Key Points
A reliable biomarker that predicts exacerbation and/or recurrence of iTTP is currently lacking.
Here we demonstrate that longitudinal assessments of ADAMTS13 biomarkers predict exacerbation or recurrence in patients with iTTP.
Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is primarily caused by immunoglobulin G (IgG)–type autoantibodies that bind and inhibit plasma ADAMTS13 activity and/or accelerate its clearance from circulation. Approximately 50% of patients with iTTP who achieve initial clinical response to therapy experience recurrence (ie, exacerbation and/or relapse); however, a reliable biomarker that predicts such an event is currently lacking. The present study determines the role of longitudinal assessments of plasma ADAMTS13 biomarkers in predicting iTTP exacerbation/recurrence. Eighty-three unique iTTP patients with 97 episodes from the University of Alabama at Birmingham Medical Center between April 2006 and June 2019 were enrolled. Plasma levels of ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG on admission showed no significant value in predicting iTTP exacerbation or recurrence. However, persistently low plasma ADAMTS13 activity (<10 U/dL; hazard ratio [HR], 4.4; 95% confidence interval [CI], 1.6-12.5; P = .005) or high anti-ADAMTS13 IgG (HR, 3.1; 95% CI, 1.2-7.8; P = .016) 3 to 7 days after the initiation of therapeutic plasma exchange was associated with an increased risk for exacerbation or recurrence. Furthermore, low plasma ADAMTS13 activity (<10 IU/dL; HR, 4.8; 95% CI, 1.8-12.8; P = .002) and low ADAMTS13 antigen (<25th percentile; HR, 3.3; 95% CI, 1.3-8.2; P = .01) or high anti-ADAMTS13 IgG (>75th percentile; HR, 2.6; 95% CI, 1.0-6.5; P = .047) at clinical response or remission was also predictive of exacerbation or recurrence. Our results suggest the potential need for a more aggressive approach to achieve biochemical remission (ie, normalization of plasma ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG) in patients with iTTP to prevent the disease recurrence.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare but potentially fatal blood disorder.1,2 It is characterized by severe thrombocytopenia and microangiopathic hemolytic anemia (eg, low hemoglobin and hematocrit, elevated serum lactate dehydrogenase, low haptoglobin, and the presence of schistocytes in the peripheral blood smear, etc.).1-3 Some patients may present with signs and symptoms of end-organ damage such as ischemic stroke,4,5 renal insufficiency,2,3 and myocardial ischemia.6,7 The primary cause of iTTP is the development of an immunoglobulin G (IgG)–type autoantibody, which binds and inhibits plasma ADAMTS13 activity8-10 and/or accelerates its clearance from the circulation as immune complexes.11,12 This results in compromised proteolysis of ultralarge von Willebrand factor (VWF) released from activated or injured endothelial cells, leading to heightened adhesion and aggregation of platelets at the sites of vascular injury, followed by the formation of disseminated thrombi in small arterioles and capillaries. This is a characteristic feature of iTTP pathology.
Therapeutic plasma exchange (TPE) remains an emergent and highly effective therapy, to date, for iTTP,13,14 which is believed to remove autoantibodies against ADAMTS13 while replenishing the missing or inhibited ADAMTS13 enzyme. TPE is often used in conjunction with other therapies including corticosteroids, rituximab, and cyclophosphamide, among others.13-16 This combination of therapies has reduced the mortality rate of iTTP to less than 10% to 20%.1,2,7 Further reduction in the mortality rate in patients with acute iTTP may be achieved with the addition of a novel anti-VWF nanobody, caplacizumab,17,18 to the standard of care, although the cost-effectiveness of adding this new treatment remains to be determined.
Despite progress being made in therapeutic strategy in past decades, ∼50% of patients with iTTP who survive their initial episode may experience 1 to several episodes of exacerbation (ie, disease recurrence within 30 days after discontinuation of TPE) and/or relapse (ie, disease recurrence 30 days after the discontinuation of TPE).19-21 Unfortunately, clinical factors and laboratory biomarkers that could reliably predict the iTTP exacerbation and/or relapse are currently inadequate.
Previous studies have demonstrated that there is an association of the admission levels of plasma ADAMTS13 activity or inhibitors, or anti-ADAMTS13 IgG or ADAMTS13-antibody immune complexes with either an exacerbation or mortality in patients with acute iTTP.3,22-24 However, these studies have largely relied on 1-time measurement of these biomarkers in the samples obtained at patient presentation. Therefore, the information obtained has limited utility. The present study seeks to determine the role of longitudinal measurements of ADAMTS13 biomarkers (eg, ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG) in predicting disease exacerbation and/or relapse in a large cohort of patients with iTTP who survived the initial acute episode and achieved clinical response/remission.
Methods
Patient selection and sample collection
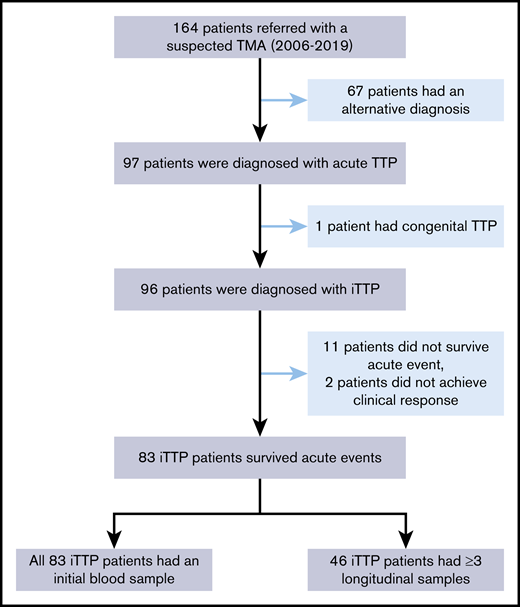
The Institutional Review Board of the University of Alabama at Birmingham approved the study protocol. A total of 164 patients with suspected thrombotic microangiopathy who were referred to the Apheresis Service for emergent TPE between April 2006 and June 2019 were screened. Of those, 67 patients had alternative diagnoses. This left 97 patients being diagnosed with acute TTP. Of these, 1 was diagnosed with congenital TTP, 11 did not survive the initial episode, and 2 did not achieve clinical response. These patients were excluded from the analysis. This left 83 unique patients with iTTP who suffered 97 acute episodes in the study. Of these, 46 patients had at least 3 longitudinal blood samples collected and 48 patients had at least 2 samples collected. One sample was collected before TPE, another at 3 to 7 days after TPE initiation, and the last at clinical response/remission (Figure 1).
Selection of patients with iTTP for the study. A total of 164 patients with suspected TTP between April 2006 and June 2019 were referred for TPE. Of those, 67 patients had alternative diagnoses, 1 patient had hereditary TTP, 11 patients died during the acute episode, and 2 patients did not respond to treatment (1 later died, and 1 was lost to follow-up). These patients were excluded from the study. This left 83 unique patients (97 episodes) for analysis. Of these, 46 patients with iTTP had serial blood samples (≥3) and 48 patients had at least 2 samples collected during the treatment.
Selection of patients with iTTP for the study. A total of 164 patients with suspected TTP between April 2006 and June 2019 were referred for TPE. Of those, 67 patients had alternative diagnoses, 1 patient had hereditary TTP, 11 patients died during the acute episode, and 2 patients did not respond to treatment (1 later died, and 1 was lost to follow-up). These patients were excluded from the study. This left 83 unique patients (97 episodes) for analysis. Of these, 46 patients with iTTP had serial blood samples (≥3) and 48 patients had at least 2 samples collected during the treatment.
The diagnostic criteria for iTTP in this study were as follows: severe thrombocytopenia and microangiopathic hemolytic anemia (ie, low hemoglobin, low hematocrit, elevated lactate dehydrogenase, and schistocytes) with or without evidence of end-organ damage,14,19 as well as plasma ADAMTS13 activity lower than 10 IU/dL and inhibitor higher than 0.4 U/mL, and/or anti-ADAMTS13 IgG higher than 15 U/mL.7,25 Demographic information, clinical presentation, comorbidities, laboratory results, and treatments were extracted from electronic medical records and entered into the Alabama TTP Registry Database for analyses. After discharge, patients were followed up at the Clinic of the University of Alabama at Birmingham, through a telephone interview, and at the annual TTP fair and education events (https://labs.uab.edu/zhengl/ttp-fair-menu/2019-ttp-fair).
Assays for plasma ADAMTS13 activity, ADAMTS13 antigen, and inhibitors or anti-ADAMTS13 IgG
Whole blood was drawn from an apheresis catheter before the initiation of TPE, anticoagulated with sodium citrate, and centrifuged at 1500g for 15 minutes. Plasma was separated from cellular components within 2 hours of collection, and stored in aliquots at −80°C until testing. Plasma ADAMTS13 activity and inhibitor on admission were determined in a reference laboratory (Versiti; Milwaukee, WI). In addition, the initial and subsequent plasma ADAMTS13 activities were determined using an in-house FRETS-VWF73 assay, as described previously.7,26 The results with the in-house assay were comparable to that performed in the reference laboratory. Pooled normal human plasma was used for the calibration, which was defined as having 100 U/dL (or 100%) of ADAMTS13 activity. Plasma ADAMTS13 antigen (R&D Systems, Minneapolis, MN) and anti-ADAMTS13 IgG (DiaPharma, West Chester Township, OH) were determined by the commercial enzyme-linked immunosorbent assay methods according to the manufacturers’ instructions. Levels of plasma anti-ADAMTS13 IgG higher than 15 U/mL, 12 to 15 U/mL, and lower than 12 U/mL were classified as positive, borderline, and negative, respectively.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR). Mann-Whitney U and Wilcoxon tests were used for paired and unpaired continuous variables, respectively. The χ2 and Fisher's exact tests were performed for the categorical variables. Spearman correlation tests were used to determine the correlations between various ADAMTS13 biomarkers. Furthermore, Kaplan-Meier survival analysis with a log-rank test was used to determine the exacerbation- or recurrence-free survival rates. Cox proportional hazard ratio regression was employed to determine the relative risk for iTTP exacerbation and/or recurrence in various groups of patients at different disease stages. All statistical analyses were performed with SPSS 25 or GraphPad Prism 7 software. The P values of <.05 and <.01 were considered to be statistically significant and highly significant, respectively.
Results
Clinical and laboratory characteristics of the Alabama iTTP cohort
Of 83 unique patients with iTTP who presented with a total of 97 episodes, the median age was 45 (IQR, 34-52.5) years at the time of enrollment. Forty-seven (56.6%) patients were female, and 36 (43.4%) were male. A majority of patients, 68 (81.9%), were black, and only 15 (18.1%) were white. Of 97 episodes, 60 (61.9%) were initial and 37 (38.1%) were relapsed. Blood group O, which is thought to be protective against TTP27 due to the reduced levels of their plasma VWF antigen and increased susceptibility of their VWF to proteolysis by ADAMTS13,28 constituted more than half (54.2%) of the patients, whereas the non-O blood group made up 45.8% of patients. The majority of patients (86.6%) presented with comorbidities, including obesity (median body mass index [BMI] of 33.6 (IQR, 29.0-41.0), hypertension (49.5%), diabetes mellitus (22.7%), systemic lupus erythematosus (18.2%), and HIV infection (9.0%). Of note, those with a positive HIV test did not have clinical manifestations or did not take antiretroviral therapy at the time of the iTTP diagnosis. More than 50% of patients presented with signs and symptoms that involved the central nervous system (eg, confusion, disorientation, coma, and seizure), 25.8% had abdominal pain, 16.5% had fever, and 13.4% complained of chest pain (Table 1).
Demographic features, laboratory parameters, treatments, and outcomes of patients with iTTP (n = 97)
| Parameters . | Values* . |
|---|---|
| Age, y (n = 97) | 45 (34-52.5) |
| Sex (n = 83) | |
| Female | 47 (56.6) |
| Male | 36 (43.4) |
| Race (n = 83) | |
| African American | 68 (81.9) |
| White | 15 (18.1) |
| Disease status (n = 97) | |
| Initial | 60 (61.9) |
| Relapse | 37 (38.1) |
| Blood group (n = 83) | |
| O | 45 (54.2) |
| Non-O | 38 (45.8) |
| BMI | 33.6 (29.0-41.0) |
| Comorbidities | |
| Hypertension (n = 97) | 48 (49.5) |
| Diabetes mellitus (n = 97) | 22 (22.7) |
| SLE (n = 44) | 8 (18.2) |
| HIV (n = 67) | 6 (9.0) |
| Symptoms (n = 97) | |
| CNS symptoms | 52 (53.6) |
| Fever | 16 (16.5) |
| Chest pain | 13 (13.4) |
| Abdominal pain | 25 (25.8) |
| Parameters . | Values* . |
|---|---|
| Age, y (n = 97) | 45 (34-52.5) |
| Sex (n = 83) | |
| Female | 47 (56.6) |
| Male | 36 (43.4) |
| Race (n = 83) | |
| African American | 68 (81.9) |
| White | 15 (18.1) |
| Disease status (n = 97) | |
| Initial | 60 (61.9) |
| Relapse | 37 (38.1) |
| Blood group (n = 83) | |
| O | 45 (54.2) |
| Non-O | 38 (45.8) |
| BMI | 33.6 (29.0-41.0) |
| Comorbidities | |
| Hypertension (n = 97) | 48 (49.5) |
| Diabetes mellitus (n = 97) | 22 (22.7) |
| SLE (n = 44) | 8 (18.2) |
| HIV (n = 67) | 6 (9.0) |
| Symptoms (n = 97) | |
| CNS symptoms | 52 (53.6) |
| Fever | 16 (16.5) |
| Chest pain | 13 (13.4) |
| Abdominal pain | 25 (25.8) |
CNS, central nervous system; O, blood type O; SLE, systemic lupus erythematosus.
Age and BMI are expressed as the median (IQR). All other parameters are expressed as n (%) of patients.
Routine and special laboratory parameters in all 97 iTTP episodes on admission are shown in Table 2. The laboratory results fully supported the diagnosis of iTTP in all patients enrolled into the study. The median platelet count was 14 × 109/L (IQR, 10-21.4 × 109/L). The median hematocrit was 25% (IQR, 22%-28%), and the median serum lactate dehydrogenase was 905 U/L (IQR, 550.5-1,363 U/L). The median serum creatinine level was 1.2 mg/dL (IQR, 0.9-1.8 mg/dL).
Presenting laboratory characteristics of 97 iTTP patient episodes
| Parameters . | Values* . |
|---|---|
| White blood cells, ×109/L | 10.3 (8.4-13.3) |
| Hemoglobin, g/dL | 8.8 (7.5-9.6) |
| Hematocrit, % | 25 (22-28) |
| Platelet count, ×109/L | 14 (10-21.4) |
| Lactate dehydrogenase, U/L | 905 (559.5-1,363) |
| Creatinine, mg/dL | 1.2 (0.9-1.8) |
| Prothrombin time, s | 14.6 (14.1-15.5) |
| Partial thromboplastin time, s | 30 (27-34) |
| Fibrinogen, mg/dL | 415 (327-494) |
| D-dimer, ng/mL | 1,869 (979.5-3973.5) |
| ADAMTS13 activity, U/dL | 2.8 (0.6-4.9) |
| ADAMTS13 antigen, ng/mL | 54.7 (27.9-105.4) |
| ADAMTS13 inhibitor, BU | 1.6 (0.6-3.4) |
| Anti-ADAMTS13 IgG, IU/mL | 4368.5 (2717.1-7565.7) |
| Parameters . | Values* . |
|---|---|
| White blood cells, ×109/L | 10.3 (8.4-13.3) |
| Hemoglobin, g/dL | 8.8 (7.5-9.6) |
| Hematocrit, % | 25 (22-28) |
| Platelet count, ×109/L | 14 (10-21.4) |
| Lactate dehydrogenase, U/L | 905 (559.5-1,363) |
| Creatinine, mg/dL | 1.2 (0.9-1.8) |
| Prothrombin time, s | 14.6 (14.1-15.5) |
| Partial thromboplastin time, s | 30 (27-34) |
| Fibrinogen, mg/dL | 415 (327-494) |
| D-dimer, ng/mL | 1,869 (979.5-3973.5) |
| ADAMTS13 activity, U/dL | 2.8 (0.6-4.9) |
| ADAMTS13 antigen, ng/mL | 54.7 (27.9-105.4) |
| ADAMTS13 inhibitor, BU | 1.6 (0.6-3.4) |
| Anti-ADAMTS13 IgG, IU/mL | 4368.5 (2717.1-7565.7) |
All values are expressed as the median (IQR).
Of 97 episodes, all (100%) received TPE plus corticosteroid therapy, and 38 (39.2%) were treated with rituximab, primarily for refractory episodes, exacerbations, or a history of relapses. The median number of days from admission to rituximab usage was 7 (IQR 4.5-8). All 97 episodes in the study patients achieved clinical response/remission defined in the methods. The median number of days required to achieve platelet normalization was 5 (IQR, 4-7). The median follow-up time for the study population was 25.6 months (IQR, 3.8-58.7 months). Of 93 episodes, 42 (45.2%) were followed by sustained remission and 55 (59.1%) exhibited disease recurrence (eg, 40.2% exacerbation, 23.7% relapse, and 6.2% relapse after the initial exacerbation; Table 3).
Treatments and outcomes of 97 iTTP patient episodes
| Parameters . | Values . |
|---|---|
| Number (%) who received TPE | 97 (100) |
| Number (%) who received rituximab before recurrence | 38 (39.2) |
| Days to platelet normalization, median (IQR) | 5 (4-7) |
| Number of TPE to achieve clinical response, median (IQR) | 4 (3-5.5) |
| Outcome, n (%) | |
| Remission | 42 (43.3) |
| Recurrence | 55 (56.7) |
| Exacerbation | 39 (40.2) |
| Relapse | 22 (22.7) |
| Relapse after exacerbation | 6 (6.2) |
| Parameters . | Values . |
|---|---|
| Number (%) who received TPE | 97 (100) |
| Number (%) who received rituximab before recurrence | 38 (39.2) |
| Days to platelet normalization, median (IQR) | 5 (4-7) |
| Number of TPE to achieve clinical response, median (IQR) | 4 (3-5.5) |
| Outcome, n (%) | |
| Remission | 42 (43.3) |
| Recurrence | 55 (56.7) |
| Exacerbation | 39 (40.2) |
| Relapse | 22 (22.7) |
| Relapse after exacerbation | 6 (6.2) |
Longitudinal changes of plasma ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG in patients with acute iTTP
On admission, the median plasma level of ADAMTS13 activity in these patients was 2.8 (IQR, 0.6-4.9) U/dL. Two recurrent cases had their plasma ADAMTS13 activity between 10 and 20 U/dL, but with elevated levels of plasma anti-ADAMTS13 IgG. Plasma levels of ADAMTS13 antigen, however, varied significantly among patients with acute iTTP, with the median plasma level of 54.7 ng/mL (IQR, 27.9-105.4 ng/mL). Seventy-nine (81.4%) of 97 episodes had detectable inhibitors, with the median inhibitor titer of 1.6 U/mL (IQR, 0.6-3.6 U/mL). In all except 1 (99%), there were elevated levels of plasma anti-ADAMTS13 IgG, with the median level of 4368.5 U/mL (IQR, 2717.1-7565.7 U/mL) (Table 2).
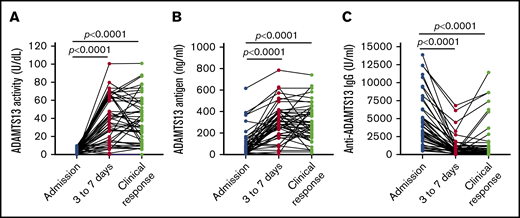
Three to 7 days after TPE, the median plasma levels of ADAMTS13 activity (Figure 2A) and ADAMTS13 antigen (Figure 2B) increased to 38.6 U/dL (IQR, 15.8-65.8 U/dL; P = .0001) and 292.5 ng/mL (IQR, 83.8-400.4 ng/mL; P = .0001), respectively. At the same time, the median plasma level of anti-ADAMTS13 IgG (Figure 2C) was significantly reduced to 463.3 U/mL (IQR, 180.9-1027.0 U/mL; P = .0001). However, among 46 patients with at least 3 longitudinal samples collected, 7 (15.2%) and 10 (21.7%) patients had persistent plasma ADAMTS13 activity lower than 10 U/dL and lower than 20 U/dL, respectively, throughout the acute episode despite standard treatment. When clinical response/remission was achieved, the median levels of plasma ADAMTS13 activity (Figure 2A) and ADAMTS13 antigen (Figure 2B) increased to 38.3 U/dL (IQR, 34.4-57.7 U/dL) and 273 ng/mL (IQR, 175.9-406.3 ng/mL), respectively. These changes were concurrent with the reduction of plasma anti-ADAMTS13 IgG (Figure 2C) to 416.7 U/mL (IQR, 192.1-1249 U/mL). These results demonstrate the tremendous heterogeneity in response to the standard treatment in achieving a biochemical remission in patients with acute iTTP.
Longitudinal changes of plasma ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG in 46 patients with iTTP. Each solid line depicts the change of plasma ADAMTS13 activity (A), ADAMTS13 antigen (B), and anti-ADAMTS13 IgG (C) over time (eg, on admission, 3-7 days after the initiation of TPE, and at clinical response/remission), as indicated in each panel. Mann-Whitney test was performed to determine the statistical difference between any 2 groups. Here, P < .0001 indicates that the difference is statistically highly significant.
Longitudinal changes of plasma ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG in 46 patients with iTTP. Each solid line depicts the change of plasma ADAMTS13 activity (A), ADAMTS13 antigen (B), and anti-ADAMTS13 IgG (C) over time (eg, on admission, 3-7 days after the initiation of TPE, and at clinical response/remission), as indicated in each panel. Mann-Whitney test was performed to determine the statistical difference between any 2 groups. Here, P < .0001 indicates that the difference is statistically highly significant.
The role of plasma ADAMTS13 activity at various clinical stages in predicting iTTP exacerbation or relapse
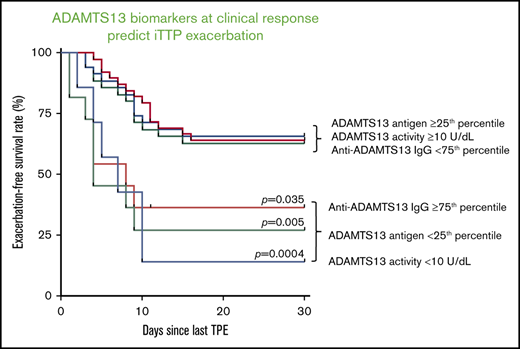
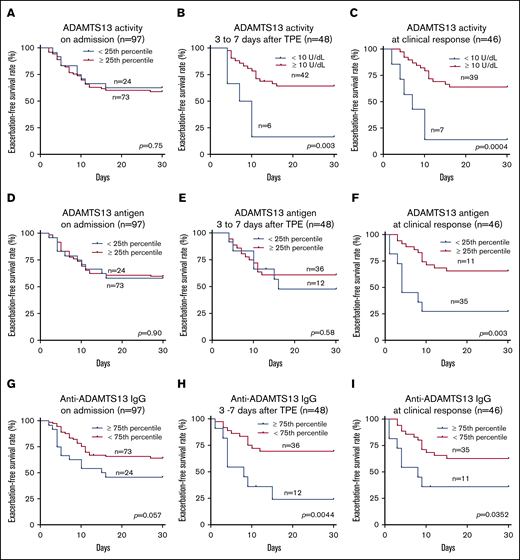
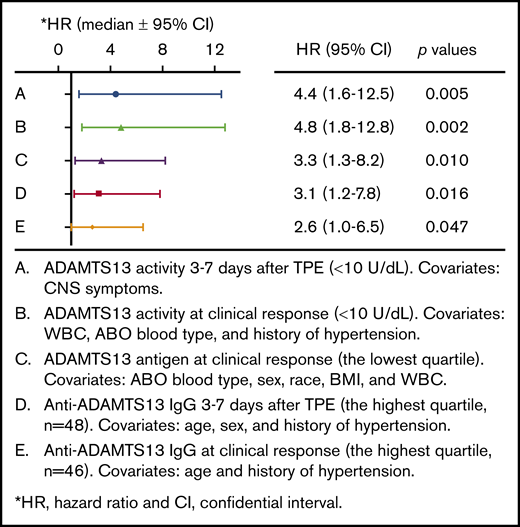
Although low ADAMTS13 activity (<10 IU/dL) on admission is critical for confirming the diagnosis of iTTP,14,29 the admission levels of plasma ADAMTS13 activity had no or little value in predicting a short-term exacerbation (P = .745; Figure 3A) or a long-term (1- or 2-year) relapse (supplemental Figure 1). Interestingly, patients with plasma ADAMTS13 activity lower than 10 U/dL 3 to 7 days after the initiation of TPE plus corticosteroids had a dramatically lower exacerbation-free survival rate than those with plasma ADAMTS13 activity of at least 10 U/dL (P = .014; Figure 3B). Such a difference remained statistically significant even after body mass index, central nervous system involvement, prothrombin time, and rituximab therapy were included as covariates in the Cox proportional hazard regression analysis (hazard ratio [HR], 4.4; 95% confidence interval [CI], 1.6-12.5; P = .005; Figure 4). Moreover, patients with plasma ADAMTS13 activity lower than 10 U/dL at clinical response/remission also exhibited a significantly lower exacerbation-free survival rate than those with plasma ADAMTS13 activity of at least 10 U/dL (P = .0004; Figure 3C). Even when ABO blood group, hypertension, and central nervous system involvement were included as covariates in the Cox proportional hazard regression analysis, the difference was still statistically highly significant (HR, 4.8; 95% CI, 1.8-12.8; P = .002; Figure 4).
Exacerbation-free survival rates in patients with iTTP based on ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG. The exacerbation-free survival rates in patients with low (<25th percentile) and high (≥25th percentile) levels of plasma ADAMTS13 activity on admission (A) or in patients with low (<10 IU/dL) and high (≥10 U/dL) levels of plasma ADAMTS13 activity 3 to 7 days after initiation of TPE (B) and at clinical response/remission (C); the exacerbation-free survival rates in patients with low (<25th percentile) and high (≥25th percentile) levels of plasma ADAMTS13 antigen on admission (D), 3 to 7 days after initiation of therapies (E), and at clinical response/remission (F); the exacerbation-free survival rates in patients with high (≥75th percentile or ≥7468.4 U/mL) and low (<75th percentile or < 7468.4 U/mL) levels of plasma anti-ADAMTS13 IgG on admission (G), 3 to 7 days after initiation of therapies (H), and at clinical response/remission (I). P values less than .05 and .01 are considered to be statistically significant and highly significant, respectively.
Exacerbation-free survival rates in patients with iTTP based on ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG. The exacerbation-free survival rates in patients with low (<25th percentile) and high (≥25th percentile) levels of plasma ADAMTS13 activity on admission (A) or in patients with low (<10 IU/dL) and high (≥10 U/dL) levels of plasma ADAMTS13 activity 3 to 7 days after initiation of TPE (B) and at clinical response/remission (C); the exacerbation-free survival rates in patients with low (<25th percentile) and high (≥25th percentile) levels of plasma ADAMTS13 antigen on admission (D), 3 to 7 days after initiation of therapies (E), and at clinical response/remission (F); the exacerbation-free survival rates in patients with high (≥75th percentile or ≥7468.4 U/mL) and low (<75th percentile or < 7468.4 U/mL) levels of plasma anti-ADAMTS13 IgG on admission (G), 3 to 7 days after initiation of therapies (H), and at clinical response/remission (I). P values less than .05 and .01 are considered to be statistically significant and highly significant, respectively.
Cox proportional hazard regression analysis identifies the parameters associated with iTTP recurrence. HRs were determined in patients with low ADAMTS13 activity (<10 U/dL) 3 to 7 days after TPE (A), low ADAMTS13 activity (<10 U/dL) at clinical response/remission (B), low levels of ADAMTS13 antigen (the lowest quartile) at clinical response/remission (C), and high levels of anti-ADAMTS13 IgG (the highest quartile) 3 to 7 days after TPE (D) and at clinical response (E). Additional covariates were added to the analysis (as indicated in panels A-E). P values less than .05 and .01 are considered to be statistically significant and highly significant, respectively. WBC, white blood cells.
Cox proportional hazard regression analysis identifies the parameters associated with iTTP recurrence. HRs were determined in patients with low ADAMTS13 activity (<10 U/dL) 3 to 7 days after TPE (A), low ADAMTS13 activity (<10 U/dL) at clinical response/remission (B), low levels of ADAMTS13 antigen (the lowest quartile) at clinical response/remission (C), and high levels of anti-ADAMTS13 IgG (the highest quartile) 3 to 7 days after TPE (D) and at clinical response (E). Additional covariates were added to the analysis (as indicated in panels A-E). P values less than .05 and .01 are considered to be statistically significant and highly significant, respectively. WBC, white blood cells.
The role of plasma ADAMTS13 antigen at various clinical stages in predicting iTTP exacerbation/relapse
Plasma levels of ADAMTS13 antigen varied widely among patients with acute iTTP, which is largely attributable to the different mechanisms underlying the severe deficiency of ADAMTS13 activity. Acute inflammation may result in reduction of hepatic or endothelial ADAMTS13 synthesis.30,31 Alternatively, anti-ADAMTS13 IgG may directly inhibit ADAMTS13 activity with or without causing an accelerated clearance of ADAMTS13 antigen and/or immune complexes.10,23,32 Low plasma levels of ADAMTS13 antigen (<25th percentile) on admission (Figure 3D) and 3 to 7 days after therapy (Figure 3E) showed no predictive value for exacerbation. However, patients with low plasma ADAMTS13 antigen (<25th percentile) at clinical response/remission exhibited a significantly lower exacerbation-free survival rate than those with plasma ADAMTS13 antigen in the other 3 quartiles (P = .029; Figure 3F). Cox proportional hazard regression analysis demonstrated that patients with ADAMTS13 antigen in the lowest quartile at clinical response/remission had dramatically increased likelihood of exacerbation or relapse (HR, 3.3; 95% CI, 1.3-8.2; P = .010). Such a difference remained statistically significant even when body mass index, race, and partial thromboplastin time were included as the covariates for analysis (Figure 4).
The role of plasma ADAMTS13 inhibitors or anti-ADAMTS13 IgG at various disease stages in predicting iTTP exacerbation or relapse
A functional assay was used to determine the ability of patients' undiluted or serially diluted plasma to reduce ADAMTS13 activity in a reference plasma. High-titer inhibitors (≥5 U/mL) on admission did not predict exacerbation (P = .094; supplemental Figure 2A), but predicted 1-year recurrence (ie, exacerbation + relapse; P = .044; supplemental Figure 2B). However, when the disease activity and rituximab therapy were included as covariates during the analysis, no statistically significant difference was detected for 1-year recurrence-free survival rate between the 2 groups (not shown). However, high-titer inhibitors (≥5 U/mL) on admission were associated with higher levels of anti-ADAMTS13 IgG on admission, lower ADAMTS13 activity 3 to 7 days after starting TPE, and lower ADAMTS13 activity, ADAMTS13 antigen, and higher levels of anti-ADAMTS13 IgG at clinical response (not shown). All these parameters were predictive of iTTP exacerbation/relapse.
The IgG-type antibodies against ADAMTS13 are primarily responsible for the inhibition of ADAMTS13 activity in patients with acute iTTP.8,9 High levels of plasma anti-ADAMTS13 IgG (≥75th percentile or ≥7568 U/mL) on admission did not predict exacerbation (P = .057; Figure 3G), 1-year (P = .057; supplemental Figure 2C), or 2-year (P = .075; supplemental Figure 2D) disease recurrence. However, high levels of anti-ADAMTS13 IgG (≥75th quartile) 3 to 7 days after TPE (Figure 3H; P = .004) or at clinical response/remission (Figure 3I; P = .035) were predictive of the disease exacerbation. When age, sex, and history of hypertension were included as covariates in the analysis, the differences remained statistically significant between the 2 groups at either point (HR, 3.1 [P = .016] or 2.6 [P = .047]; Figure 4). Consistent with these findings, patients with anti-ADAMTS13 IgG in the upper quartile on admission were younger and had higher inhibitor titers, lower ADAMTS13 activity and antigen 3 to 7 days after therapy, lower plasma ADAMTS13 activity and ADAMTS13 antigen, and higher anti-ADAMTS13 IgG at clinical response/remission. These patients often required more TPE sessions to achieve the clinical response/remission (not shown).
Correlations between ADAMTS13 activity and ADAMTS13 antigen or anti-ADAMTS13 IgG in patients with iTTP at various stages of clinical course
To determine the relationships between ADAMTS13 activity and ADAMTS13 antigen and/or autoantibodies against ADAMTS13, a Spearman correlation analysis was performed. In the samples obtained on admission, there was no correlation between ADAMTS13 activity and ADAMTS13 antigen levels (supplemental Figure 3A; r = 0.057; P = .58) or anti-ADAMTS13 IgG levels (supplemental Figure 3B; r = 0.2; P = .15). However, there was an inverse correlation between ADAMTS13 antigen levels and anti-ADAMTS13 IgG levels (r = −0.33; P = .001). In the samples obtained 3 to 7 days after the initiation of TPE, plasma ADAMTS13 activity was significantly correlated with its antigen levels (r = 0.66; P = .0001; supplemental Figure 3D), but inversely correlated with plasma anti-ADAMTS13 IgG (r = −0.51; P = .0002; supplemental Figure 3E), and there was an inverse correlation between ADAMTS13 antigen levels and anti-ADAMTS13 IgG levels (r = −0.49; P = .0005; supplemental Figure 3F). When clinical response/remission was achieved, the correlations became even stronger between plasma ADAMTS13 activity levels and ADAMTS13 antigen levels (r = 0.69; P < .0001; supplemental Figure 3G) or anti-ADAMTS13 IgG levels (r = −0.54; P = .0001; supplemental Figure 3H), as well as between ADAMTS13 antigen levels and anti-ADAMTS13 IgG levels (r = −0.50; P = .0005; supplemental Figure 3I). These results indicate a potentially different mechanism underlying severe deficiency of plasma ADAMTS13 activity in patients with iTTP during the acute setting before and after therapeutic intervention.
Discussion
Our study demonstrates the critical role of longitudinal assessments of plasma ADAMTS13 biomarkers including ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG in predicting iTTP exacerbation and/or recurrence. Advances in early diagnosis and effective therapeutic strategies have significantly reduced the overall mortality rate in patients with acute iTTP.3,24,33 Risk factors such as black race, older age, myocardial infarction or elevated troponin I, elevated prothrombin time, cerebrovascular disease, renal failure, congestive heart failure, cancer, and sepsis have all been shown to be associated with the increased mortality rate.7,17,24,33,34
Clinical factors and biomarkers predicting iTTP recurrence have largely focused on their initial admission levels or a random measurement during remission of plasma ADAMTS13 activity and inhibitors or anti-ADAMTS13 IgG.21,22,24 Systematic and longitudinal assessments of plasma ADAMTS13 biomarkers during standard therapies or at the time of clinical response/remission are lacking.
Here, we demonstrate that plasma ADAMTS13 activity on admission has no value in predicting iTTP exacerbation/recurrence. This makes sense because all patients with iTTP enrolled into the study had admission levels of plasma ADAMTS13 activity lower than 10 U/dL, which approaches the lowest limit of detection with the FRETS-based assay. The assay is incapable of discriminating 0 from a level below 10 U/dL in most cases. Interestingly, persistently low plasma ADAMTS13 activity (<10 U/dL) 3 to 7 days after the initiation of standard therapies is associated with an increased risk for exacerbation, resulting in re-initiation of TPE or readmission of the patient to the hospital within 30 days. Moreover, patients with low plasma ADAMTS13 activity (<10 U/dL) at clinical response/remission are 4.3-fold more likely to have experienced an exacerbation than those with their plasma ADAMTS13 activity at least 10 IU/dL.
Plasma ADAMTS13 antigen is not routinely determined in patients with suspected thrombotic microangiopathy because of its unknown utility. Alwan et al reported that those with plasma ADAMTS13 antigen in the lowest quartile had an increased mortality rate (18%) compared with those (3.8%) in their highest quartile.23 Yang et al also reported that patients with iTTP who did not survive an acute episode had lower ADAMTS13 antigen on admission than those who survived.35 Although we did not examine the association between plasma ADAMTS13 antigen and the mortality rate, we demonstrated that the levels of plasma ADAMTS13 antigen in the lowest quartile, not on admission, but during clinical response/remission in particular, were associated with a 3.2-fold increase in the rate of clinical exacerbation when compared with those who have ADAMTS13 antigen levels in the other 3 quartiles. Together, the data from ours and others support the potential role of assessing plasma ADAMTS13 antigen on admission, 3 to 7 days after starting therapy, and at clinical response/remission for predicting both mortality and exacerbation.
High-titer inhibitors on admission may3,36 or may not22,24 be associated with exacerbation/relapse. Our data demonstrated that high-titer (>5 U/mL) inhibitors on admission were associated with an increased rate of 1-year relapse, but not exacerbation, suggesting that the presenting inhibitors may be a prognostic factor for a long-term but not short-term outcome. In supporting such a hypothesis, patients with plasma anti-ADAMTS13 IgG levels in the highest quartile (≥75%) 3 to 7 days after initiation of TPE or at clinical response/remission are more likely to have experienced an exacerbation with a hazard ratio of 3.1 and 2.6, respectively. These results are consistent with 2 other studies,22,36 but not with another study37 previously published. For instance, Bettoni et al reported that low plasma ADAMTS13 activity or antigen (<10%) and the presence of ADAMTS13 inhibitor or IgG during remission was significantly associated with an increased rate of recurrence.22 Peyvandi et al also reported that severe deficiency of plasma ADAMTS13 activity (<10%) was associated with a higher likelihood of recurrence (odds ratio, 2.9). Anti-ADAMTS13 IgG was more prevalent in patients with recurrent iTTP (odds ratio, 3.1). In addition, the presence of both severe ADAMTS13 deficiency and anti-ADAMTS13 IgG during remission increased the likelihood of recurrence by 3.6 times.36 However, Jin et al reported that although lower ADAMTS13 activity and younger age were significantly associated with higher risk for relapse within 3 months, the anti-ADAMTS13 IgG levels were not predictive of iTTP relapses.37 Such discrepancy may be explained by the differences in the time of sampling and testing, the sensitivity/specificity of the assays, and the subtypes of IgG tested in the assays. For instance, IgG4 levels, but not other IgG subtypes, are higher in those who relapsed than in those who did not.38,39
With the rapid expansion of high-cost, but perhaps efficacious, pharmacological therapies such as rituximab40,41 and caplacizumab,17,18,42,43 in addition to standard of care (eg, TPE and corticosteroids), a reliable biomarker that predicts the likelihood of exacerbation and/or recurrence may help guide the timing, dosage, and duration of usage of such expensive therapeutic modalities, particularly in low-resource regions. Rituximab targets CD20 on B-lymphocytes, resulting in elimination of clonal B cells that may produce inhibitory antibodies toward ADAMTS13. In general, it takes about a month for rituximab (at a dose of 375 mg/m2, weekly) to have a full clinical effect, leading to restoration of plasma ADAMTS13 activity and elimination of ADAMTS13 antibodies.44-46 Caplacizumab, in contrast, specifically targets the VWF A1 domain, directly blocking the platelet and VWF interaction, thus reducing the formation of platelet-rich thrombi. Caplacizumab does not correct the underlying deficiency of plasma ADAMTS13 activity and eliminte anti-ADAMTS13 IgGs.17,47,48 Therefore, an early discontinuation of treatment with caplacizumab in patients with severe deficiency of plasma ADAMTS13 activity resulting from autoantibodies may result in an exacerbation and a relapse of iTTP.17
On the basis of our data and others, we recommend that plasma ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG levels be tested 3 to 7 days after the initiation of TPE and at clinical response/remission (or before patient discharge). If given during the acute episode, caplacizumab may be continued for additional 30 days or longer if a patient remains severely deficient of ADAMTS13 activity (<10 U/dL),49 has low ADAMTS13 antigen (<158 ng/mL), or has high anti-ADAMTS13 IgG (>7568 U/mL) at the time of discharge. This may prevent exacerbations and hospital readmission in which a new round of TPE would otherwise be initiated. In addition, a standard dose (375 mg/m2)44,50 or a low dose (100 mg/m2)51,52 of rituximab may be given or continued weekly until normalization of plasma ADAMTS13 activity and/or antigen or elimination of anti-ADAMTS13 IgG is achieved.
To conclude, longitudinal assessments of plasma ADAMTS13 activity, antigen, and anti-ADAMTS13 IgG, particularly 3 to 7 days after the initiation of standard therapies and/or at clinical response/remission (or before patient discharge), are crucial for predicting the relative risk for exacerbation and/or relapse in patients with acute iTTP following the standard of care. It should be cautious when interpreting plasma levels of ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG after the initiation of plasma infusion or exchange for diagnostic purpose. Plasma infusion or exchange may affect plasma levels of ADAMTS13 activity, antigen, and IgG autoantibodies to various extents.3,35 However, when plasma ADAMTS13 activity remains lower than 10 U/dL with detectable autoantibodies, despite daily TPE plus adjunctive therapies, it remains diagnostic for iTTP.3,35,53 and important for prognosis. Our findings, if confirmed in a large multicenter study, could change how we manage patients with iTTP in the future.
Acknowledgments
J.S. is a visiting scholar from the Department of Hematology of Yantai Yu Huang Ding Hospital Affiliated to Qingdao University, Shandong province, China. The work constitues part of her dissertation research at the University of Alabama at Birmingham, leading to a PhD degree from Shandong University, Qingdao, China.
The study was supported in part by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL126724 and HL144552-01A1) and the Answering TTP Foundation (X.L.Z.). The authors thank our pathology residents, fellows, apheresis nurses, and medical technologists in the coagulation laboratory for their assistance in obtaining informed consent and blood samples, and sample preparation and storage.
Authorship
Contribution: J.S. and X.L.Z. designed the research, performed experiments, and analyzed the results, as well as wrote the manuscript; all other authors contributed to patient recruitment, informed consents, and sample collections; and all authors have reviewed and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: X.L.Z. is a speaker or consultant for Alexion, Ablynx/Sanofi, and Shire/Takeda and is also the founder of Clotsolution, Inc. The remaining authors declare no competing financial interests.
Correspondence: X. Long Zheng, Division of Laboratory Medicine, Department of Pathology, The University of Alabama at Birmingham, WP230K, 619 19th St South, Birmingham, AL 35249; e-mail: xzheng@uabmc.edu or longzheng01@gmail.com.
References
Author notes
Send data sharing requests via e-mail to the corresponding author, X. Long Zheng (xzheng@uabmc.edu or longzheng01@gmail.com).
The full-text version of this article contains a data supplement.