Key Points
Type I and II are 2 novel subpopulations of immunoglobulin G aPS/PT with different mechanisms of prothrombin recognition and function.
APS patients can be classified into 2 groups, A and B, according to the presence of type I and II aPS/PT autoantibodies.
Abstract
Anti-phosphatidylserine/prothrombin (aPS/PT) antibodies are often detected in patients with antiphospholipid syndrome (APS), but how aPS/PT engage prothrombin at the molecular level remains unknown. Here, the antigenic determinants of immunoglobulin G aPS/PT were investigated in 24 triple-positive APS patients at high risk of thrombosis by using prothrombin mutants biochemically trapped in closed and open conformations, and relevant fragments spanning the entire length of prothrombin. Two novel unexpected findings emerged from these studies. First, we discovered that some aPS/PT are unique among other anti-prothrombin antibodies insofar as they efficiently recognize prothrombin in solution after a conformational change requiring exposure of fragment-1 to the solvent. Second, we identified and characterized 2 previously unknown subpopulations of aPS/PT, namely type I and type II, which engage fragment-1 of prothrombin at different epitopes and with different mechanisms. Type I target a discontinuous density-dependent epitope, whereas type II engage the C-terminal portion of the Gla-domain, which remains available for binding even when prothrombin is bound to the phospholipids. Based on these findings, APS patients positive for aPS/PT were classified into 2 groups, group A and group B, according to their autoantibody profile. Group A contains mostly type I antibodies whereas group B contains both type I and type II antibodies. In conclusion, this study offers a first encouraging step toward unveiling the heterogeneity of anti-prothrombin antibodies in correlation with thrombosis, shedding new light on the mechanisms of antigen–autoantibody recognition in APS.
Introduction
In the absence of genetic signatures, the biological hallmark of the antiphospholipid syndrome (APS) is represented by a thrombotic episode and the constant presence in plasma (>12 weeks) of a heterogeneous family of autoantibodies known as antiphospholipid antibodies (aPL).1-3 How aPL elicit thrombosis and obstetric complications in patients with APS is not very well understood at the molecular level,4-6 and equally challenging remains the identification of the classes of aPL that best correlate with the clinical manifestations of the disease.7-9 In fact, not only aPL interact with a variety of lipid and protein antigens but, owing to the molecular flexibility of the antigens themselves, subpopulations of aPL targeting the same antigen have been documented in patients with APS.10,11
Among the types of aPL commonly found in patients with APS, antibodies targeting prothrombin in complex with phosphatidylserine (aPS/PT) have been gaining popularity in recent years because of their strong positive correlation with thrombosis.12-15 Using enzyme-linked immunosorbent assays (ELISA), aPS/PT have been found primarily in 2 types of APS patients: (1) patients positive solely for lupus anticoagulant13,16-18 (as discussed in Table 1 of the review by Amengual et al19 ); and (2) patients positive for lupus anticoagulant, anti-cardiolipin (aCL), and anti–β2-glycoprotein I (anti-β2GPI) antibodies who carry the highest risk of thrombosis and recurrence, the so-called “triple-positive.”12,14,20 Because of these clinical observations and earlier research conducted in animal models of APS-induced thrombosis,21-23 it has been hypothesized that aPS/PT may be responsible for some of the vascular and obstetric manifestations observed in patients with APS; testing for aPS/PT could therefore be requested by physicians to confirm or reinforce an APS diagnosis in selected patients or even adopted as a new test to identify novel APS patients at higher risk of thrombosis who would otherwise go undetected with the use of current testing methods.
Whether aPS/PT merit inclusion in the official classification criteria as a new biomarker of APS is a very complex and important decision that would benefit tremendously from prospective multicenter studies and from an in-depth knowledge of the molecular signatures that differentiate aPS/PT from the other classes of aPL. Although clinical validation is on the rise,24 little is known about the mechanisms of prothrombin recognition by aPS/PT.19,25 To fill this gap in our fundamental knowledge, the goal of the current study was to elucidate how immunoglobulin G (IgG) aPS/PT recognize prothrombin at the molecular level.
Materials and methods
Protein production and purification
Prothrombin wild-type (proTWT) and mutants were expressed in mammalian cells as previously described.26 Prethrombin-2 was expressed in Escherichia coli.27 Prethrombin-1, fragment-1, kringle-1, and kringle-2 were obtained by limited proteolysis using thrombin and factor Xa.28 The purity and chemical identity of each fragment were verified by using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and N-terminal sequencing. Small unilamellar vesicles composed of phosphatidylcholine (PC) or phosphatidylcholine and phosphatidylserine (PS) in a 3:1 molar ratio (PC:PS) were prepared by extrusion using 100-nm polycarbonate membranes (Avanti Polar Lipids), kept at 4°C, and used within 7 days.26 Protein concentrations were determined by reading at 280 nm with molar extinction coefficients adjusted based on the amino acid sequence. All other chemicals were purchased from MilliporeSigma.
Patients and total IgG extracts
Venous blood was collected at the University of Padua in 3.8% sodium citrate (9:1) and centrifuged twice at 2000g for 15 min at 4°C. Plasma was stored in 25 μL aliquots at −80°C ready for individual use. All subjects gave their informed consent to the present study. Twenty-seven patients with APS and triple positivity according to the Sapporo Classification3,29 and 2 healthy donors were included in this study. Lupus anticoagulant was assayed as previously described.30 Anti-cardiolipin IgG antibodies (aCL) and anti–β2-glycoprotein I IgG antibodies (anti-β2GPI) were detected by using QUANTA Lite ACA IgG III and QUANTA Lite β2 GPI IgG (Inova Diagnostics) following the manufacturing instructions. The laboratory characteristics of the cohort are reported in Table 1. Total IgG extracts were purified by using Protein G spin columns (Thermo Fisher Scientific) followed by size exclusion chromatography to obtain pure IgG monomers. Each preparation was >98% pure as judged by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The IgG concentration was determined by reading at 280 nm with a molar extinction coefficient of 1.0 mg-1cm−1.
Laboratory characteristics of the study cohort
| Patient no. . | aCL . | Anti-β2GPI . | aPS/PT . | aPT-A . |
|---|---|---|---|---|
| P1 | + | + | +++ | + |
| P3 | ++ | ++++ | ++++ | +++ |
| P4 | ++ | ++ | ++++ | + |
| P6 | +++ | +++ | +++ | - |
| P7 | + | + | ++++ | ++++ |
| P8 | +++ | +++ | ++ | - |
| P9 | ++ | + | ++++ | ++++ |
| P10 | + | +++ | +++ | - |
| P11 | + | ++ | + | - |
| P12 | ++ | +++ | + | - |
| P13 | +++ | ++++ | +++ | ++ |
| P14 | +++ | +++ | ++++ | + |
| P15 | +++ | +++ | + | - |
| P16 | + | + | +++ | - |
| P17 | +++ | ++++ | ++++ | - |
| P18 | +++ | +++ | + | - |
| P19 | + | ++ | +++ | - |
| P20 | + | + | +++ | + |
| P21 | ++ | ++ | ++++ | + |
| P23 | ++ | + | ++++ | +++ |
| P24 | ++ | +++ | ++++ | + |
| P25 | + | ++ | +++ | - |
| P26 | +++ | ++++ | ++++ | + |
| P27 | + | + | ++++ | + |
| Patient no. . | aCL . | Anti-β2GPI . | aPS/PT . | aPT-A . |
|---|---|---|---|---|
| P1 | + | + | +++ | + |
| P3 | ++ | ++++ | ++++ | +++ |
| P4 | ++ | ++ | ++++ | + |
| P6 | +++ | +++ | +++ | - |
| P7 | + | + | ++++ | ++++ |
| P8 | +++ | +++ | ++ | - |
| P9 | ++ | + | ++++ | ++++ |
| P10 | + | +++ | +++ | - |
| P11 | + | ++ | + | - |
| P12 | ++ | +++ | + | - |
| P13 | +++ | ++++ | +++ | ++ |
| P14 | +++ | +++ | ++++ | + |
| P15 | +++ | +++ | + | - |
| P16 | + | + | +++ | - |
| P17 | +++ | ++++ | ++++ | - |
| P18 | +++ | +++ | + | - |
| P19 | + | ++ | +++ | - |
| P20 | + | + | +++ | + |
| P21 | ++ | ++ | ++++ | + |
| P23 | ++ | + | ++++ | +++ |
| P24 | ++ | +++ | ++++ | + |
| P25 | + | ++ | +++ | - |
| P26 | +++ | ++++ | ++++ | + |
| P27 | + | + | ++++ | + |
Optical density values at 450 nm for aCL, aβ2GPI, aPS/PT, and aPT-A. All patients showed lupus anticoagulant, measured as prolongation of the clotting time.3,29 P2, P5, and P22 had levels of aPS/PT below the cutoff and were therefore omitted. Scoring system for the ELISA assays: + = from 0.25 to 0.5; ++ = from 0.5 to 1.0; +++ = from 1.0 to 2.0; ++++ = from 2.0 to maximum. White = Group A; Blue = Group B.
Detection of aPS/PT
The levels of aPS/PT were detected by using QUANTA Lite aPS/PT IgG (Inova Diagnostics) following the manufacturer’s instructions. Patients’ plasma was added to the plates alone (100 μL, 1:100 vol/vol, control) or was preincubated side-by-side with each prothrombin competitor at the specified concentration (50 μL, 1:50 vol/vol, and 50 μL, 0-30 μM proT) for 30 minutes before determining the residual level of autoantibodies. The optical density at 450 nm was measured on a SpectraMax i3x multimode detection platform (Molecular Devices). All experiments were performed in duplicate with at least 2 lots of pure recombinant proteins and protein fragments. Each point represents the mean of at least 4 individual determinations. For each set of experiments, positive and negative controls provided by the manufacturer were included.
Detection of aPT-A
The levels of IgG anti-prothrombin antibodies (aPT-A) were detected by using an in-house ELISA in which proTWT (1 μg/well) was absorbed onto NUNC Maxisorp 96-well microtiter plates (Thermo Fisher Scientific), as reported previously.31
Surface plasmon resonance experiments
Binding affinities were measured by using an L1 sensor chip on a BIAcore-S200 instrument (GE Healthcare) at 25°C in which liposomes were immobilized at 1600 response units. Dose-dependent experiments were performed by injecting increasing concentrations (0-2 μM) of proTWT, prothrombin Y93A (proTY93A), double-mutant proTS101C/A470C (proTCC), and Gla-domainless prothrombin (GD-proT) (as a control) in running buffer (10 mM Tris pH 7.4, 150 mM NaCl, 10 mM CaCl2, 0.1% wt/wt BSA) at a flow rate of 25 μL/min. The equilibrium dissociation constants were obtained as a fitting parameter by plotting the value of the response units at the steady state for each concentration by using the BIAevaluation software (GE Healthcare) and OriginPro 2015 (OriginLab Corporation).
Statistical analysis
OriginPro 2015 was used for statistical analysis. Comparisons between 2 groups were performed by using a 2-sample Student t test. Results were considered significant at P < .05 (*P < .05, **P < .001, n.s. = not significant).
Results
IgG aPS/PT display superior reactivity toward the open conformation of prothrombin in solution
Prothrombin exists in 2 forms at equilibrium, “closed” and “open,” due to the flexibility of the linker connecting the kringle domains26,32 (Figure 1). The closed form adopts a compact shape and is characterized by an intramolecular interaction between residues Y93 in kringle-1 and W547 in the protease domain. This form is believed to predominate under physiological conditions and can be irreversibly trapped by proTCC, which introduces an artificial disulfide bond between residues S101 in kringle-1 and A470 in the protease domain.26 Unlike the closed form, the structure of the open form is more elongated and can be artificially stabilized by the single point mutation of residue Y93 to alanine in the prothrombin mutant proTY93A,33 the removal of 14 residues from linker-2 in the prothrombin mutant Δ154-167,34 and the addition of the thrombin-specific active site inhibitor argatroban to the closed form.26,33
Prothrombin structures. Prothrombin is a multidomain protein that adopts closed (A) and open (B) states. It comprises the N-terminal Gla-domain (blue), 2 kringles (kringle 1 [K1], red; kringle 2 [K2], green), and a serine protease domain (SP, yellow). The X-ray crystal structures of the prothrombin mutant proTCC (Protein Data Bank Identifier: 6C2W,26 left panel) and proTΔ154-167(Protein Data Bank Identifier: 5EDM,34 right panel) have been solved recently. Mutation of residues S101 and A470 to cysteine in proTCC stabilizes the closed form. The location of these 2 residues is shown in cyan. Conversely, mutation of residue Y93 to alanine in the mutant proTY93A, deletion of 14 of 26 residues of Lnk2 in the mutant proTΔ154-167, or addition of argatroban stabilizes the open form. The location of these residues is shown in magenta. The Gla-domain of both structures has been slightly modified from the original structures and adopts the calcium-bound, physiologically relevant conformation.47
Prothrombin structures. Prothrombin is a multidomain protein that adopts closed (A) and open (B) states. It comprises the N-terminal Gla-domain (blue), 2 kringles (kringle 1 [K1], red; kringle 2 [K2], green), and a serine protease domain (SP, yellow). The X-ray crystal structures of the prothrombin mutant proTCC (Protein Data Bank Identifier: 6C2W,26 left panel) and proTΔ154-167(Protein Data Bank Identifier: 5EDM,34 right panel) have been solved recently. Mutation of residues S101 and A470 to cysteine in proTCC stabilizes the closed form. The location of these 2 residues is shown in cyan. Conversely, mutation of residue Y93 to alanine in the mutant proTY93A, deletion of 14 of 26 residues of Lnk2 in the mutant proTΔ154-167, or addition of argatroban stabilizes the open form. The location of these residues is shown in magenta. The Gla-domain of both structures has been slightly modified from the original structures and adopts the calcium-bound, physiologically relevant conformation.47
Given the considerable variation of solvent-accessible surface area associated with the closed-to-open transition and the well-established conformational heterogeneity of aPL, especially anti-β2GPI antibodies,7,8,11,35,36 we hypothesized that IgG aPS/PT may display different reactivity in solution toward prothrombin mutants biochemically stabilized in closed (proTCC) and open (proTY93A, prothrombin Δ154-167 [proTΔ154-167]) conformations. This hypothesis was tested by performing ELISA competition experiments using the plasma of 24 triple-positive APS patients with levels of IgG aPS/PT >30 units (Figure 2A).
Reactivity of aPS/PT toward closed and open conformations of prothrombin in solution. (A) IgG aPS/PT were researched in 27 APS triple-positive patients (P1-P27). Two healthy donors (HD1 and HD2), one negative control (– CTRL), and 1 positive control (+ CTRL) were included. The dashed red line (optical density at 450 nm [OD450nm] = 0.32 or 30 units) identifies the cutoff value. (B-D) Competition experiments. (B) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTCC, proTY93A and proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays. The effect of each competitor is reported as the percentage of inhibition calculated by using the following: 100 × ((OD1 – OD2)/OD1), where optical density 1 (OD1) and OD2 are the values of absorbance in the absence and presence of competitor, respectively. (C) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). Data were analyzed with a simple binding equation, S = S0 + (S∞ I/IC50)/(1 + I/IC50), and the values of IC50 are reported in Table 2. (D) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT in the absence or presence of 200 μM argatroban (Arg) for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays as described before. (E-F) Reactivity of aPT-A toward closed and open conformations of prothrombin in solution. (E) Diluted plasma samples (50 μL, 1:50 vol/vol) from 13 APS patients positive for aPT-A were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT, proTCC, proTY93A, or proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPT-A were calculated as described above. (F) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). IC50 values are reported in Table 2. (G) Subpopulations of aPS/PT. Two-dimensional representation of the inhibitory effect determined in Figure 2B for proTCC (closed, x-axis) and proTY93A (open, y-axis) in the 24 APS patients positive for aPS/PT. Dashed black lines divide the plot into 4 quadrants. Group A patients (red box) are mostly located in the upper left quadrant, with a few outliers. Group B patients falls into the upper right quadrant. No aPS/PT were detected in the lower right quadrant.
Reactivity of aPS/PT toward closed and open conformations of prothrombin in solution. (A) IgG aPS/PT were researched in 27 APS triple-positive patients (P1-P27). Two healthy donors (HD1 and HD2), one negative control (– CTRL), and 1 positive control (+ CTRL) were included. The dashed red line (optical density at 450 nm [OD450nm] = 0.32 or 30 units) identifies the cutoff value. (B-D) Competition experiments. (B) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTCC, proTY93A and proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays. The effect of each competitor is reported as the percentage of inhibition calculated by using the following: 100 × ((OD1 – OD2)/OD1), where optical density 1 (OD1) and OD2 are the values of absorbance in the absence and presence of competitor, respectively. (C) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). Data were analyzed with a simple binding equation, S = S0 + (S∞ I/IC50)/(1 + I/IC50), and the values of IC50 are reported in Table 2. (D) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT in the absence or presence of 200 μM argatroban (Arg) for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays as described before. (E-F) Reactivity of aPT-A toward closed and open conformations of prothrombin in solution. (E) Diluted plasma samples (50 μL, 1:50 vol/vol) from 13 APS patients positive for aPT-A were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT, proTCC, proTY93A, or proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPT-A were calculated as described above. (F) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). IC50 values are reported in Table 2. (G) Subpopulations of aPS/PT. Two-dimensional representation of the inhibitory effect determined in Figure 2B for proTCC (closed, x-axis) and proTY93A (open, y-axis) in the 24 APS patients positive for aPS/PT. Dashed black lines divide the plot into 4 quadrants. Group A patients (red box) are mostly located in the upper left quadrant, with a few outliers. Group B patients falls into the upper right quadrant. No aPS/PT were detected in the lower right quadrant.
We found that the addition of 5 μM of proTCC to patients’ plasma resulted in a small but significant 18% reduction in the optical density (Figure 2B). In contrast, mutants biochemically stabilized in the open form bound much tighter to IgG aPS/PT, showing median inhibitory values of 60% for proTY93A and 67% for proTΔ154-167, as well as 50% inhibitory concentration (IC50) values on average 15-fold lower compared with proTCC (Figure 2C; Table 2). This scenario shows that proTY93A and proTΔ154-167 are stronger competitors in solution.
Values of IC50 (micromolar) for proTWT, proTCC, proTY93A, and proTΔ154-167 for IgG aPS/PT and IgG aPT-A
| Variable . | proTWT . | proTCC . | proTY93A . | proTΔ154-167 . | Ratio (closed/open) . |
|---|---|---|---|---|---|
| aPS/PT | |||||
| P7 | 71 ± 10 | >80 | 4.5 ± 0.8 | 4.5 ± 0.8 | 18 |
| P13 | >80 | >80 | 4.1 ± 1.1 | 3.5 ± 0.8 | 19 |
| P24 | 22.4 ± 5 | 25 ± 5 | 3.2 ± 0.9 | 2.8 ± 0.8 | 8 |
| aPT-A | |||||
| P7 | 2.6 ± 0.5 | 2.4 ± 0.5 | 2.7 ± 0.4 | 1.2 ± 0.3 | 0.9 |
| P13 | 3.8 ± 1.0 | 4.8 ± 1.1 | 3.7 ± 0.5 | 3.5 ± 0.5 | 1.3 |
| P24 | 1.7 ± 0.3 | 1.5 ± 0.5 | 2.8 ± 0.3 | 2.5 ± 0.8 | 0.5 |
| Variable . | proTWT . | proTCC . | proTY93A . | proTΔ154-167 . | Ratio (closed/open) . |
|---|---|---|---|---|---|
| aPS/PT | |||||
| P7 | 71 ± 10 | >80 | 4.5 ± 0.8 | 4.5 ± 0.8 | 18 |
| P13 | >80 | >80 | 4.1 ± 1.1 | 3.5 ± 0.8 | 19 |
| P24 | 22.4 ± 5 | 25 ± 5 | 3.2 ± 0.9 | 2.8 ± 0.8 | 8 |
| aPT-A | |||||
| P7 | 2.6 ± 0.5 | 2.4 ± 0.5 | 2.7 ± 0.4 | 1.2 ± 0.3 | 0.9 |
| P13 | 3.8 ± 1.0 | 4.8 ± 1.1 | 3.7 ± 0.5 | 3.5 ± 0.5 | 1.3 |
| P24 | 1.7 ± 0.3 | 1.5 ± 0.5 | 2.8 ± 0.3 | 2.5 ± 0.8 | 0.5 |
Each titration was repeated twice, and the values of IC50 were determined by global fit using a simple binding equation (see "Materials and methods"). The ratio was calculated by divining the IC50 values of proTCC (closed) and proTY93A (open).
Similar results were attained with proTWT in the absence and presence of argatroban (Figure 2D). A weak reactivity (15%) was detected with proTWT alone, but a much stronger inhibition (54%) was observed in the presence of proTWT and argatroban. Given the similarity between proTWT in complex with argatroban with proTY93A and proTΔ154-167, we concluded that IgG aPS/PT require a significant structural reorganization of prothrombin for proper recognition in solution and that the superior reactivity of the antibodies toward the open form of prothrombin is unlikely to arise from an artifact caused by the mutations. Importantly, this interpretation is in agreement with recent findings showing that proTCC and proTWT adopt similar conformations in solution26 and fully consistent with the presence of the preexisting conformational equilibrium for proTWT. In principle, higher levels of competition could be expected for proTWT because it exists in equilibrium between open and closed conformations, whereas proTCC is locked in the closed state. However, the equilibrium is heavily biased toward the closed form, which will greatly reduce the apparent antibody binding constants. Using the estimates of relevant binding constants (Table 2), the calculated expected values of competition under our experimental conditions for proTWT and proTCC (supplemental Figure 1) should differ by no more than 5% (ie, within experimental error).
Additional support to the proposed conformation-dependent mechanism of ligand recognition of IgG aPS/PT was researched by investigating the reactivity of a different family of anti-prothrombin antibodies, namely aPT-A, against closed and open forms of prothrombin in solution. aPT-A are detected by immobilizing prothrombin alone onto hydrophilic plastic plates, without phospholipids, and are believed to recognize epitopes located primarily in kringle-2 and the protease domain.37–39 In competition experiments, proTWT, proTCC, and proTY93A displayed similar levels of inhibition and comparable IC50 values against aPT-A (Figure 2E-F; Table 2). The difference between proTCC and proTΔ154-167, however, was statistically significant, suggesting that the distal portion of Lnk2 may be recognized by aPT-A. This interpretation is consistent with the structural data shown in Figure 1 in which kringle-2, Lnk2, and the majority of the protease domain (unlike fragment-1) are exposed to the solvent regardless of the conformation of prothrombin. Because of the chemical diversity of Lnk2 in human and bovine prothrombin,34 these data could also help rationalize previous observations.40
Identification of 2 novel subpopulations of APS patients positive for IgG aPS/PT
Despite the superior reactivity of IgG aPS/PT toward the open form in solution, proTCC was found to inhibit >50% the binding of IgG aPS/PT to immobilized prothrombin in roughly one-half of the patient population. This finding was unexpected and led us to hypothesize that different subpopulations of autoantibodies within aPS/PT may exist. To investigate this theory, we compared the levels of inhibition produced by closed (proTCC, x-axis) vs open (proTY93A, y-axis) forms of prothrombin for each patient and plotted the result of the analysis in Figure 2G. The 24 data points analyzed with this method clustered in 2 well-separated quadrants of the plot, dividing the patient population into 2 groups that we labeled group A and group B. Group A (n = 15), on the upper left quadrant, was characterized by IgG aPS/PT featuring low (<30%) inhibition in the presence of the closed but significant inhibition in the presence of the open (>45%) form. Group B (n = 9), on the upper right quadrant, was characterized by IgG aPS/PT displaying a significant inhibition by the closed form (>50%) that was enhanced <25% by the open form. Unfortunately, no correlation existed between the total levels of IgG aPS/PT detected by using ELISA and autoantibody types, thus precluding the identification of these 2 subclasses simply based on the total plasma levels of autoantibodies. Hence, competition experiments remain, thus far, the only way to identify these 2 subpopulations of APS patients.
Epitope mapping studies uncover type I and type II antibodies
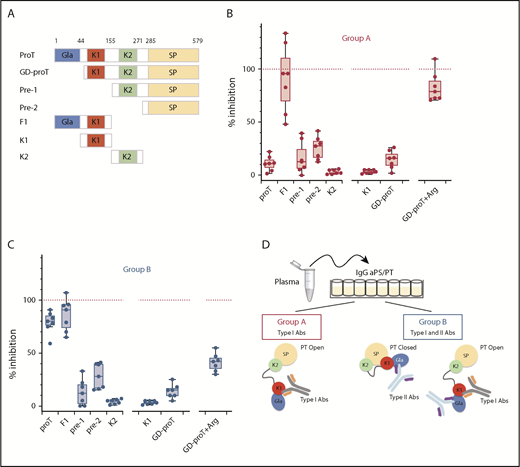
Assuming that IgG aPS/PT from group A and B recognize prothrombin at distinct sites, using different binding mechanisms, epitope mapping experiments were performed to identify the antigenic determinants of each group. Specifically, proTWT and 6 fragments spanning the entire length of the molecule were used in competition experiments (Figure 3A) along with total IgG extracts purified from 7 patients from group A and 7 patients from group B.
Epitope mapping of aPS/PT antibodies from groups A and B. (A) Prothrombin fragments: GD-proT (residues 44-579); prethrombin-1, pre-1 (156-579); prethrombin-2, pre-2 (residues 285-579); fragment-1, F1 (residues 1-154); kringle-1, K1 (residues 44-155); and kringle-2, K2 (residues 156-271). Total IgG extracts from 7 patients from group A (B) (50 μL) (P4, P7, P9, P13, P14, P24, and P27) and 7 patients from group B (C) (50 μL) (P1, P3, P6, P17, P19, P21, and P25) were mixed with a solution (50 μl, 10 μM) of proTWT or specified fragment for 30 minutes at room temperature. Argatroban (Arg) was mixed with GD-proT at a concentration of 250 μM. The residual level of aPS/PT antibodies from group A (B) and group B (C) was quantified by using ELISA assays as described before. The effect of each competitor is reported as the percentage of inhibition relative to the effect of proTY93A (100%, dashed red line). (D) Visual abstract summarizing the main finding of the epitope mapping studies.
Epitope mapping of aPS/PT antibodies from groups A and B. (A) Prothrombin fragments: GD-proT (residues 44-579); prethrombin-1, pre-1 (156-579); prethrombin-2, pre-2 (residues 285-579); fragment-1, F1 (residues 1-154); kringle-1, K1 (residues 44-155); and kringle-2, K2 (residues 156-271). Total IgG extracts from 7 patients from group A (B) (50 μL) (P4, P7, P9, P13, P14, P24, and P27) and 7 patients from group B (C) (50 μL) (P1, P3, P6, P17, P19, P21, and P25) were mixed with a solution (50 μl, 10 μM) of proTWT or specified fragment for 30 minutes at room temperature. Argatroban (Arg) was mixed with GD-proT at a concentration of 250 μM. The residual level of aPS/PT antibodies from group A (B) and group B (C) was quantified by using ELISA assays as described before. The effect of each competitor is reported as the percentage of inhibition relative to the effect of proTY93A (100%, dashed red line). (D) Visual abstract summarizing the main finding of the epitope mapping studies.
We found that both antibodies in groups A and B reacted very poorly against the C-terminal fragment prethrombin-1 (residues 156-579) that was obtained by cleaving prothrombin at R155 with thrombin (Figure 3B-C). They also showed weak reactivity toward prethrombin-2 (residues 285-579) and kringle-2 (residues 156-271), which are proteolytic fragments of prethrombin-1. In contrast, binding of IgG aPS/PT from groups A and B to immobilized prothrombin was strongly inhibited by the N-terminal fragment, fragment-1 (residues 1-155). This outcome led us to conclude that, in solution, the main epitope of IgG aPS/PT is located in the N-terminal portion of the prothrombin molecule.
Fragment-1 comprises the Gla-domain (residues 1-44) and kringle-1 (residues 44-155). To narrow down the location of the epitope, antibodies were reacted against kringle-1 and GD-proT (residues 44-579). As done before for the full-length protein, argatroban was added to GD-proT to define, in this case, the contribution of the Gla-domain in the open form.26,33 Antibodies of group A (Figure 3B) did not bind to kringle-1, interacted weakly with GD-proT (perhaps slightly better than proT), and bound well to GD-proT in complex with argatroban. Antibodies of group B (Figure 3C) lost their ability to interact with proT in solution after the removal of the Gla-domain (from 84% to 15%), reacted modestly toward GD-proT in the presence of argatroban and, similar to antibodies of group A, did not recognize kringle-1. In summary, IgG aPS/PT target fragment-1 of prothrombin at 2 different epitopes, using 2 different mechanisms (Figure 3D). The first epitope, or epitope 1, requires a conformational change of the antigen in solution to become available. The second epitope, or epitope 2, is exposed to the solvent in both closed and open conformations and seems to be restricted to the Gla-domain. Importantly, the different inhibitory profile of GD-proT with and without argatroban in the 2 groups suggests that epitope 1–directed antibodies, or type I antibodies (type I Abs), are present in both groups. At variance, epitope 2 targeting antibodies, or type II Abs, may be a unique characteristic of group B.
Role of the membranes on the formation of aPS/PT complexes
Several studies have shown that IgG aPS/PT best recognize prothrombin when it is bound to negatively charged phospholipids.17,38,40–44 Our experiments thus far have shown that some IgG aPS/PT prefer the open conformation of prothrombin in solution. A possible explanation to this effect would be that binding of prothrombin to the phospholipids induces opening of the prothrombin structure, thus facilitating autoantibody recognition. Another reason would be that opening of the prothrombin structure somehow mimics the formation of high-affinity immunogenic complexes attained by increasing the local concentration of the antigen onto the membranes. In contrast to the former model, the latter mechanism does not require a structural rearrangement of the protein upon binding to the membranes to explain the enhanced binding affinity as it relies on the bivalent nature of IgG molecules. This model is often referred to as “phospholipid-induced oligomerization.”38,41,45,46
To elucidate the binding mechanism of type I Abs, we took advantage of the unique biochemical features of proTCC and proTY93A. We reasoned that if the opening of the prothrombin structure is responsible for the increased reactivity of IgG aPS/PT toward lipid-bound prothrombin, more inhibition of type I Abs should be observed in the presence of liposomes only for proTWT but not for proTCC and proTY93A. In fact, proTCC is covalently locked in the closed form and cannot open up upon binding to the membranes; in contrast, proTY93A is already open and primed for autoantibody binding.
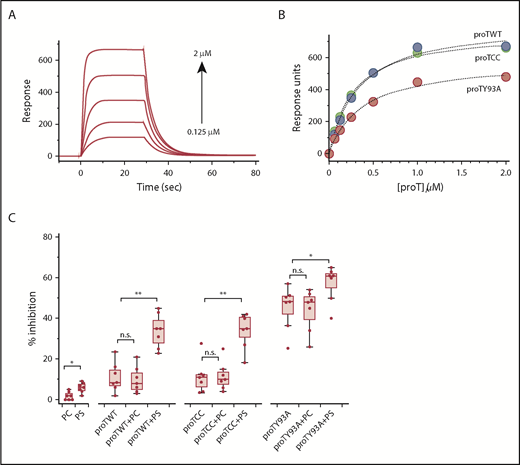
At first, we used surface plasmon resonance to confirm that proTWT, proTCC, and proTY93A interact with liposomes made of a 75:25 PC:PS mixture with the same affinity, and they do (Figure 4A-B). We next incubated proTWT, proTCC, and proTY93A in the absence or presence of saturating concentration of PC:PS liposomes with type I Abs and determined the level of inhibition according to ELISA (Figure 4C). Liposomes with different chemical compositions with and without proteins served as controls. In the presence of PC:PS, the inhibitory potency of proTWT and proTCC significantly increased and was comparable to the effect of proTY93A. The addition of phospholipids to proTY93A also improved inhibition, although to a much lower extent. These results vouch for a phospholipid-induced oligomerization model whereby clustering of prothrombin onto the membranes drives the enhanced reactivity of type I Abs toward phospholipid-bound prothrombin. Importantly, conformational changes after the initial complex formation may be possible and important for complex stabilization and cannot be ruled out by these data.
Effect of the membranes. Binding of proTWT (green circles), proTCC (blue circles), and proTY93A (red circles) to PS-containing liposomes monitored by surface plasmon resonance. (A) Representative sensograms for proTCC (0.125-2 μM, 1:2 dilution). (B) Plot of response units (RUs) as a function of prothrombin concentration. Solid lines were drawn according to a simple binding equation with best-fit parameters: proTWT, RU0 = 0, RU∞ = 773 ± 14, dissociation constants (Kd) = 270 ± 15 nM; proTCC, RU0 = 0, RU∞ = 822 ± 14, Kd = 329 ± 10 nM; proTY93A, RU0 = 0, RU∞ = 581 ± 14, Kd = 370 ± 21nM. (C) Competitive inhibition of proTWT, proTCC, and proTY93A at 5 μM in the absence or presence of liposomes (100 μM).
Effect of the membranes. Binding of proTWT (green circles), proTCC (blue circles), and proTY93A (red circles) to PS-containing liposomes monitored by surface plasmon resonance. (A) Representative sensograms for proTCC (0.125-2 μM, 1:2 dilution). (B) Plot of response units (RUs) as a function of prothrombin concentration. Solid lines were drawn according to a simple binding equation with best-fit parameters: proTWT, RU0 = 0, RU∞ = 773 ± 14, dissociation constants (Kd) = 270 ± 15 nM; proTCC, RU0 = 0, RU∞ = 822 ± 14, Kd = 329 ± 10 nM; proTY93A, RU0 = 0, RU∞ = 581 ± 14, Kd = 370 ± 21nM. (C) Competitive inhibition of proTWT, proTCC, and proTY93A at 5 μM in the absence or presence of liposomes (100 μM).
To further explore this possibility and test whether epitope 2–directed antibodies may have different functional effects than epitope 1–directed antibodies, phospholipid binding experiments were performed (Figure 5). Using surface plasmon resonance, we found that autoantibodies from three group A patients seemed to stimulate a time-dependent accumulation of prothrombin onto the membranes, whereas autoantibodies from two group B patients seemed to inhibit the in vitro binding of prothrombin to immobilized liposomes. Specifically, proTWT binding to PC:PS was characterized by 3 phases: a sharp association phase (phase I), a flat equilibrium phase (phase II), and a very rapid dissociation phase (phase III). Incubation of proTWT with total IgG extracts from group A patients containing type I Abs gave rise to a time-dependent accumulation of prothrombin onto the membranes, as shown by a linear increase in phase II. Importantly, phase III slowed down, suggestive of formation of dimeric (multimeric) complexes. This type of profile is consistent with a phospholipid-induced oligomerization model and is similar to data previously published by Field et al45 and Willems et al46 for antiprothrombin antibodies using surface plasmon resonance and ellipsometry, respectively. Type II Abs in group B, in contrast, inhibited the in vitro binding of proTWT to phospholipids, as captured by the lower response in phase I for P1, P10, and P25. Of note, P10 and P25 displayed a mixed behavior, as expected for group B patients due to the presence of both type I and type II Abs.
Effect of IgG anti-prothrombin antibodies on the binding of prothrombin to phospholipids. PC:PS (75:25 wt/wt) liposomes were immobilized onto an L1 chip, and proTWT was injected at a concentration of 0.5 μM (or 0.036 mg/mL) in the absence (solid green line) or presence (0.10-0.15 mg/mL, solid red or blue line) of IgGs. Panels A-C display the effect of IgGs isolated from P4, P24, and P27 in group A (solid red line). Panels D-F display the effect of IgGs isolated from P1, P10, and P25 in group B (solid blue line).
Effect of IgG anti-prothrombin antibodies on the binding of prothrombin to phospholipids. PC:PS (75:25 wt/wt) liposomes were immobilized onto an L1 chip, and proTWT was injected at a concentration of 0.5 μM (or 0.036 mg/mL) in the absence (solid green line) or presence (0.10-0.15 mg/mL, solid red or blue line) of IgGs. Panels A-C display the effect of IgGs isolated from P4, P24, and P27 in group A (solid red line). Panels D-F display the effect of IgGs isolated from P1, P10, and P25 in group B (solid blue line).
Discussion
Two unexpected findings distinguish this study from previous research. First, we identified critical functional differences between subpopulations of anti-prothrombin antibodies, namely IgG aPS/PT and IgG aPT-A, and established that the diversity between these populations lies in the mechanism to engage the antigen and not merely on the strength of the interaction, which, overall, is weak (Table 2). Specifically, some IgG aPS/PT require a significant structural reorganization of the antigen for proper recognition in solution, whereas IgG aPT-A target epitopes that are constitutively exposed to the solvent in both closed and open forms of prothrombin.37–39 Given that IgG aPS/PT, and not IgG aPT-A, significantly correlate with thrombosis and obstetric complications,13,14,20 these findings also raise the interesting hypothesis that excessive stabilization of the open form of prothrombin in vivo may contribute to the onset and progression of APS. Second, we discovered that IgG aPS/PT are heterogeneous, and APS patients positive for IgG aPS/PT can be classified into 2 groups (group A and group B) according to their autoantibody profile. Group A contains mostly IgG aPS/PT targeting epitope 1, or type I Abs; group B, in addition to type I Abs, also contains IgG aPS/PT recognizing epitope 2, or type II Abs (Figure 3D).
Epitope mapping experiments document that epitope 2 is located in the Gla-domain, more precisely toward the C-terminal portion. This scenario occurs because (1) type II Abs failed to react with GD-ProT and (2) by inserting into the phospholipid bilayer, the N-terminal end of the Gla-domain is not available for binding when prothrombin is bound to the membranes.47,48 Epitope 1, by contrast, is likely to be discontinuous and should be partly located in kringle-1. This explanation is the most reasonable given the inhibitory profile reported in Figure 3B. If epitope 1 were entirely confined in kringle-1, kringle-1 alone should have been a strong competitor for type I Abs in solution, but it is not. However, if kringle-1 were not an epitope for type I Abs at all, then the addition of argatroban to GD-proT should have been inconsequential but was instead necessary and sufficient to rescue the otherwise poor inhibitory potency of this fragment. Additional cryptic epitopes in the serine protease domain could, in principle, explain the effect of argatroban. However, this hypothesis is less likely given the profound inhibitory effect of fragment-1 and the very poor inhibition observed for the prethrombin-1 and prethrombin-2 fragments, which contain the whole serine protease domain.
Another significant observation emerging from our studies is that epitope 1–directed antibodies require phospholipid-induced dimerization of prothrombin for efficient antigen recognition. It follows that the enhanced reactivity of type I Abs toward the open form arises from either a spontaneous tendency of the open form to dimerize in solution, the presence of 2 identical epitopes within the same molecule that become available only in the open form, or stabilization of an alternative prothrombin conformation upon complex formation that resembles the phospholipid bound form. In solution, this conformation could be accessible to the open form, but not to the closed form, due to its enhanced molecular flexibility. Interestingly, previous studies found that fragment-1 self-associates in solution,49-51 the same amino acid sequence ENFCRNPD is found in both kringle-1 and kringle-2,52 and bovine prothrombin undergoes a conformational change upon binding to the lipids53 resulting in a conformation that may differ from the ones so far captured crystallographically.
In addition to advancing our basic understanding of the molecular mechanisms of antigen-antibody recognition in APS, we believe our findings have translational significance and broad implications for the APS field twofold. First, they may lead to the design of soluble competitors that neutralize the effect of pathogenic anti-prothrombin autoantibodies in vivo. This strategy has been successfully applied for anti-β2GPI antibodies before.5,54,55 Second, they may support the development of novel, robust, and cost-effective diagnostic tests for the identification of subpopulations of antiprothrombin antibodies in association with thrombosis. This approach is particularly important when it comes to the identification of a novel biomarker to diagnose seronegative APS patients.24,56,57
A final comment goes to the potential clinical significance of our findings. Because of their different mechanism of prothrombin binding, type I and II Abs might have different origin and pathologic effects. In this study, we found no significant correlation between the autoantibody types and sex, age, thrombotic and obstetric complications, the levels of aCL, anti-β2GPI and aPS/PT antibodies, and the strength of lupus anticoagulant. We also examined the circulating levels of prothrombin in all 24 patients. We found that the levels were not reduced, suggesting that neither antibody type is neutralizing. Although this outcome is expected for type I Abs because they cannot interact with the circulating form of prothrombin, the lack of complex formation for type II Abs is probably due to the weak micromolar affinity for prothrombin in solution. It is important, however, to note that our study has limitations and, in particular, it examined a limited number of triple-positive APS patients at high risk of thrombosis. In these patients, the presence of other and perhaps dominant pathogenic aPL such as aCL and anti-β2GPI antibodies could indeed obscure the true contribution of IgG aPS/PT.39,58 In this regard, the results reported in Figure 5 in the context of current knowledge suggest what could be some of the mechanisms for lupus anticoagulant and prothrombotic state in the presence of type I and type II autoantibodies. By massively recruiting prothrombin onto the membranes, type I Abs could lead to delayed recruitment of other clotting factors, which may slow down the initiation and propagation of the clotting cascade in vitro, similar to anti-β2GPI antibodies.59 In vivo, however, autoantibody-induced accumulation of prothrombin onto endothelial cells could accelerate thrombin generation, thus leading to a thrombotic phenotype that could be sustained by upregulation of tissue factor and E-selectin, as shown before for the human monoclonal aPT-A IS6.21,42,60 In contrast, by reducing the amount of prothrombin bound to the lipids, type II Abs may limit the conversion of prothrombin to thrombin by the prothrombinase complex. As with anti-β2GPI targeting domain V,61 type II Abs might, therefore, be protective in vivo, and the thrombotic manifestations observed in these patients could be due to compensatory effects, including the presence of other autoantibodies types or cross-reactivity of type II Abs with other plasma proteins containing a Gla-domain such as the anticoagulant protein C.62,63 Future studies using isolated type I and type II Abs will likely overcome the current limitations of these findings and may be useful to shed new light on the role of aPS/PT on the pathogenesis of APS.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Enrico Di Cera for sharing the plasmids encoding prothrombin, Gla-domainless prothrombin, and prethrombin-2 as well as for granting access to the SpectraMax i3x multimode detection platform. The authors are also grateful to Tomasz Heyduk for helpful discussions and for critically reading the manuscript. Finally, the authors acknowledge Dave Wood for the mass spectrometry analysis of the prothrombin fragments and Eliza Ruben for performing preliminary experiments.
This work was supported in part by the American Heart Association 15SDG25550094 (N.P.) and the President’s Research Fund, Saint Louis University (N.P.).
Authorship
Contribution: N.P., M.C., and W.P. performed the research; V.P. provided patients’ plasma samples and analyzed the data; N.P. and M.C. analyzed the data; N.P. designed the research and wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola Pozzi, Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, St. Louis, MO 63104; e-mail: nicola.pozzi@health.slu.edu.


![Figure 1. Prothrombin structures. Prothrombin is a multidomain protein that adopts closed (A) and open (B) states. It comprises the N-terminal Gla-domain (blue), 2 kringles (kringle 1 [K1], red; kringle 2 [K2], green), and a serine protease domain (SP, yellow). The X-ray crystal structures of the prothrombin mutant proTCC (Protein Data Bank Identifier: 6C2W,26 left panel) and proTΔ154-167(Protein Data Bank Identifier: 5EDM,34 right panel) have been solved recently. Mutation of residues S101 and A470 to cysteine in proTCC stabilizes the closed form. The location of these 2 residues is shown in cyan. Conversely, mutation of residue Y93 to alanine in the mutant proTY93A, deletion of 14 of 26 residues of Lnk2 in the mutant proTΔ154-167, or addition of argatroban stabilizes the open form. The location of these residues is shown in magenta. The Gla-domain of both structures has been slightly modified from the original structures and adopts the calcium-bound, physiologically relevant conformation.47](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/3/11/10.1182_bloodadvances.2019030932/2/m_advances030932f1.png?Expires=1767748523&Signature=maYHjyNerHMURSSFacFsuIeTcTp4~QhXROsnRvkketD0oAT-3oVvCVYQdMxqQ8-1CKadtGT0Hf7f~RHR0Wu65p-KxqSFQ5ZUIVaEImpji0G1yJkqDYSUO8YFjcUBh2g9PJd7AbDDOK1ZlkLy4Tlkpf~ZyRqDvFbGr5VOP~GJ7NEyj2Q7d~QWVGpK98GMdqrrs4i3GEx63bjIUu6TAU1XYnQ2Opp5vWSHGOAO3l3HIzmnlOYAN2Jf-~FuizUpIBcR2CRAnGLnsWj70EjsT0iuoO~5bOHmSH-i0hWy7nuzD8MFNr4eWQatFczB6~IvEx8AyCQMDzqv5KPjxs4GqFntEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Reactivity of aPS/PT toward closed and open conformations of prothrombin in solution. (A) IgG aPS/PT were researched in 27 APS triple-positive patients (P1-P27). Two healthy donors (HD1 and HD2), one negative control (– CTRL), and 1 positive control (+ CTRL) were included. The dashed red line (optical density at 450 nm [OD450nm] = 0.32 or 30 units) identifies the cutoff value. (B-D) Competition experiments. (B) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTCC, proTY93A and proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays. The effect of each competitor is reported as the percentage of inhibition calculated by using the following: 100 × ((OD1 – OD2)/OD1), where optical density 1 (OD1) and OD2 are the values of absorbance in the absence and presence of competitor, respectively. (C) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). Data were analyzed with a simple binding equation, S = S0 + (S∞ I/IC50)/(1 + I/IC50), and the values of IC50 are reported in Table 2. (D) Plasma samples (50 μL, 1:50 vol/vol) from 24 APS patients positive for aPS/PT were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT in the absence or presence of 200 μM argatroban (Arg) for 30 minutes at room temperature. The residual levels of aPS/PT were quantified by using ELISA assays as described before. (E-F) Reactivity of aPT-A toward closed and open conformations of prothrombin in solution. (E) Diluted plasma samples (50 μL, 1:50 vol/vol) from 13 APS patients positive for aPT-A were incubated side-by-side with a solution (50 μL, 0.72 mg/mL, 10 μM) of proTWT, proTCC, proTY93A, or proTΔ154-167 for 30 minutes at room temperature. The residual levels of aPT-A were calculated as described above. (F) Dose-dependent effect of proTCC (blue circles) and proTY93A (red circles) (0-15 μM) as shown for a representative patient (P7). IC50 values are reported in Table 2. (G) Subpopulations of aPS/PT. Two-dimensional representation of the inhibitory effect determined in Figure 2B for proTCC (closed, x-axis) and proTY93A (open, y-axis) in the 24 APS patients positive for aPS/PT. Dashed black lines divide the plot into 4 quadrants. Group A patients (red box) are mostly located in the upper left quadrant, with a few outliers. Group B patients falls into the upper right quadrant. No aPS/PT were detected in the lower right quadrant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/3/11/10.1182_bloodadvances.2019030932/2/m_advances030932f2.png?Expires=1767748523&Signature=Nn9Sa-4r0ensm7tJu-4Y7hzpLhtfyywoEsZy7pILjrQ5-1IPtwamkH-oGgt60ci45A7xAX5i7ZhFP54QFdq3sb78JGGJNVeJOYA3xJqAQmNT7uL1P-Ve3psh2FNaXfOb60dLyvmpuZpSW6B~jJzd1B5siobnRgPj1-qazBUGPggLlmHTiLymzSofCT2Nr~qQiY1~KkS3dtIean0-5B93TZW~faJ-~T9FwQ2wmkn-EFpC3bIc9UTjqly6s6Zpf2FQDeHaOIwICITX-mYu6XwHWN6GVzSM38ZQY-Oyq~wEZp4tmV4mIcipx3mIMZ7WUjGuDgx1jzVn6FGgJBzZ5WuJfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)