Key Points
z-Butylidenephthalide and z-ligustilide act as more potent HbF allosteric modulators than 2,3-BPG in lowering the oxygen affinity of HbF.
z-Butylidenephthalide and z-ligustilide aid HbF induction therapy for β-hemoglobinopathies by tuning HbF function closer to that of HbA.
Abstract
Fetal hemoglobin (HbF) induction therapy has become the most promising strategy for treating β-hemoglobinopathies, including sickle-cell diseases and β-thalassemia. However, subtle but critical structural difference exists between HbF and normal adult hemoglobin (HbA), which inevitably leads to reduced binding of the endogenous modulator 2,3-bisphosphoglycerate (2,3-BPG) to HbF and thus increased oxygen affinity and decreased oxygen transport efficiency of HbF. We combined the oxygen equilibrium experiments, resonance Raman (RR) spectroscopy, and molecular docking modeling, and we discuss 2 phthalides, z-butylidenephthalide and z-ligustilide, that can effectively lower the oxygen affinity of HbF. They adjust it to a level closer to that of HbA and make it a more satisfactory oxygen carrier for adults. From the oxygen equilibrium curve measurements, we show that the 2 phthalides are more effective than 2,3-BPG for modulating HbF. The RR spectra show that phthalides allosterically stabilize the oxygenated HbF in the low oxygen affinity conformation, and the molecular docking modeling reveals that the 2 chosen phthalides interact with HbF via the cleft around the γ1/γ2 interface with a binding strength ∼1.6 times stronger than that of 2,3-BPG. We discuss the implications of z-butylidenephthalide and z-ligustilide in boosting the efficacy of HbF induction therapy to mitigate the clinical severities of β-hemoglobinopathies.
Introduction
Fetal hemoglobin (HbF) induction therapy has become the most promising strategy for treating β-hemoglobinopathies, including sickle cell diseases and β-thalassemia.1-14 However, despite their common oxygen-carrying capabilities, critical structural difference exists between HbF and normal adult hemoglobin (HbA), preventing HbF from fully substituting HbA in transporting oxygen.15 The major difference between HbF and HbA lies in their binding to the endogenous modulator 2,3-bisphosphoglycerate (2,3-BPG). Although 2,3-BPG effectively modulates HbA via its β1/β2 cavity,16-18 2 of 3 crucial 2,3-BPG binding sites on HbA (βVal1 and βHis143) are replaced by glycine and serine on the γ globins of HbF, respectively (βVal1→γGly1 and βHis143→γSer143),15,19 resulting in significantly weaker 2,3-BPG binding to HbF and therefore higher oxygen affinity for HbF. Although the higher affinity of HbF ensures that the fetus can extract sufficient oxygen from the maternal blood, when HbF is re-induced in adults, the higher oxygen affinity makes HbF less efficient than HbA in releasing oxygen to organs and tissue cells. To bring the HbF induction therapy to its full potential, it is essential to identify suitable HbF modulators to optimize its oxygen transport efficiency to a level comparable to that of HbA.
Extensive studies have been made in past decades to identify and/or synthesize potent allosteric modulators for regulating HbA oxygen affinity20-25 and to understand the HbA allosteric transition mechanism.17,26-31 Despite the tremendous early efforts to study the HbA allosteric modulators, relatively few studies have been reported for the allosteric modulators of HbF. The only reported study regarding the HbF allosteric modulators was carried out by Papassotiriou et al32 who investigated the oxygen affinity modulatory effects of several bezafibrate derivatives, including clofibrate, bezafibrate, and RSR-4 (2-[4-[2-(3,5-dichloroanilino)-2-oxoethyl]phenoxy]-2-methylpropanoic acid) on Hb, from which RSR-4 was found to be a strong allosteric modulator capable of decreasing the oxygen affinity of HbF. The P50 value (a characteristic measure of oxygen affinity defined as the oxygen partial pressure [PO2] at which Hb becomes half oxygenated and half deoxygenated) of HbF-containing red blood cells was found to increase from 18.7 mm Hg to 37.3 mm Hg upon treatment with 0.5 mM RSR-4. However, an overly strong HbF modulatory effect may result in insufficient oxygen loading in the lung. For instance, for an oxygen equilibrium curve (OEC) with a P50 value of 37.3 mm Hg, the oxygen saturation ratio is only ∼0.85 at the alveolar partial pressure of oxygen of 104 mm Hg, which significantly restricts its medical potential. Thus, an ideal HbF modulator with a proper modulatory capacity to adjust the HbF oxygen affinity to match that of HbA is lacking.
By combining oxygen equilibrium experiments, resonance Raman (RR) spectroscopy, and the molecular docking modeling, we showed that 2 phthalide derivatives, z-butylidenephthalide (Figure 1A) and z-ligustilide (Figure 1B) (recently proposed as 2,3-BPG substitutes for HbA25 ) can serve as potent modulators for HbF. Here, we discuss the implications of our work in boosting the efficacy of HbF induction therapy for treating β-hemoglobinopathies.
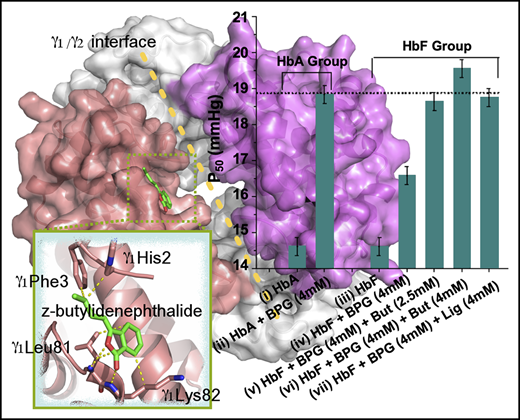
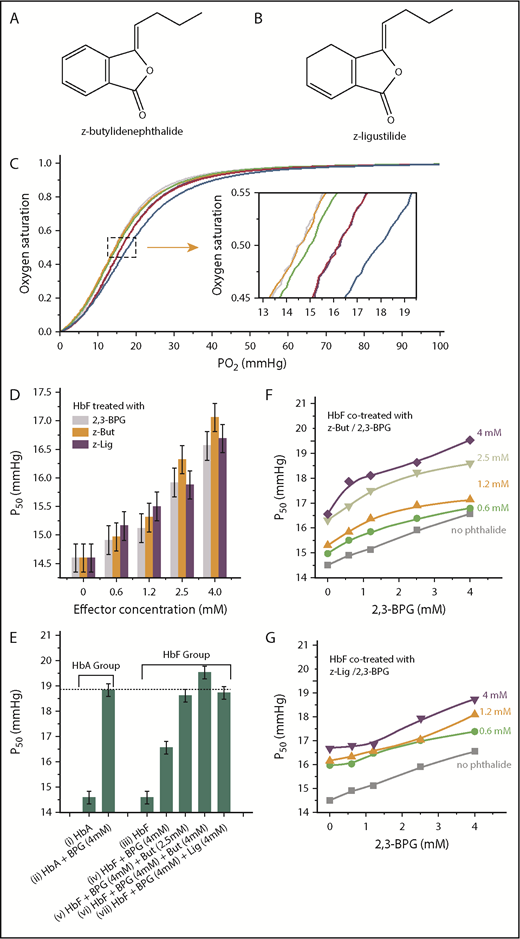
Phthalides are more potent modulators than 2,3-BPG in lowering oxygen affinity of HbF. (A) Molecular structure of z-butylidenephthalide (z-But). (B) Molecular structure of z-ligustilide (z-Lig). (C) OEC curves of purified HbF (orange), purified HbA (gray), HbF treated with 0.6 mM 2,3-BPG (green), HbA treated with 0.6 mM 2,3-BPG (purple), HbF treated with 0.6 mM z-butylidenephthalide (pink), and HbF treated with 4.0 mM z-butylidenephthalide (blue). (C) Inset shows an enlarged view of the area specified by gray dashed lines. (D) P50 values for HbF treated with 2,3-BPG (gray columns), z-butylidenephthalide (orange columns) and z-ligustilide (purple columns) at varying levels of treatment ranging between 0.6 and 4.0 mM. (E) A comparison of P50 for pure HbA (i), HbA treated with 4 mM 2,3-BPG (ii), pure HbF (iii), HbF treated solely with 4 mM 2,3-BPG (iv), and HbF cotreated with 4 mM 2,3-BPG and 2.5 to 4 mM phthalides (v-vii), which explicitly show that additional phthalide treatments can help raise the P50 for HbF to a level similar to that of P50 for HbA treated solely with the same level of 2,3-BPG. (F) P50 evolution for HbF treated with 2,3-BPG only (gray curve) and cotreated with z-butylidenephthalide at 0.6 mM (green curve), 1.2 mM (orange curve), 2.5 mM (lime green curve), and 4.0 mM (purple curve), along with varying levels of 2,3-BPG. (G) P50 evolution for HbF treated with 2,3-BPG only (gray curve), and cotreated with z-ligustilide of 0.6 mM (green curve), 1.2 mM (orange curve), and 4.0 mM (purple curve) along with varying levels of 2,3-BPG.
Phthalides are more potent modulators than 2,3-BPG in lowering oxygen affinity of HbF. (A) Molecular structure of z-butylidenephthalide (z-But). (B) Molecular structure of z-ligustilide (z-Lig). (C) OEC curves of purified HbF (orange), purified HbA (gray), HbF treated with 0.6 mM 2,3-BPG (green), HbA treated with 0.6 mM 2,3-BPG (purple), HbF treated with 0.6 mM z-butylidenephthalide (pink), and HbF treated with 4.0 mM z-butylidenephthalide (blue). (C) Inset shows an enlarged view of the area specified by gray dashed lines. (D) P50 values for HbF treated with 2,3-BPG (gray columns), z-butylidenephthalide (orange columns) and z-ligustilide (purple columns) at varying levels of treatment ranging between 0.6 and 4.0 mM. (E) A comparison of P50 for pure HbA (i), HbA treated with 4 mM 2,3-BPG (ii), pure HbF (iii), HbF treated solely with 4 mM 2,3-BPG (iv), and HbF cotreated with 4 mM 2,3-BPG and 2.5 to 4 mM phthalides (v-vii), which explicitly show that additional phthalide treatments can help raise the P50 for HbF to a level similar to that of P50 for HbA treated solely with the same level of 2,3-BPG. (F) P50 evolution for HbF treated with 2,3-BPG only (gray curve) and cotreated with z-butylidenephthalide at 0.6 mM (green curve), 1.2 mM (orange curve), 2.5 mM (lime green curve), and 4.0 mM (purple curve), along with varying levels of 2,3-BPG. (G) P50 evolution for HbF treated with 2,3-BPG only (gray curve), and cotreated with z-ligustilide of 0.6 mM (green curve), 1.2 mM (orange curve), and 4.0 mM (purple curve) along with varying levels of 2,3-BPG.
Methods
HbF was purified from placental umbilical cord blood by using standard procedures.25,33 Treatments of HbF using z-butylidenephthalide and z-ligustilide were performed by mixing various volumes of the specified effector at 2.4 × 10−2 M with 100 μL of purified HbF solution at 3.8 × 10−4 M. After mixing with HbF, the effector concentrations ranged between 0.2 and 12 mM. The HbF oxygen affinity was characterized via the OECs measured by using a Hemox Analyzer (TCS Scientific Corp., New Hope, PA). The structural properties of HbF were investigated via RR spectroscopy at 532 nm (WITec Inc., Ulm, Germany). The active sites and binding strength of HbF (PDB:1FDH [Protein Data Bank: human foetal deoxyhaemoglobin])19 to phthalides were assessed via molecular docking modeling (AutoDock 4.2.6)34 (see supplemental Methods for details.)
Results and discussion
To investigate the effects of z-butylidenephthalide and z-ligustilide on HbF oxygen affinity, we first measured the OECs from which the P50 value can be obtained. A right-shifted OEC corresponds to an increased P50 and reduced oxygen affinity. Without any effectors, the OECs for pure HbF and HbA were nearly superimposed (Figure 1C, orange and gray curves and its inset), with the same P50 value of 14.60 ± 0.25 mm Hg for both pure HbA and HbF. Upon treating with 0.6 mM 2,3-BPG, the OEC of HbA treated with 2,3-BPG (Figure 1C, purple curve) right-shifted more significantly than that of HbF treated with 2,3-BPG (Figure 1C, green curve). The P50 increased from 14.60 to 16.24 ± 0.25 mm Hg for HbA treated with 0.6 mM 2,3-BPG, but increased only slightly to 15.05 mm Hg for HbF treated with the same level of 2,3-BPG. However, by additionally administering 0.6 mM z-butylidenephthalide to HbF pretreated with 0.6 mM 2,3-BPG, its OEC (Figure 1C, pink curve) shifted farther to the right and became nearly superimposed on that of HbA treated solely with 0.6 mM 2,3-BPG (Figure 1C, purple curve). The P50 of 16.30 ± 0.25 mm Hg was derived, indicating that z-butylidenephthalide can lower the oxygen affinity of HbF to bring it closer to that of HbA treated with 2,3-BPG only.
The P50 values for HbF treated with 0.6 to 4.0 mM 2,3-BPG, z-butylidenephthalide, and z-ligustilide (gray, orange, and purple columns in Figure 1D) show that both z-butylidenephthalide and z-ligustilide exhibit a stronger modulatory effect than 2,3-BPG. A comparison of P50 is made for pure HbA, HbA treated with 4 mM 2,3-BPG, pure HbF, HbF treated solely with 4 mM 2,3-BPG, and HbF cotreated with 2.5 to 4.0 mM phthalides along with 4 mM 2,3-BPG (Figure 1E), showing that additional phthalide treatments can progressively raise the P50 of HbF to match that of HbA treated only with 2,3-BPG of the same levels (Figure 1E, dashed line). The above findings clearly demonstrate that the 2 chosen phthalides help boost the oxygen transport ability of HbF to a level closer to that of HbA under the same level of 2,3-BPG (Table 1). 2,3-BPG is normally present in erythrocytes at an in vivo concentration of 5 to 8 mM. To ensure that the inherent 2,3-BPG does not impede the modulating capacity of the 2 phthalides, we investigated the P50 evolution for HbF treated with 0.6 to 4.0 mM z-butylidenephthalide (Figure 1F) and z-ligustilide (Figure 1G) under varying levels of 2,3-BPG. It explicitly reveals that the P50 for HbF can be progressively increased with increasing phthalide treatments, and the presence of 2,3-BPG does not impair the HbF modulatory ability of phthalides (Table 2). Moreover, the cooperativity of HbF, expressed by Hill’s coefficient (n50), was also derived from the OECs (Table 2).35 The n50 values range between 2.51 and 2.71, indicating that the cooperativity of HbF was not impaired upon treatment with the phthalides.
Comparison of P50 values of HbF cotreated with 2,3-BPG and phthalides vs that of HbA treated solely with 2,3-BPG
| Effector . | HbA . | HbF . | ΔP50, mm Hg . |
|---|---|---|---|
| 0.6 mM 2,3-BPG | 16.24 | 15.05 | −1.19 |
| 0.6 mM 2,3-BPG + 0.6 mM z-butylidenephthalide | – | 16.30 | 0.06 |
| 4 mM 2,3-BPG | 18.83 | 16.56 | −2.27 |
| 4 mM 2,3-BPG + 2.5 mM z-butylidenephthalide | – | 18.63 | 0.20 |
| 4 mM 2,3-BPG + 4 mM z-butylidenephthalide | – | 19.54 | 0.71 |
| 4 mM 2,3-BPG + 4 mM z-ligustilide | – | 18.74 | 0.09 |
| Effector . | HbA . | HbF . | ΔP50, mm Hg . |
|---|---|---|---|
| 0.6 mM 2,3-BPG | 16.24 | 15.05 | −1.19 |
| 0.6 mM 2,3-BPG + 0.6 mM z-butylidenephthalide | – | 16.30 | 0.06 |
| 4 mM 2,3-BPG | 18.83 | 16.56 | −2.27 |
| 4 mM 2,3-BPG + 2.5 mM z-butylidenephthalide | – | 18.63 | 0.20 |
| 4 mM 2,3-BPG + 4 mM z-butylidenephthalide | – | 19.54 | 0.71 |
| 4 mM 2,3-BPG + 4 mM z-ligustilide | – | 18.74 | 0.09 |
▵P50 = (P50 of HbF cotreated with 2,3-BPG and chosen phthalide) – (P50 of HbA treated solely with 2,3-BPG at the same level).
P50 and n50 values for HbF treated with phthalide and 2,3-BPG under various treatment conditions
| . | No phthalide . | Phthalide . | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.6 mM . | 1.2 mM . | 4.0 mM . | ||||||
| P50 . | n50 . | P50 . | n50 . | P50 . | n50 . | P50 . | n50 . | |
| z-Butylidenephthalide | ||||||||
| No 2,3-BPG | 14.60 | 2.59 | 15.87 | 2.62 | 16.68 | 2.62 | 17.06 | 2.56 |
| 2,3-BPG | ||||||||
| 0.60 mM | 14.91 | 2.59 | 16.11 | 2.65 | 16.35 | 2.7 | 17.86 | 2.56 |
| 1.2 mM | 15.12 | 2.58 | 16.42 | 2.66 | 16.37 | 2.68 | 18.11 | 2.52 |
| 2.5 mM | 15.92 | 2.59 | 16.62 | 2.66 | 17.4 | 2.71 | 18.63 | 2.52 |
| 4.0 mM | 16.56 | 2.59 | 17.37 | 2.63 | 17.29 | 2.68 | 19.54 | 2.54 |
| z-Ligustilide | ||||||||
| No 2,3-BPG | 14.60 | 2.59 | 15.98 | 2.54 | 16.16 | 2.54 | 16.69 | 2.51 |
| 2,3-BPG | ||||||||
| 0.60 mM | 14.91 | 2.59 | 16.03 | 2.64 | 16.34 | 2.7 | 16.79 | 2.66 |
| 1.2 mM | 15.12 | 2.58 | 16.48 | 2.65 | 16.58 | 2.7 | 16.84 | 2.67 |
| 2.5 mM | 15.92 | 2.59 | 17.01 | 2.67 | 17.03 | 2.68 | 17.93 | 2.69 |
| 4.0 mM | 16.56 | 2.59 | 17.39 | 2.64 | 18.09 | 2.71 | 18.74 | 2.7 |
| . | No phthalide . | Phthalide . | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.6 mM . | 1.2 mM . | 4.0 mM . | ||||||
| P50 . | n50 . | P50 . | n50 . | P50 . | n50 . | P50 . | n50 . | |
| z-Butylidenephthalide | ||||||||
| No 2,3-BPG | 14.60 | 2.59 | 15.87 | 2.62 | 16.68 | 2.62 | 17.06 | 2.56 |
| 2,3-BPG | ||||||||
| 0.60 mM | 14.91 | 2.59 | 16.11 | 2.65 | 16.35 | 2.7 | 17.86 | 2.56 |
| 1.2 mM | 15.12 | 2.58 | 16.42 | 2.66 | 16.37 | 2.68 | 18.11 | 2.52 |
| 2.5 mM | 15.92 | 2.59 | 16.62 | 2.66 | 17.4 | 2.71 | 18.63 | 2.52 |
| 4.0 mM | 16.56 | 2.59 | 17.37 | 2.63 | 17.29 | 2.68 | 19.54 | 2.54 |
| z-Ligustilide | ||||||||
| No 2,3-BPG | 14.60 | 2.59 | 15.98 | 2.54 | 16.16 | 2.54 | 16.69 | 2.51 |
| 2,3-BPG | ||||||||
| 0.60 mM | 14.91 | 2.59 | 16.03 | 2.64 | 16.34 | 2.7 | 16.79 | 2.66 |
| 1.2 mM | 15.12 | 2.58 | 16.48 | 2.65 | 16.58 | 2.7 | 16.84 | 2.67 |
| 2.5 mM | 15.92 | 2.59 | 17.01 | 2.67 | 17.03 | 2.68 | 17.93 | 2.69 |
| 4.0 mM | 16.56 | 2.59 | 17.39 | 2.64 | 18.09 | 2.71 | 18.74 | 2.7 |
P50 units are mm Hg.
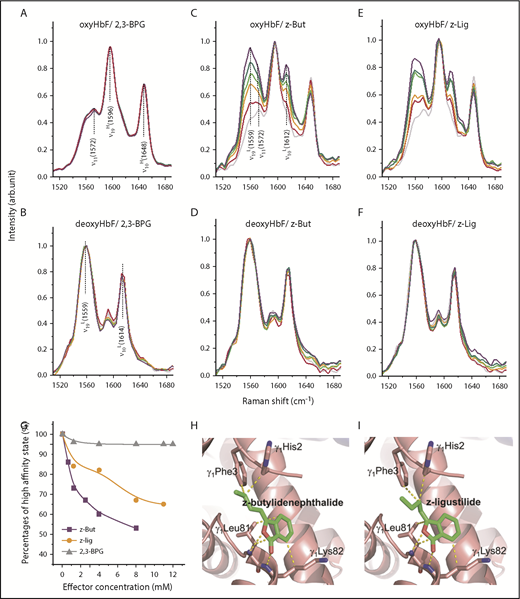
To gain insights into the possible mechanism underlying the pronounced HbF modulatory effects of phthalides, we used RR spectroscopy at 532 nm to assess the structural characteristics for HbF treated with varying levels of 2,3-BPG (Figure 2A-B), z-butylidenephthalide (Figure 2C-D), and z-ligustilide (Figure 2E-F) under both oxygenated (oxy) and deoxygenated (deoxy) conditions. Upon oxygenation or deoxygenation, HbF transformed between the high-oxygen-affinity oxyHbF state (manifested as the high-affinity ν19H and ν10H modes at 1596 and 1648 cm−1 in Figure 2A), and the low-oxygen-affinity deoxyHbF state (manifested as the low-affinity ν19L and ν10L modes at 1558 and 1614 cm−1 in Figure 2B).20 Although 2,3-BPG exhibits a negligible effect in HbF allostery (Figure 2A-B), both z-butylidenephthalide and z-ligustilide exhibit a dose-dependent effect on oxyHbF, as revealed by the progressively increased proportion of low-affinity modes (ν19L and ν10L at 1558 and 1614 cm−1 in Figure 2C,E). In contrast to 2,3-BPG (Figure 2G, gray curve), the relative proportion of the high-affinity state with respect to its low-affinity counterpart was substantially suppressed with treatment using increasing levels of z-butylidenephthalide and z-ligustilide (Figure 2G, purple and orange curves), indicating that both z-butylidenephthalide and z-ligustilide can allosterically stabilize oxyHbF in the low-affinity state. The active sites of HbF upon phthalide treatment were investigated via molecular docking modeling (AutoDock 4.2.6).34 Because of their structural resemblance, z-butylidenephthalide and z-ligustilide both seem to interact with HbF via the cleft around the γ1/γ2 interface, with γ1His2, γ1Phe3, γ1Leu81, and γ1Lys82 identified as the active sites (Figure 2H-I; supplemental Table 1). It is noteworthy that this active area exactly corresponds with the area responsible for the reduced binding of 2,3-BPG to HbF.15 The binding strength of z-butylidenephthalide and z-ligustilide to HbF was estimated to be 1.57 and 1.56 times stronger, respectively, than that of 2,3-BPG to HbF.
Phthalides stabilize oxyHbF in the low-oxygen-affinity state. RR spectroscopy of oxyHbF treated with varying amounts of 2,3-BPG (A), z-butylidenephthalide (B), and z-ligustilide (C) and deoxyHbF treated with varying amounts of 2,3-BPG (D), z-butylidenephthalide (E), and z-ligustilide (F). Color code for panels A-F: pure HbF (gray), HbF treated with the specified effector of 1 mM (red), 4 mM (orange), 8 mM (green), and 12 mM (violet). (G) Percentages of the high-affinity state for oxyHbF treated with z-butylidenephthalide (purple curve), z-ligustilide (orange curve), and 2,3-BPG (gray curve) with increasing degrees of treatment. (H) Active sites of HbF upon treatment with z-butylidenephthalide, with the intermolecular interactions denoted as yellow dashed lines. (I) Active sites of HbF upon treatment with z-ligustilide, with the intermolecular interactions denoted as yellow dashed lines.
Phthalides stabilize oxyHbF in the low-oxygen-affinity state. RR spectroscopy of oxyHbF treated with varying amounts of 2,3-BPG (A), z-butylidenephthalide (B), and z-ligustilide (C) and deoxyHbF treated with varying amounts of 2,3-BPG (D), z-butylidenephthalide (E), and z-ligustilide (F). Color code for panels A-F: pure HbF (gray), HbF treated with the specified effector of 1 mM (red), 4 mM (orange), 8 mM (green), and 12 mM (violet). (G) Percentages of the high-affinity state for oxyHbF treated with z-butylidenephthalide (purple curve), z-ligustilide (orange curve), and 2,3-BPG (gray curve) with increasing degrees of treatment. (H) Active sites of HbF upon treatment with z-butylidenephthalide, with the intermolecular interactions denoted as yellow dashed lines. (I) Active sites of HbF upon treatment with z-ligustilide, with the intermolecular interactions denoted as yellow dashed lines.
This study proposes 2 phthalide derivatives, z-butylidenephthalide and z-ligustilide, as new types of HbF modulators, capable of modulating HbF allostery via its γ1/γ2 cleft to stabilize oxyHbF in the low-affinity configuration and make it a more satisfactory oxygen carrier for adults. This study sheds new light on boosting the efficacy of HbF induction therapy for treating β-hemoglobinopathies.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Ming-Tsang Wu of Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan, for help with blood sample collection (Institutional Review Board number KMUHIRB-SV(II)-20170014).
This work is supported by grants from Ministry of Science and Technology (MOST) in Taiwan under project numbers MOST 104-2113-M-110-011, MOST 105-2119-M-110-002, and MOST 106-2319-B-492-002.
Authorship
Contribution: C.C.W. designed and supervised the experiments, supervised the data analysis, and wrote the manuscript; W.-R.C. performed the experiments and data analysis; and C.-C.C. performed the molecular docking computation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chia C. Wang, Department of Chemistry and Aerosol Science Research Center, National Sun Yat-sen University, 70 Lien-Hai Rd, Kaohsiung, Taiwan 80424, Republic of China; e-mail: chiawang@mail.nsysu.edu.tw.