Key Points
There was no significant difference in response rates, PFS, or OS among patients that developed resistance to different lenalidomide doses.
Longer duration of prior lenalidomide and a longer lenalidomide-free interval are associated with better outcomes with pomalidomide.
Abstract
To gain insights into the characteristics of clinical resistance to lenalidomide, we evaluated the outcomes of 147 consecutive patients with multiple myeloma (MM) homogeneously treated with immunomodulatory imide drugs (IMiDs) pomalidomide and dexamethasone (Pd) for relapsed and/or refractory MM (median, 3 prior lines of treatment). We focused our analysis on the effect of the lenalidomide dose at which resistance was developed, the duration of lenalidomide exposure, and lenalidomide-free interval. On intent to treat, 33% of patients achieved ≥partial remission (PR) with Pd. When Pd was given immediately after lenalidomide, ≥PR was 32% (vs 37% after bortezomib). The response rates were similar for patients that received 5 to 15 mg vs 25 mg of lenalidomide (38.5% vs 30.5%, P = .329). Response rates were higher for patients that had received at least 12 months of lenalidomide (44% vs 27%) and for those with ≥18 months from last lenalidomide dose to pomalidomide dose (65% vs 23%). Median progression-free survival (PFS) and overall survival (OS) were 5 and 12.1 months, respectively, which was similar for patients who received lenalidomide, bortezomib or other regimens just before Pd and similar for patients who were receiving different doses of lenalidomide. IMiD-free interval ≥18 months was associated with longer PFS (10.3 vs 3.9 months, P = .003) and OS (27.1 vs 9.3, P = .008) as well as duration of last lenalidomide therapy ≥12 months (PFS: 7.8 vs 3.2, P = .023; OS: 16.5 vs 7.9, P = .005) even after adjustment for the number of prior therapies, duration of disease, and last lenalidomide dose.
Introduction
Multiple myeloma (MM) remains an incurable disease and is characterized by multiple relapses requiring the use of different treatments to control plasma cell clone expansion. In most patients, every relapse is associated with shorter duration of remission and, finally, the development of resistance to all available therapies is unavoidable in most cases. As we gain insight into the biology of the disease, the importance of the presence of subclones with different sensitivity or resistance to therapy and the development of clonal tides and of clonal evolution1-5 have been identified as important parameters leading to the development of disease progression and need to change therapy. With the extensive use of lenalidomide-containing combinations,6,7 it has become increasingly important to characterize the development of resistance to lenalidomide and explore treatment options for patients who progress while on lenalidomide.
New treatments that either target new pathways or further improve the efficacy on previously identified targets have been developed and have led to the approval of several different active agents. Pomalidomide is a second-generation immunomodulatory imide drug (IMiD) that has been postulated to exert increased immunomodulatory and antimyeloma activity over lenalidomide and thalidomide.8 Pomalidomide has been explored extensively and showed significant activity in patients with refractory myeloma, so that pomalidomide with low-dose dexamethasone (Pd) is now a standard treatment regimen for patients who have failed both lenalidomide and bortezomib.9-14 These studies also suggested that Pd was active irrespective of the number of prior therapies and whether resistance to lenalidomide or bortezomib had developed.9,14 Recent randomized studies have explored the addition of a third agent to Pd backbone for patients with resistance or relapse after lenalidomide,15-24 but it remains unclear what the activity of Pd or Pd combinations is in different settings of refractoriness to lenalidomide. For example, when resistance to lenalidomide was developed at lower or full doses of lenalidomide, whether it was given following secondary resistance (ie, progression after initial response to lenalidomide) or immediately after lenalidomide-based therapy or after a lenalidomide-free period, or whether progression followed a short or prolonged period of lenalidomide therapy. The aims of the current study were to explore the importance of these factors in patients treated homogeneously with the doublet of pomalidomide with dexamethasone and provide data that could help characterize the types of lenalidomide resistance.
Patients and methods
The current analysis included 147 consecutive patients with myeloma, all of whom had been exposed to lenalidomide and proteasome inhibitors (bortezomib or carfilzomib), and who were treated in the Department of Clinical Therapeutics, National and Kapodistrian University of Athens. All patients received pomalidomide at a dose of 4 mg on days 1 through 21 of 28-day cycles with weekly dexamethasone (at a dose of 20 to 40 mg). All patients received the Pd doublet; patients that received Pd with a third agent were excluded from the analysis. In all patients, data regarding prior lenalidomide doses and duration and regimen details, responses and relapse, prior disease history, and other characteristics were available.
International Myeloma Working Group (IMWG) criteria for the definition of refractoriness, response and time to event were used in the current analysis.25,26 According to the IMWG criteria,25 relapsed and refractory myeloma was defined as progression on therapy in patients who achieve minor response (MR) or better, or progress within 60 days of their last therapy. Patients who never achieved at least an MR to any prior therapy and progress while on therapy were defined as primary refractory. Relapsed myeloma was defined as disease that was previously treated, and there was evidence of progressive disease as previously defined and at the time of relapse did not meet the criteria for relapsed and refractory or primary refractory myeloma.26 For this analysis, patients who did not achieve at least an MR during any line of lenalidomide-based therapy were rated as primary resistant to lenalidomide. The collection, analysis, and publication of the data has been approved by the Institutions Scientific Committee/Institutional Review Board Alexandra Hospital.
Statistical analysis
All analyses were on an intent-to-treat basis. Comparisons for categorical variables among different groups were made with the χ2 test, using Fisher’s exact test when appropriate. Time to event (progression, death) was calculated from the date of the first dose of pomalidomide, time-to-event curves were plotted with the Kaplan-Meier method, and comparisons among groups were made using the log-rank test. For multivariate analysis, Cox proportional hazards models were developed using forward conditional selection. To identify optimal cutoffs for duration of last lenalidomide-based therapy and for duration of IMiD-free interval, we performed a receiver operating characteristic analysis with achievement of at least partial remission at 3 months as a binary end point; cutoffs were chosen based on maximization of Youden’s index; the cutoff was then rounded to the closest clinically relevant time point. The analysis was performed using SPSS v.20 software.
Results
Patients and prior therapies
The median age of the patients in the analysis was 64 years (range, 34-97). The median number of prior treatments was 3 (range, 1-9); 47% of them had received prior autologous stem cell transplantation, 71% were refractory to the last bortezomib-containing regimen, 92% were refractory to the last lenalidomide-containing regimen, and 8% had discontinued lenalidomide for reasons other than disease progression. Per IMWG definition, 9 (6%) patients had primary refractory myeloma and 138 (94%) had relapsed and refractory myeloma. Most of the patients had disease that had relapsed and was refractory to lenalidomide-containing therapy (n = 70), whereas 65 (44%) patients never achieved a PR or better to lenalidomide. Only 12 (8%) patients had disease that had relapsed to lenalidomide.
The median time from start of first-line therapy to start of pomalidomide was 55 months (range, 2-225 months). All patients had disease that was refractory to the last regimen; however, 48% had achieved at least a PR to their most recent regimen (18% complete remission or very good partial remission [VGPR] and 30% PR) before the development of progressive disease. The last regimen before Pd included a proteasome inhibitor (PI; bortezomib/carfilzomib) in 71 (48%) patients, lenalidomide in 62 (42.5%) patients, and conventional chemotherapy with steroids in 21 (14%) patients; 7 patients received both bortezomib and lenalidomide at their last regimen. The last dose of lenalidomide at which patients developed lenalidomide-refractory disease was 5 mg in 9 (6%), 10 mg in 27 (18%), 15 mg in 16 (11%), and 25 mg in 95 (65%). Median duration of last lenalidomide-based therapy was 9.3 months (range, 2-76.5). The last dexamethasone dose that was given together with lenalidomide was 160 mg/mo in 56%, 80 to 128 mg/mo in 37%, and no dexamethasone was given in 9 (6%); among patients that were receiving lenalidomide without dexamethasone, 2 patients were receiving 10 mg of lenalidomide, 3 patients 15 mg of lenalidomide, and 4 patients 25 mg of lenalidomide. Other characteristics of the patients are depicted in Table 1.
Characteristics of the patients in the analysis (N = 147)
| Characteristics of the patients . | Data . |
|---|---|
| Age, median (range), y | 64 (38-86) |
| Age >70 y | 51 (35) |
| Male/female, % | 51/49 |
| Median time from diagnosis to pomalidomide (range), y | 4.6 (0.7-15) |
| Median no. of prior treatments (range) | 3 (1-9) |
| 1-2 prior lines | 31 (21) |
| 3 prior lines | 49 (33) |
| >3 prior lines | 67 (46) |
| Prior ASCT | 78 (53) |
| Prior bortezomib | 143 (97) |
| Refractory to last bortezomib regimen | 104 (71) |
| Prior lenalidomide | 147 (100) |
| Prior thalidomide | 103 (70) |
| Refractory to last lenalidomide regimen | 135 (92) |
| Primary refractory | 9 (6) |
| Relapsed and refractory | 138 (94) |
| Last lenalidomide dose | |
| 5/10/15/25, mg | 9 (6)/27 (18)/16 (11)/95 (65) |
| Last regimen before Pd | |
| Bortezomib-containing | 71 (48) |
| Lenalidomide-containing | 62 (42.5) |
| Other (conventional chemo) | 21 (14) |
| Best response to last regimen, before progression ≥VGPR/PR/NR or PD, % | 18/30/52 |
| Response (≥PR) to last lenalidomide regimen | 82 (56) |
| Time from last lenalidomide dose to pomalidomide, mo | 6.7 |
| Time from last lenalidomide dose to pomalidomide ≥18 mo | 37 (25) |
| Duration of last lenalidomide-based regimen | 9.3 |
| Duration of last lenalidomide-based regimen ≥12 mo | 61 (42) |
| eGFR <60 mL/min | 50 (34) |
| Platelet counts <100 × 109/L | 35 (24) |
| Hemoglobin <10 g/dL | 69 (47) |
| LDH > ULN | 61 (41.5) |
| Calcium >10.5 g/dL | 21 (14) |
| ISS 1/2/3 | 21 (14)/67 (46)/59 (40) |
| t(4,14)* | 13 (21) |
| t(14,16)* | 5 (8) |
| del17p* | 11 (17.5) |
| amp/add1q21* | 20 (30.5) |
| High-risk cytogenetics*,† | 23 (36) |
| Characteristics of the patients . | Data . |
|---|---|
| Age, median (range), y | 64 (38-86) |
| Age >70 y | 51 (35) |
| Male/female, % | 51/49 |
| Median time from diagnosis to pomalidomide (range), y | 4.6 (0.7-15) |
| Median no. of prior treatments (range) | 3 (1-9) |
| 1-2 prior lines | 31 (21) |
| 3 prior lines | 49 (33) |
| >3 prior lines | 67 (46) |
| Prior ASCT | 78 (53) |
| Prior bortezomib | 143 (97) |
| Refractory to last bortezomib regimen | 104 (71) |
| Prior lenalidomide | 147 (100) |
| Prior thalidomide | 103 (70) |
| Refractory to last lenalidomide regimen | 135 (92) |
| Primary refractory | 9 (6) |
| Relapsed and refractory | 138 (94) |
| Last lenalidomide dose | |
| 5/10/15/25, mg | 9 (6)/27 (18)/16 (11)/95 (65) |
| Last regimen before Pd | |
| Bortezomib-containing | 71 (48) |
| Lenalidomide-containing | 62 (42.5) |
| Other (conventional chemo) | 21 (14) |
| Best response to last regimen, before progression ≥VGPR/PR/NR or PD, % | 18/30/52 |
| Response (≥PR) to last lenalidomide regimen | 82 (56) |
| Time from last lenalidomide dose to pomalidomide, mo | 6.7 |
| Time from last lenalidomide dose to pomalidomide ≥18 mo | 37 (25) |
| Duration of last lenalidomide-based regimen | 9.3 |
| Duration of last lenalidomide-based regimen ≥12 mo | 61 (42) |
| eGFR <60 mL/min | 50 (34) |
| Platelet counts <100 × 109/L | 35 (24) |
| Hemoglobin <10 g/dL | 69 (47) |
| LDH > ULN | 61 (41.5) |
| Calcium >10.5 g/dL | 21 (14) |
| ISS 1/2/3 | 21 (14)/67 (46)/59 (40) |
| t(4,14)* | 13 (21) |
| t(14,16)* | 5 (8) |
| del17p* | 11 (17.5) |
| amp/add1q21* | 20 (30.5) |
| High-risk cytogenetics*,† | 23 (36) |
Data are n (%) unless otherwise noted.
eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; ISS, International Staging System; ULN, upper limit of normal.
Available in 64 patients.
Defined as presence of any of t(4,14) or t(14,16) del17p.
On intent to treat, 49 (33%) patients achieved at least a PR after Pd therapy, including stringent complete response/complete response in 14 (9.5%), VGPR in 24 (16%), and PR in 11 (8%). First, we evaluated the impact of therapy that was given just before Pd: in patients who received lenalidomide-based therapy just before Pd (n = 62), 32% achieved ≥PR. Thirty-seven percent of patients responded (≥PR) who received bortezomib-based therapy just before Pd, as did 24% of patients who received other chemotherapy-based regimens (P = .546 vs lenalidomide-based). Table 1 summarizes responses to Pd across different groups.
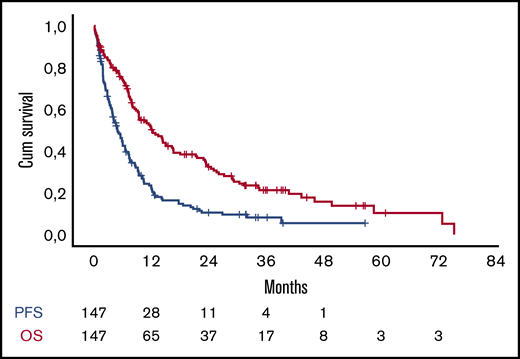
At the date of data cutoff, 125 (86%) patients have either progressed or died and 110 (75%) have died (Figure 1). The median progression-free survival (PFS) for all patients was 5 months (95% confidence interval [CI]. 3.5-6.2). Patients who received lenalidomide in their last regimen before Pd had similar PFS to patients who received either bortezomib or other regimens (4.6 vs 5.9 months, P = .749). The median overall survival after initiation of Pd for all patients was 12.1 months (95% CI, 8.4-15.8) (Figure 1). The survival of patients who received lenalidomide immediately before Pd was 12 months vs 12.1 months for the rest of the patients (P = .834).
PFS (blue line) and OS (red line) of the patients in the analysis. Cum, cumulative.
PFS (blue line) and OS (red line) of the patients in the analysis. Cum, cumulative.
Effect of lenalidomide dose and prior response to lenalidomide to efficacy of pomalidomide/dexamethasone
The response rates were similar for patients that were receiving 5 to 15 mg (n = 52) or 25 mg (n = 95) of lenalidomide in their last lenalidomide-based regimen (either just before Pd or earlier) (38.5% vs 30.5%, P = .329). Among those who received lenalidomide just before Pd, the response rates among those that were receiving 25 mg of lenalidomide (n = 33) was 27% and among those that were receiving 5 to 15 mg (n = 29) was 37% (P = .370). The response rates among the small number of patients who were not receiving dexamethasone were higher than those receiving dexamethasone with lenalidomide within the last lenalidomide-based regimen (78% for no dexamethasone vs 33% for dexamethasone doses up to 80 mg, 31% for doses of 160 mg/mo, P = .015).
Among patients who failed to achieve at least a PR to any prior lenalidomide therapy (n = 65), the response rate (≥PR) was 31.3% vs 35% of those who had achieved at least a PR to any prior lenalidomide-based therapy (n = 82) (P = .601). We also examined whether response to lenalidomide before the development of lenalidomide resistance, in the subgroup of patients that was given lenalidomide just prior to Pd, affected response: at least a PR was achieved by 31% vs 36% for those who had <PR as their best response to lenalidomide just before Pd (P = .783). Thus, response rates to Pd were similar for patients who had never achieved or had achieved at least a PR to lenalidomide and for those progressing with 5 to 15 mg or 25 mg of lenalidomide.
The PFS was not significantly different for patients who developed refractoriness to different doses of lenalidomide, and the median PFS was 4.9 vs 5 months (P = .929) for patients that received 5 to 15 mg vs 25 mg of lenalidomide at their last lenalidomide-based regimen (at any time before Pd) (Figure 2). In the subgroup of patients that received lenalidomide just before Pd (n = 62), the PFS was 3.8 months vs 4.8 months for patients receiving 5 to 15 mg (n = 29) vs 25 mg (n = 33) of lenalidomide (P = .664). Regarding the last dose of dexamethasone (per month) that was given with lenalidomide, PFS was 20.6 months if dexamethasone was not used, 4 months for those that were receiving up to 120 mg, and 4.8 months for those receiving 160 mg/mo (P = .030).
PFS according to lenalidomide (Len) duration, IMiD-free interval, and last Len dose. (A) PFS according to a Len duration <12 months (blue line) or ≥12 months (red line). (B) PFS according to an IMiD-free interval <18 months (blue line) or ≥18 months (red line). (C) PFS according to last Len dose (5-15 mg) (blue line) vs 25 mg (red line).
PFS according to lenalidomide (Len) duration, IMiD-free interval, and last Len dose. (A) PFS according to a Len duration <12 months (blue line) or ≥12 months (red line). (B) PFS according to an IMiD-free interval <18 months (blue line) or ≥18 months (red line). (C) PFS according to last Len dose (5-15 mg) (blue line) vs 25 mg (red line).
Regarding patients who had achieved at least a PR while they were treated with lenalidomide, and before development of resistance, PFS was 6.5 months vs 4.1 months for those who had at least a PR to lenalidomide (P = .078). Among those that received lenalidomide just before Pd, the PFS was 3.9 vs 4.7 months (P = .302) for primary refractory vs those that had not achieved at least PR before progression, respectively.
The overall survival (OS) according to the last dose of lenalidomide for patients that received their last lenalidomide regimen at doses of 5 to 15 mg vs 25 mg was 11.9 months vs 12.8 months, respectively (P = .194) (Figure 3). In the subgroup of patients that received lenalidomide just before Pd, the OS was 8 months for those on 5 to 15 mg of lenalidomide vs 13 months for those on 25 mg (P = .317). The OS has not been reached for those not on dexamethasone while receiving lenalidomide vs 9 and 11.7 months for those that were receiving up to 120 mg or 160 mg/mo, respectively (P = .077).
OS according to Len duration, IMiD-free interval, and last Len dose. (A) OS according to a Len duration <12 months (blue line) or ≥12 months (red line). (B) OS according to an IMiD-free interval <18 months (blue line) or ≥18 months (red line). (C) OS according to last Len dose (5-15 mg) (blue line) vs 25 mg (red line).
OS according to Len duration, IMiD-free interval, and last Len dose. (A) OS according to a Len duration <12 months (blue line) or ≥12 months (red line). (B) OS according to an IMiD-free interval <18 months (blue line) or ≥18 months (red line). (C) OS according to last Len dose (5-15 mg) (blue line) vs 25 mg (red line).
IMiD-free interval and duration of prior exposure to lenalidomide
Because the effect of therapy on clonal evolution and subsequent development of resistance may be important, we evaluated whether duration of prior exposure to lenalidomide and the duration of the IMiD-free interval may affect efficacy of Pd. IMiD-free interval was defined as the time from the last dose of lenalidomide to the first dose of pomalidomide. The median duration of exposure to lenalidomide before Pd was 9.3 months (range, 2-76.5) and the median IMiD-free interval was 6.7 months (range, 2 days to 64 months; interquartile range, 1.4-19 months). To define clinically relevant and applicable cutoffs, we performed a receiver operating characteristic analysis with achievement of at least PR at 3 months as a binary end point, rounded to the closest clinically relevant time point. Both the duration of prior lenalidomide exposure and IMiD-free interval were associated with response to Pd: patients with longer exposure to lenalidomide (>12 months) more frequently achieved at least PR (44% vs 27% for those with <12 months of lenalidomide exposure, P = .03). The PR rate for patients with IMiD-free interval of ≥18 months was 65% vs 23% for those with <18 months from last lenalidomide dose (P < .001). In univariate analysis, a duration of lenalidomide therapy before Pd of >12 months was associated with longer PFS on Pd (7.8 vs 3.2 months, P = .023); an IMiD-free interval of ≥18 months (upper quartile of IMiD-free interval in our cohort) was associated with a PFS of 10.3 vs 3.9 months (P = .003). For the subgroup of patients receiving lenalidomide just before Pd (N = 62), the median duration of lenalidomide therapy was 9.9 months and the PFS, according to a duration of >12 or <12 months, was 4.6 vs 2.7 months (P = .052). Other factors associated with PFS are presented in Table 2.
Univariate analysis of factors associated with response, PFS, and OS
| . | ORR . | P . | PFS . | P . | OS . | P . |
|---|---|---|---|---|---|---|
| Age >70 y | 29% vs 36% | .393 | 4.9 vs 5 | .667 | 8.4 vs 14.3 | .761 |
| Hemoglobin <10 g/dL | 26% vs 36% | .221 | 4 vs 5.5 | .015 | 7.8 vs 22.8 | <.001 |
| Platelets <100 × 109/L | 20% vs 37.5% | .055 | 4 vs 5.4 | .005 | 7.2 vs 14.4 | <.001 |
| LDH > ULN | 35% vs 31% | .636 | 3.7 vs 6 | .001 | 7.9 vs 16.6 | <.001 |
| Calcium >10.5 mg/dL | 34% vs 30% | .734 | 2.85 vs 4.9 | .016 | 7.2 vs 14.3 | <.001 |
| eGFR <60 mL/min/1.73 m2 | 34% vs 32% | .806 | 4.8 vs 5.1 | .597 | 9.3 vs 12.8 | .650 |
| ISS 1 vs 2 vs 3 | 35% vs 30.5% | .552 | 5.8 vs 3.7 | .008 | 8 vs 16.2 | .028 |
| Last dose of lenalidomide 5-15 mg vs 25 mg | 38.5% vs 30.5% | .329 | 4.9 vs 5 | .929 | 11.9 vs 12.8 | .194 |
| Dexamethasone dose 0 vs <80 mg vs 160 mg | 78% vs 33% vs 31% | .015 | 20.6 vs 4 vs 4.8 | .03 | NR vs 9 vs 11.7 | .077 |
| Lenalidomide vs bortezomib/other PI vs chemo before Pd | 32% vs 37% vs 24% | .546 | 4.6 vs 5.9 vs 4.1 | .491 | 13.4 vs 11.9 vs 7.4 | .189 |
| Disease refractory to bortezomib | 30% vs 42% | .158 | 4.9 vs 5.5 | .112 | 11.4 vs 23.1 | .109 |
| 1-2 vs 3 vs >3 lines of prior therapy | 42% vs 37% 27% | .306 | 2.8 vs 4.8 vs 6 | .919 | 16 vs 12 vs 11.8 | .727 |
| Del17p* | 36% vs 37% | .991 | 4.6 vs 11.5 | .413 | 46 vs 12.2 | .233 |
| T(4;14)** | 42% vs 33% | .591 | 5.1 vs 4.1 | .973 | 7.5 vs 23 | .074 |
| Amp1q21* | 39% vs 37% | .866 | 5.1 vs 4.6 | .542 | 7.1 vs 16.5 | .400 |
| Any high-risk cytogenetics*,† | 42% vs 32% | .386 | 4 vs 6.5 | .455 | 22.8 vs 16.5 | .850 |
| Duration of last lenalidomide therapy <12 mo | 27% vs 44% | .03 | 3.2 vs 7.8 | .023 | 7.9 vs 16.5 | .005 |
| IMiD-free interval <18 mo | 23% vs 65% | <.001 | 3.9 vs 10.3 | .003 | 9.3 vs 27.1 | .008 |
| . | ORR . | P . | PFS . | P . | OS . | P . |
|---|---|---|---|---|---|---|
| Age >70 y | 29% vs 36% | .393 | 4.9 vs 5 | .667 | 8.4 vs 14.3 | .761 |
| Hemoglobin <10 g/dL | 26% vs 36% | .221 | 4 vs 5.5 | .015 | 7.8 vs 22.8 | <.001 |
| Platelets <100 × 109/L | 20% vs 37.5% | .055 | 4 vs 5.4 | .005 | 7.2 vs 14.4 | <.001 |
| LDH > ULN | 35% vs 31% | .636 | 3.7 vs 6 | .001 | 7.9 vs 16.6 | <.001 |
| Calcium >10.5 mg/dL | 34% vs 30% | .734 | 2.85 vs 4.9 | .016 | 7.2 vs 14.3 | <.001 |
| eGFR <60 mL/min/1.73 m2 | 34% vs 32% | .806 | 4.8 vs 5.1 | .597 | 9.3 vs 12.8 | .650 |
| ISS 1 vs 2 vs 3 | 35% vs 30.5% | .552 | 5.8 vs 3.7 | .008 | 8 vs 16.2 | .028 |
| Last dose of lenalidomide 5-15 mg vs 25 mg | 38.5% vs 30.5% | .329 | 4.9 vs 5 | .929 | 11.9 vs 12.8 | .194 |
| Dexamethasone dose 0 vs <80 mg vs 160 mg | 78% vs 33% vs 31% | .015 | 20.6 vs 4 vs 4.8 | .03 | NR vs 9 vs 11.7 | .077 |
| Lenalidomide vs bortezomib/other PI vs chemo before Pd | 32% vs 37% vs 24% | .546 | 4.6 vs 5.9 vs 4.1 | .491 | 13.4 vs 11.9 vs 7.4 | .189 |
| Disease refractory to bortezomib | 30% vs 42% | .158 | 4.9 vs 5.5 | .112 | 11.4 vs 23.1 | .109 |
| 1-2 vs 3 vs >3 lines of prior therapy | 42% vs 37% 27% | .306 | 2.8 vs 4.8 vs 6 | .919 | 16 vs 12 vs 11.8 | .727 |
| Del17p* | 36% vs 37% | .991 | 4.6 vs 11.5 | .413 | 46 vs 12.2 | .233 |
| T(4;14)** | 42% vs 33% | .591 | 5.1 vs 4.1 | .973 | 7.5 vs 23 | .074 |
| Amp1q21* | 39% vs 37% | .866 | 5.1 vs 4.6 | .542 | 7.1 vs 16.5 | .400 |
| Any high-risk cytogenetics*,† | 42% vs 32% | .386 | 4 vs 6.5 | .455 | 22.8 vs 16.5 | .850 |
| Duration of last lenalidomide therapy <12 mo | 27% vs 44% | .03 | 3.2 vs 7.8 | .023 | 7.9 vs 16.5 | .005 |
| IMiD-free interval <18 mo | 23% vs 65% | <.001 | 3.9 vs 10.3 | .003 | 9.3 vs 27.1 | .008 |
Available in 64 patients.
Defined as presence of any of t(4,14) or t(14,16) del17p.
In multivariate analysis for PFS, adjusting for lines of therapy, last lenalidomide dose, and dose of dexamethasone, we found that longer duration of lenalidomide exposure and longer IMiD-free interval were the most important prognostic factors for longer PFS (Table 3).
Multivariate analysis for PFS and for OS (final models)
| . | HR . | 95% CI for HR . | . | |
|---|---|---|---|---|
| Lower . | Upper . | P . | ||
| PFS | ||||
| Duration of last lenalidomide regimen <12 mo | 1.485 | 1.009 | 2.186 | .045 |
| Time since last lenalidomide dose <18 mo | 1.970 | 1.271 | 3.054 | .002 |
| OS | ||||
| Duration of last lenalidomide regimen <12 mo | 1.661 | 1.102 | 2.503 | .015 |
| Time since last lenalidomide dose <18 mo | 1.775 | 1.107 | 2.845 | .017 |
| . | HR . | 95% CI for HR . | . | |
|---|---|---|---|---|
| Lower . | Upper . | P . | ||
| PFS | ||||
| Duration of last lenalidomide regimen <12 mo | 1.485 | 1.009 | 2.186 | .045 |
| Time since last lenalidomide dose <18 mo | 1.970 | 1.271 | 3.054 | .002 |
| OS | ||||
| Duration of last lenalidomide regimen <12 mo | 1.661 | 1.102 | 2.503 | .015 |
| Time since last lenalidomide dose <18 mo | 1.775 | 1.107 | 2.845 | .017 |
HR, hazard ratio.
The OS of patients with a duration of lenalidomide therapy before Pd <12 months was 7.9 months vs 16.5 months for patients with longer duration of their last lenalidomide regimen (P = .005). For the patients that received lenalidomide just before Pd (n = 62), OS was 12.2 vs 7.5 months (P = .045), according to a duration of lenalidomide therapy >12 or <12 months. Patients with an IMiD-free interval <18 months had an OS of 9.3 months vs 27.1 months for those with at least an 18-month interval (P = .008) (Figure 3).
In multivariate analysis adjusting for lines of therapy, last lenalidomide dose, and dose of dexamethasone, we found that longer duration of lenalidomide exposure and longer IMiD-free interval were independent factors associated with longer OS (Table 3).
Treatment after pomalidomide
After Pd, 84 (57.5%) patients received further therapy; 19 (13%) received PI with an IMiD, 15 (10%) conventional chemotherapy, 8 (5.5%) IMiD with chemotherapy, 11 (7.5%) bortezomib with chemotherapy, 10 (7%) carfilzomib with dexamethasone, and 17 (12%) an anti-CD38 antibody. The median OS after progression to Pd for the patients who actually received subsequent therapy was 8.3 months; the OS, however, was 6 months for patients who received combinations of bortezomib with IMiDs or chemotherapy, it was 11 months for those that received carfilzomib with dexamethasone, and has not been reached for those that received an anti-CD38 antibody (87% OS at 12 months and 53% at 24 months, with 11/17 patients alive).
Discussion
In the current analysis, we aimed to understand patterns of lenalidomide resistance by analyzing a homogeneously treated population of relapsed/refractory patients with MM (all received pomalidomide with dexamethasone) without the addition of a third agent that could confound the analysis. Our major findings included the importance of the duration of exposure to lenalidomide, the impact of IMiD-free interval on response and survival after the combination of pomalidomide with dexamethasone, and the lack of a relationship of lenalidomide dose with resistance.
As lenalidomide maintenance becomes more extensively used, development of disease progression while on lenalidomide has become a common clinical scenario. It remains debatable if progression during low-dose lenalidomide (as during maintenance) is associated with the same degree of resistance as progression during full-dose lenalidomide. Pomalidomide is a major treatment option for patients who have failed a proteasome inhibitor and an IMiD27,28 and thus is an option also for patients progressing on lenalidomide maintenance or other lenalidomide-based therapies, such as lenalidomide/dexamethasone, which is approved and widely used in elderly patients. However, the optimal sequence of therapies for patients with myeloma with disease progressing while on a drug class has not been deciphered yet.27,28 Following the approval of lenalidomide at doses 10 to 15 mg as a maintenance therapy and the extensive use of this strategy in patients with MM after ASCT, it has become pressing to define lenalidomide resistance: is relapse while on 5, 10, or 15 mg of lenalidomide true resistance to lenalidomide? Our data indicate that the outcomes of patients that are resistant to lenalidomide are not dependent on the dose used during progression; thus, we state that relapse while on lower doses (5-15 mg) of lenalidomide still is associated with poor outcome and actually means true resistance to lenalidomide. However, because very few of our patients progressed while on lenalidomide monotherapy, our data are not directly applicable to the clinical cases of disease progression while on lenalidomide maintenance. Given the lack of data for these patients, however, we believe that our results provide significant information that is relevant in many clinical scenarios.
In this study, we also evaluated the efficacy of pomalidomide with dexamethasone, focusing on timing of prior lenalidomide exposure and duration of exposure to lenalidomide. The incentive for this analysis is related to the mechanism of resistance to lenalidomide and potentially other IMiDs. Our data indicate that longer durations of prior lenalidomide therapy were associated with better outcomes when pomalidomide is given. This may lead to the hypothesis that these patients may have had a more “IMiD-sensitive” disease and thus may benefit more when switching IMiDs, or at least keeping IMiDs in the backbone of therapy. In our analysis, we also observed the major importance of a longer IMiD-free interval in our patients treated with different IMiDs (from lenalidomide to pomalidomide). These results provide a clinical translation of previous observations on the clonal evolution in MM, occurring during the treatment of the disease1-3,29-32 and leading to the emergence of IMiD-resistant clones. Thus, the association of a longer IMiD-free interval with higher response rates and longer PFS and OS may be an indirect indication of repression of resistant clones or an inherently more sensitive disease. The cutoff of 18 months for an IMiD-free interval cannot be absolute because it was chosen based on the characteristics of our patients and an analysis based on probability of at least PR at 3 months after initiation of Pd. This cutoff is only indicative of the effect of a longer IMiD-free interval and should only be considered a rough measure; further validation is required in an independent cohort.
Because there may be a bias in selecting patients who have failed multiple lines of therapy within a short time, we adjusted for the number of prior lines of therapy for the duration of the disease from initial diagnosis, the last dose of lenalidomide, and for the use of dexamethasone: still longer duration of lenalidomide therapy and longer IMiD-free interval remained important and independent prognostic factors for better outcomes. These data should not lead to the conclusion that pomalidomide must not be used in patients failing lenalidomide, but physicians should be aware of potential lower activity if IMiD resistance has recently developed and IMiD-free interval is short. Our data rather provide arguments for the use of triplet combinations such as those with the addition of cyclophosphamide,15-17 bortezomib,33,34 carfilzomib,16,20,35,36 ixazomib,19 elotuzumab,22 or daratumumab,37,38 which have been explored and may overcome IMiD resistance and further improve outcomes. In addition, pomalidomide is probably able to partly overcome this resistance (reflected in the responses rates not being significantly different), but escape of the IMiD-resistant clones results in shorter PFS and OS. In addition, our data could be used to evaluate the results of larger phase 3 studies of various pomalidomide combinations and explain potential differences.
Responses and PFS in our cohort were similar to the results that have been published in the large phase 39 and 3B14 studies. This is not surprising because our patients had similar characteristics regarding prior treatments, exposure, and drug refractoriness to the patients that were treated in these 2 studies. Our patients did not have extensive prior exposure to anti-CD38 antibodies; however, today there are patients that have been exposed to anti-CD38 before exposure to lenalidomide. In such patients, the patterns of disease resistance to IMiDs may differ, as has been shown in a small study from our group that indicates a potential synergistic effect when daratumumab and an IMiD, in which resistance has developed, are combined.39
Our study has certain limitations. First, this is a retrospective study. Second, the number of patients may be relatively low for the different subgroups of patients that we analyzed, but the use of a common therapy reduces the confounding effect of treatment. Pomalidomide with dexamethasone is commonly used for patients failing lenalidomide, but addition of a third agent improves responses rates and PFS15,17,18,22,40 ; however, in this case, the additional effect of the third drug would confound the analysis. Third, not all patients received lenalidomide just before Pd, but this allowed us to evaluate the importance of an IMiD-free interval. Fourth, very few of our patients progressed while on lenalidomide maintenance (ie, on lower doses of lenalidomide but without dexamethasone), so our results should be viewed with caution in this patient population. Finally, we believe that further validation is required to better understand and define lenalidomide resistance, which should also include patients on lenalidomide maintenance.
In summary, pomalidomide with dexamethasone is an active therapy in patients with myeloma that is refractory to lenalidomide; however, resistance to lenalidomide is not dose dependent. Patients with a longer duration of prior lenalidomide therapy, probably as a marker of inherent IMiD sensitivity, and longer IMiD-free interval, suggestive of potential reemergence of IMiD-sensitive clones, have longer PFS and OS irrespective of prior lines of therapy. These data should be evaluated in independent cohorts to identify optimal treatment strategies for patients with resistance to IMiDs and other agents.
Please contact the corresponding author for data sharing requests.
Authorship
Contribution: E.K., M.R., M.A.D., and E.T. collected data, performed analysis, and drafted the manuscript; and M.G., N.K., M.M., E.E.-P., D.C.Z., D.F., I.N.-S., I.D., S.G., P.T., A.X., S.D., D.M., and M.S.-V. collected data and reviewed and approved the manuscript.
Conflict-of-interest disclosure: E.K. reports honoraria and advisory board work for Genesis Pharma, Takeda, Janssen, and Amgen. E.T. reports honoraria and advisory boards for Janssen, Celgene, Takeda, Genesis Pharma, and Amgen. M.A.D. reports honoraria and advisory boards for BMS, Janssen, Celgene, Amgen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Meletios A. Dimopoulos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, 80 Vas. Sofias Ave, 11528 Athens, Greece; e-mail: mdimop@med.uoa.gr.