospitals. During follow-up, MRD negativity was determined when phenotypically aberrant plasma cells were absent, with a sensitivity of at least 10−4.
At UCSF, fresh bone marrow samples from patients were sent to Adaptive Biotechnologies (Seattle, WA), and MRD assessment was performed by commercially available next-generation sequencing (NGS) of immunoglobulin genes. Patients in whom a high-frequency myeloma clone (>5%) was not identified were excluded from the MRD analysis. MRD was assessed in patients with a high-frequency myeloma clone using the IGH-VDJH and IGK or IGH-VDJH, IGH-DJH, IGK, and IGL assays. Once the absolute amount of total cancer-derived molecules present in a sample was determined, a final MRD measurement was calculated, providing the number of cancer-derived molecules per 1 million cell equivalents. In cases with 2 or more tumor clones, the clone with the highest MRD value was reported.18
MRD assessments were performed before starting maintenance treatment and/or at the achievement of complete response (CR). Additional assessments were subsequently performed on an annual basis until sustained MRD negativity was confirmed. MRD-negative status was achieved at less than 10−4. Overall, 387 bone marrow samples from the patients included in this study were analyzed by MFC or NGS.
Response and toxicity criteria
Statistical analysis
All data were included in a REDCap database (Vanderbilt University, Nashville, TN) and Microsoft Excel files. Statistical analysis was performed with the SPSS program (Statistical Package for Social Sciences Inc., Chicago, IL; version 22.0). Continuous variables were presented as means ± standard deviation (SD) or median ± interquartile range (IQR), and relative and absolute frequencies were used for qualitative variables. PFS and OS were defined as the duration from the start of maintenance to the occurrence of the event (progression or death for PFS and death for OS). Survival curves were plotted by the Kaplan-Meier method and the log-rank test was used to estimate the statistical significance of differences observed between plots. Univariable and multivariable analyses were performed by using an adjusted stepwise Cox proportional regression hazard model. The χ2 and Fisher’s exact 2-sided tests were used for comparisons between categorical variables, and analysis of variance, Mann-Whitney U test, Student t test, or Wilcoxon rank sum test were used for continuous variables. Results reaching P < .05 were significant.
Results
Descriptive analysis
The median age for the entire cohort was 60 years (IQR, 53-66 years). The median duration of lenalidomide maintenance was 21 months, with 25% of patients receiving <11 months of maintenance and 25% with treatment longer than 31 months. The treatment had been discontinued in 64.7% (n = 90) of the patients when the analysis was performed. For the entire cohort of 139 patients, median PFS was 61 months, and 5-year OS was achieved in 82.6%. Overall, 18.7% of patients (n = 26) relapsed while they were receiving maintenance treatment.
Maintenance and depth of response
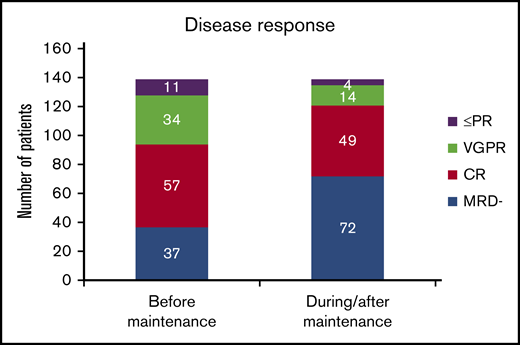
When disease response was assessed before the beginning of maintenance, 32.4% of the patients (n = 45) had not achieved CR, and in the remaining 67.6% with CR (n = 94), 37 patients had achieved MRD-negative status (26.6% of the whole series).
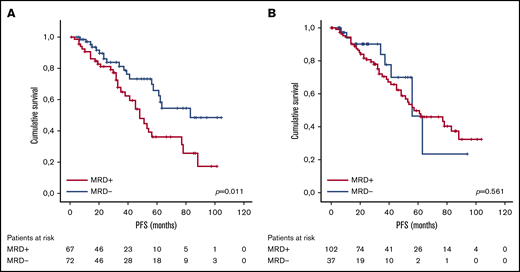
With lenalidomide maintenance, the percentage of patients with <CR was reduced to 12.9% (n = 18), and the final number of patients who achieved MRD negativity increased from 37 to 72 (51.8% of patients included; Figure 1). Of note, this result indicates that 38.1% of patients (n = 53) achieved maximal response during the maintenance period, and 34.3% (n = 35) of those who were MRD positive before maintenance achieved MRD negativity over the course of lenalidomide maintenance. For those patients who became MRD negative during maintenance, the median time to the achievement of MRD-negative status was 18.5 months from the start of maintenance (IQR, 9.0-35.5 months). No significant association was detected between the scheme of induction treatment administered and the achievement of MRD negativity at maximal response (P = .983; Table 2). We did not find significant differences in PFS between patients who achieved MRD negativity, as assessed by MFC (n = 38) or NGS (n = 34; P = .115). Globally, achievement of MRD-negative status at any time (before or during maintenance) was associated with improved PFS (median PFS, 83 months for MRD negative vs 48 months for MRD positive, P = .01; Figure 2A), with no differences in PFS between patients who achieved MRD-negative status before or during maintenance treatment (P = .34). We detected a significant impact of MRD negativity on PFS when considering MRD status only at maximal response, but not at the start of maintenance (Figure 2B). Still, 19 of the 72 patients (26.4%) who achieved MRD negativity ultimately relapsed.
Comparison of response status at the beginning of lenalidomide maintenance and at maximal response. PR, partial response; VGPR, very good partial response.
Comparison of response status at the beginning of lenalidomide maintenance and at maximal response. PR, partial response; VGPR, very good partial response.
Patient characteristics at baseline grouped according to MRD stratification at maximal response
| Characteristic . | MRD+ . | MRD− . | P . |
|---|---|---|---|
| Male/female, n (%) | 35 (52.2)/32 (47.8) | 38 (52.8)/34 (47.2) | .949 |
| Mean age (SD), y | 61.4 (8.5) | 57.8 (10.2) | .029 |
| Serum creatinine ≥2 mg/dL, n (%) | 8 (11.9) | 17 (23.6) | .073 |
| HR cytogenetics, n (%) | 15 (41.7) | 14 (32.6) | .403 |
| ISS I/II/III, n (%) | 22 (35.5)/22 (35.5)/18 (29) | 21 (31.3)/17 (25.4)/29 (43.3) | .215 |
| Positive PET-CT, n (%) | 5 (8.8) | 10 (15.4) | .262 |
| Characteristic . | MRD+ . | MRD− . | P . |
|---|---|---|---|
| Male/female, n (%) | 35 (52.2)/32 (47.8) | 38 (52.8)/34 (47.2) | .949 |
| Mean age (SD), y | 61.4 (8.5) | 57.8 (10.2) | .029 |
| Serum creatinine ≥2 mg/dL, n (%) | 8 (11.9) | 17 (23.6) | .073 |
| HR cytogenetics, n (%) | 15 (41.7) | 14 (32.6) | .403 |
| ISS I/II/III, n (%) | 22 (35.5)/22 (35.5)/18 (29) | 21 (31.3)/17 (25.4)/29 (43.3) | .215 |
| Positive PET-CT, n (%) | 5 (8.8) | 10 (15.4) | .262 |
PFS according to MRD status. (A) At maximal response during or after maintenance treatment with lenalidomide. (B) At the beginning of maintenance treatment with lenalidomide.
PFS according to MRD status. (A) At maximal response during or after maintenance treatment with lenalidomide. (B) At the beginning of maintenance treatment with lenalidomide.
An additional subanalysis was performed, including 35 patients (25.2%) who reached sustained MRD negativity during maintenance (confirmed in at least 2 assessments). In this subgroup, the outcome was compared between those who discontinued lenalidomide maintenance after sustained MRD negativity was confirmed (n = 20) and those who maintained treatment (n = 15). No difference in PFS was detected between the groups (respectively, 4-year PFS 75.5% vs 74.5%; P = .823).
Subgroup analysis in patients with prior ASCT
A separate analysis was performed selecting only the 116 patients who received ASCT before the maintenance therapy to confirm the results in this more homogeneous cohort. For this subgroup, the median age was 59.5 years (IQR, 52.0-65.0 years) with a median duration of maintenance of 20.5 months (IQR, 11.0-31.0 months). In this subgroup, median PFS was 77 months and 5-year OS was 88.3%.
In the assessment performed after ASCT and before the start of maintenance, there were 34 patients (29.4%) whose disease status was less than CR, and of the 82 patients with CR, 41.5% (n = 34) had achieved MRD negativity. During maintenance, the number of patients in CR increased to 102 (87.9% of total), with 58.8% of them MRD negative (n = 60). Overall, 35.3% of patients (n = 41) obtained maximal response during the maintenance period, with a median time to maximal response of 16 months (IQR, 9.5-31.0 months), including 26 patients who achieved MRD negativity at a median time of 21.5 months (IQR, 11-37.3 months).
In this subgroup, the achievement of MRD-negative status was associated with improved PFS outcome compared with those patients with detectable MRD (median PFS not reached vs 48 months, respectively; P = .02). There was no significant difference in PFS between those who achieved MRD negativity before or during the maintenance period (P = .073).
Cox regression analyses
A univariate approach involving a Cox proportional hazards model was used to individually test whether different categorized variables of interest were associated with improved PFS. In this analysis, only the achievement of MRD negativity and absence of active disease shown by PET-CT, both considered at maximal response for each patient, showed a significant impact on PFS, as did the cytogenetic risk according to IMWG consensus recommendations (Table 3), but not other factors, such as age, induction therapy, or previous ASCT. In the final model obtained in the subsequent multivariable analysis, HR cytogenetics (hazard ratio, 3.69; 95% confidence interval [CI], 1.35-10.10), MRD negativity (hazard ratio, 0.30; 95% CI, 0.10-0.87), and PET-CT negativity (hazard ratio, 0.07; 95% CI, 0.01-0.48) at maximal response remained predictive of PFS.
Risk factors for PFS: univariable and multivariable Cox regression analyses
| . | . | PFS (univariable) . | PFS (multivariable) . | ||||
|---|---|---|---|---|---|---|---|
| Variables . | Category . | Hazard ratio . | CI . | P . | Hazard ratio . | CI . | P . |
| Age | ≤70 y | 1 | — | .028 | — | — | — |
| >70 y | 2.48 | 1.11-5.56 | |||||
| Sex | Male | 1 | — | .197 | — | — | — |
| Female | 0.69 | 0.40-1.21 | |||||
| ISS | 1 | 1 | — | — | — | — | — |
| 2 | 1.14 | 0.56-2.32 | .712 | ||||
| 3 | 1.22 | 0.61-2.46 | .577 | ||||
| Serum creatinine | <2 mg/dL | 1 | — | .544 | — | — | — |
| ≥2 mg/dL | 0.79 | 0.37-1.69 | |||||
| Myeloma isotype | IgG | 1 | — | — | — | — | — |
| IgA | 0.77 | 0.38-1.57 | .472 | ||||
| Light chains | 1.15 | 0.56-2.36 | .700 | ||||
| Cytogenetic risk | SR | 1 | — | .001 | 1 | — | .011 |
| HR | 3.81 | 1.73-8.39 | 3.69 | 1.35-10.10 | |||
| Induction scheme | Others | 1 | — | — | — | — | — |
| PI-based | 0.15 | 0.02-1.12 | .151 | ||||
| IMiD-based | 1.05 | 0.56-1.95 | .148 | ||||
| IMiD+PI | 0.81 | 0.25-1.65 | .810 | ||||
| ASCT | No prior ASCT | 1 | — | .137 | — | — | — |
| Prior ASCT | 0.62 | 0.33-1.17 | |||||
| Consolidation treatment | No consolidation | 1 | — | .280 | — | — | — |
| Consolidation | 0.34 | 0.46-2.44 | |||||
| PET* | PET+ | 1 | — | <.001 | 1 | — | .007 |
| PET− | 0.16 | 0.06-0.41 | 0.07 | 0.01-0.48 | |||
| MRD* | MRD+ | 1 | — | .013 | 1 | — | .027 |
| MRD− | 0.49 | 0.27-0.86 | 0.30 | 0.10-0.87 | |||
| . | . | PFS (univariable) . | PFS (multivariable) . | ||||
|---|---|---|---|---|---|---|---|
| Variables . | Category . | Hazard ratio . | CI . | P . | Hazard ratio . | CI . | P . |
| Age | ≤70 y | 1 | — | .028 | — | — | — |
| >70 y | 2.48 | 1.11-5.56 | |||||
| Sex | Male | 1 | — | .197 | — | — | — |
| Female | 0.69 | 0.40-1.21 | |||||
| ISS | 1 | 1 | — | — | — | — | — |
| 2 | 1.14 | 0.56-2.32 | .712 | ||||
| 3 | 1.22 | 0.61-2.46 | .577 | ||||
| Serum creatinine | <2 mg/dL | 1 | — | .544 | — | — | — |
| ≥2 mg/dL | 0.79 | 0.37-1.69 | |||||
| Myeloma isotype | IgG | 1 | — | — | — | — | — |
| IgA | 0.77 | 0.38-1.57 | .472 | ||||
| Light chains | 1.15 | 0.56-2.36 | .700 | ||||
| Cytogenetic risk | SR | 1 | — | .001 | 1 | — | .011 |
| HR | 3.81 | 1.73-8.39 | 3.69 | 1.35-10.10 | |||
| Induction scheme | Others | 1 | — | — | — | — | — |
| PI-based | 0.15 | 0.02-1.12 | .151 | ||||
| IMiD-based | 1.05 | 0.56-1.95 | .148 | ||||
| IMiD+PI | 0.81 | 0.25-1.65 | .810 | ||||
| ASCT | No prior ASCT | 1 | — | .137 | — | — | — |
| Prior ASCT | 0.62 | 0.33-1.17 | |||||
| Consolidation treatment | No consolidation | 1 | — | .280 | — | — | — |
| Consolidation | 0.34 | 0.46-2.44 | |||||
| PET* | PET+ | 1 | — | <.001 | 1 | — | .007 |
| PET− | 0.16 | 0.06-0.41 | 0.07 | 0.01-0.48 | |||
| MRD* | MRD+ | 1 | — | .013 | 1 | — | .027 |
| MRD− | 0.49 | 0.27-0.86 | 0.30 | 0.10-0.87 | |||
IMiD, immunomodulatory drug; PI, proteasome inhibitor.
For these variables, the results of PET and MRD at maximal response were examined.
Maintenance and cytogenetic risk
Considering patients with available fluorescence in situ hybridization data at diagnosis, those with SR cytogenetics (n = 50) showed better survival outcomes than those with HR cytogenetics (n = 29; median PFS not reached vs 33 months; P < .001). The rate of MRD conversion in patients with detectable MRD before beginning maintenance therapy was higher in the SR subgroup (n = 28) than in the HR subgroup (n = 15), but the difference was not significant (respectively, 39.3% vs 26.7%; P = .623). Median PFS of patients with HR cytogenetics who achieved MRD negativity was 34 months.
Maintenance and PET-CT
In addition, 119 patients included in our series had a PET-CT evaluation in the assessment performed before the start of maintenance therapy. In 15 of those, persistent active disease was identified at that point, and PET-CT follow-up was implemented on an annual basis. Interestingly, 53.3% of these patients (n = 8) achieved suppression of FDG uptake during maintenance, with improved survival compared with those with sustained uptake (n = 7) (respectively, median PFS 61 months vs 11 months; P = .023). In 4 of those 7 patients, MRD negativity was achieved in bone marrow despite the persistence of active lesions shown on PET-CT. On the other hand, 2 of the 8 patients who achieved suppression of FDG uptake during maintenance showed persistent MRD positivity.
Serial measurements of MRD
To better understand the clinical value of MRD kinetics during treatment, we performed a further subgroup analysis with 52 patients who were MRD positive at the beginning of maintenance. In this subgroup, the evolution of MRD was tracked on an annual basis, which allowed us to classify patients according to the evolutionary pattern of MRD values. Of this subgroup, 25 achieved MRD-negative status at some point during maintenance, and this was confirmed with subsequent MRD assessments. Also, 12 patients with initial MRD positivity showed decreasing kinetics in subsequent MRD measurements (magnitude of more than 1 logarithm, compared with the initial MRD assessment) without achieving MRD negativity. Fifteen patients who were initially MRD positive subsequently did not show a significant reduction. Of note, in those patients with improvement in MRD values, the differences in PFS outcomes were not significant between those who did and did not ultimately achieve MRD-negative status (median PFS, 83 vs 88 months, respectively; P = .513). Furthermore, those patients with improving MRD who did not achieve MRD-negative status exhibited a survival benefit relative to those whose MRD values were not decreasing or were increasing (median PFS, 88 vs 54 months, respectively; P = .013). This finding was also true of those patients who ultimately achieved MRD negativity compared with patients with nondecreasing or increasing MRD values (P = .019). Of note, we identified 4 patients who became MRD positive after achieving MRD negativity, confirmed in 2 assessments without exhibiting criteria for progression; all 4 patients ultimately experienced disease relapse (3 of them during the following 6 months, but more than 2 years for the other ones).
Safety profile
We were performed safety analyses on 133 patients. A therapy-related AE grade >2 was observed in 34.6% of these patients (n = 46). The most common AEs included neutropenia (13.5%), thrombocytopenia (5.3%), fatigue (5.3%), and diarrhea (5.3%). There was 1 case of a grade 5 AE (infection). A thrombotic event of any grade was reported in 5.8% (n = 8) of patients during the maintenance period, despite the use of antithrombotic prophylaxis, which was adjusted to individual thrombotic/hemorrhagic risks. Nevertheless, treatment discontinuation was related to toxicity in 22.3% of patients (n = 31), with a median time to discontinuation in these cases of 20 months (IQR, 11-31 months). The occurrence of significant toxicity (AE grade >2) did not seem to have an impact on PFS (P = .392). Moreover, dose reduction of lenalidomide was required in 27.8% of patients (n = 37) during maintenance, but it did not seem to affect the achievement of MRD negativity (P = .498) or PFS outcomes (P = .894). An SPN was detected in 5.8% (n = 8) of patients (myelodysplastic syndrome; acute lymphoblastic leukemia; pituitary adenoma; and papillary thyroid, pancreatic, rectal, prostate, and breast carcinomas), with a median time to the diagnosis of a tumor of 44.0 months (IQR, 22.0-48.5).
Discussion
The use of lenalidomide as maintenance therapy in MM has been extensively studied and demonstrates an improvement in survival outcomes6,7,13,21,22 ; however, its effect on the depth and dynamics of disease response has not been thoroughly investigated. Indeed, there are limited data in nonclinical trial settings on the proportion of patients without MRD negativity at the start of maintenance therapy who show an improvement in disease response or who achieve MRD-negative status with maintenance treatment.13,23-25 Although our study is subject to the usual constraints of a retrospective observational study, the lack of data about the effect of lenalidomide maintenance on the evolution of MRD and the clinical significance of the MRD kinetics in this phase of MM treatment justifies our analysis, which could serve as a starting point for the inclusion of long-term serial MRD assessment in future prospective studies focused on maintenance therapy in MM.
The percentage of MRD negativity was 26.6% in our series before maintenance was started, which is in the range of that used in published series,17,25,26 with the proviso that induction treatments were heterogeneous. We found that more than one-third of all the patients included in our series achieved their maximum response during the maintenance period, including not only an improved depth of MRD response for patients in CR, but also an improvement in the response category for patients with less than CR before maintenance. Indeed, 34.3% of MRD-positive patients at the beginning of maintenance achieved MRD negativity with lenalidomide, which is in the range of what has been reported in previous studies.25 Survival outcomes were not significantly different between these patients and those who started the maintenance period with MRD negativity. A similar effect has been reported in the IFM 2009 clinical trial24 in patients who obtained MRD negativity during the first year of maintenance compared with those who were MRD negative before the beginning of maintenance. Also, in the context of the PRIMeR study,25 a subanalysis of the STaMINA trial showed that MRD negativity after 1 year of maintenance after ASCT was prognostic, not only for PFS, but also for OS. This finding may mean that lenalidomide not only maintains the response reached after induction with or without ASCT, but also significantly increases the depth of response, thus representing extended treatment rather than maintenance. In part because of the low power of our study, we did not observe significant differences in PFS according to MRD status before maintenance in contrast to what occurs if we consider MRD status at maximal response. This finding may be justified by the increased depth of response during maintenance, reinforcing the notion that lenalidomide maintenance significantly improves the prognosis of some patients without MRD negativity after induction/consolidation.
In addition, we have confirmed in our series that the achievement of MRD negativity in the maintenance setting translates into longer PFS. It is interesting to note that MRD negativity tended to occur after a prolonged treatment period (median time to MRD negativity was 18.5 months; IQR, 9.0-35.5 months); indeed, almost 30% of patients (n = 10) who achieved MRD-negative status during maintenance required more than 30 months of therapy. We believe this finding supports the importance of prolonging lenalidomide therapy for as long as possible in patients who are MRD positive (the longer, the better), as has been suggested.27,28 This may reflect not only the stabilization of disease, but also the deepening of response, as measured by MRD. In support of this conclusion, we noted that some patients with sequential MRD monitoring experienced a slow decrease in MRD over time, never achieving MRD negativity, but still obtaining a benefit in survival. That there was no difference in PFS between this group of patients with favorable kinetics without immunophenotypic and molecular remission and those who ultimately obtained MRD negativity is encouraging, as well as surprising. It would be interesting to test whether any differences in survival outcomes become evident with longer follow-up, or whether these patients ultimately achieve MRD-negative status after prolonged maintenance. (In this series we observed achievement of MRD negativity up to 65 months after the initiation of maintenance.) On the other hand, it is interesting to note the lack of difference, confirmed in at least 2 assessments, in PFS between patients with MRD negativity who continued maintenance treatment compared with those who discontinued lenalidomide at this point. Nevertheless, we are cautious about definitive recommendations on the length of maintenance for this subgroup of patients with good prognosis because of the limited sample size; but it would be of great interest to investigate this matter in depth in future studies.
Concerning sequential MRD assessments during maintenance, we observed that progressively declining MRD with maintenance therapy identifies a subgroup of patients who had favorable prognosis, even though they did not achieve MRD-negative status during the follow-up period. In addition, we detected a conversion from MRD negative to positive without a change in monoclonal protein or clinical symptoms suggestive of progression in 4 of 19 patients who relapsed after they had achieved MRD negativity. These observations underscore the importance of long-term sequential MRD monitoring, as it can indicate the continued value of the current therapy and discriminate subgroups of patients with differential prognoses. In some cases, it would also be useful to be alert about incipient relapses and the need to begin preemptive treatment.
Patients included in this study were assessed for MRD by MFC or NGS, depending on the center, after we confirmed in our series that survival outcomes were not affected by the technique used. It is important to note in this regard that previous studies have established the high degree of correlation between both techniques, with a similar performance in MRD assessment.29-31 Of interest, according to the multivariable analysis, the prognostic value of disease response determined by MRD assessment (MFC or NGS) and imaging techniques (PET-CT) remains more predictive than the type of induction treatment administered or other conventional risk factors, as has been proposed in previous studies supporting the complementarity of both assessments.16,32,33 In fact, prolonged therapy with lenalidomide achieved PET-CT negativity in more than 50% of patients who had pathological FDG uptake before maintenance, with those patients with persistent active lesions in PET-CT having an unfavorable prognosis. Nevertheless, the reduced number of patients with active disease in the initial PET-CT limited the possibility of performing a comprehensive analysis on its correlation with MRD results, in accordance with the findings of the aforementioned studies. These results reinforce the idea that an improvement in survival outcomes can be achieved with the use of prolonged and well-tolerated treatment and the achievement of deeper responses. Moreover, our results indicated that HR cytogenetics continues to represent a prognosis factor. Even when the absence of differences in the rate of MRD conversion between the HR and SR subgroups must be regarded cautiously because of the reduced sample size, there is still more than 29% of patients with HR cytogenetics who may improve their response and achieve MRD negativity during maintenance. Yet, in our series, lenalidomide maintenance did not overcome the unfavorable prognosis conferred by HR cytogenetics.
The safety profile of lenalidomide maintenance observed in our series is similar to that reported in previous clinical trials,6,8,21,34-36 including the incidence of SPN.6,7,35,37 Unfortunately, because of the design of the study, we did not have a control group for comparison. Although more than one-third of the assessed patients experienced grade >2 therapy-related AEs, the events were manageable and represented only 22% of the discontinuations. We found that most of the toxicity-related discontinuations were related to reduction of long-term tolerability, so they were performed late (median time to toxicity-related discontinuation, 21 months), usually after a certain level of stability of disease response had been confirmed. This finding may explain why we did not observe a significant impact on PFS outcomes in patients who experienced toxicity.
In conclusion, our findings confirm, in real-world clinical practice, the importance of lenalidomide maintenance, not only to maintain the response obtained after induction/consolidation therapy in newly diagnosed MM, but also to improve the depth and quality of this response and, consequently, to improve survival outcomes. This study should encourage MRD monitoring in patients receiving maintenance therapy, as it may identify subgroups of patients with different prognoses, and it could even help to inform clinical decisions. The identification of the category of favorable MRD kinetics without immunophenotypic or molecular response seems relevant because of the good PFS outcomes with improved prognosis compared with those with nondecreasing values, even when MRD negativity is not achieved. Our findings support the inclusion of serial MRD monitoring during maintenance as a prognostic tool and may justify the inclusion of MRD as a clinical and surrogate end point for future studies. Although this study is retrospective and requires prospective validation, the data confirm the suspected clinical significance of prolonged lenalidomide treatment in increasing the depth of disease response in MM.
Original data are available by e-mail request to the corresponding author.
Acknowledgments
The work was performed without financial support from any third party.
The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Authorship
Contribution: R.A., J.-J.L., and J.M.-L. designed the research; M.-T.C. contributed vital analytical tools; R.A., R.R.-T., C.G., J.M.M., J.L.-J., N.B., A.V., R.S., T.M., and J.M.-L. collected the data; R.A., M.-T.C., S.W., N.S., L.C.-Y., J.W., J.-J.L., and J.M.-L. analyzed and interpreted the data; and R.A., J.W., J.-J.L., and J.M.-L. wrote the manuscript.
Conflict-of-interest disclosure: N.S. reports consultancy and honoraria from Celgene, Janssen, Bluebird Bio, Sutro Biopharma, Genentech, Seattle Genetics, Oncopeptides, Karoypharm, Surface Oncology, Precision Biosciences, GSK, Nektar, Amgen, Indapta Therapeutics, Sanofi, and Indapta Therapeutics. R.R.-T. reports consulting or advisory role for Janssen and Celgene. J.M.-L. reports grants from Celgene, Janssen, and BMS, received during the current study, but not applied to the work; consultancy and honoraria from Incyte, Roche, Novartis, and BMS; and holds a patent (Método de cuantificación de Enfermedad Mínima Residual en un sujeto) for quantifying the level of MRD in a subject and is receiving royalties. The remaining authors declare no competing financial interests.
Correspondence: Joaquín Martínez-López, Hospital Universitario 12 de Octubre, Servicio de Hematología, Av De Córdoba s/n, Centro de Actividades Ambulatorias, Planta 3ª, Bloque D, 28041 Madrid, Spain; e-mail: jmarti01@med.ucm.es.
References
Author notes
J.-J.L. and J.M.-L. are joint senior authors of this study.