Key Points
Serum-enhanced labile plasma iron in patients undergoing allogeneic HSCT is critical for Aspergillus fumigatus growth in vitro.
Transferrin iron in serum is inaccessible for A fumigatus, and uptake of iron in the form of eLPI involves fungal siderophores.
Introduction
Apart from relapse and graft-versus-host disease, prevention of life-threatening infections remains the most important challenge in clinical care of patients undergoing hematopoietic stem cell transplantation (HSCT). Despite improved antifungal prophylaxis regimens, invasive aspergillosis (IA) still poses a major threat, with mortality rates up to 80% among infected HSCT patients.1 Thus, novel biomarkers to identify patients at risk and a better understanding of underlying pathomechanisms are needed.
In healthy individuals, plasma iron accessible for cells is bound to transferrin (Tf). However, under pathologic conditions, once Tf’s binding capacity is exceeded, enhanced labile plasma iron (eLPI) develops.2 Indeed, iron-overloaded patients with myelodysplastic syndrome or acute myeloid leukemia undergoing HSCT are at risk of developing eLPI as a result of chemotherapy-induced shut-down of erythropoiesis and tissue damage causing iron release from apoptotic cells, as well as frequent red blood cell transfusion therapy for anemia.3-6 Recently, the Allogeneic Iron Investigators (ALLIVE) trial demonstrated a causative link between the occurrence of eLPI and an increased incidence of infection-associated nonrelapse mortality in patients undergoing allogeneic HSCT.3 In particular, eLPI’s promoting role for the dissemination of selected bacteria has been determined.7,8 Referring to IA, studies have proposed iron overload as a risk factor for the occurrence of IA after transplantation.9,10 Furthermore, iron is known to be essential for Aspergillus fumigatus (AFU) growth and virulence.11 However, the relevance of eLPI for AFU as an iron source is unknown. Here we specifically investigated the role of eLPI for AFU outgrowth, using serum samples of patients with acute myeloid leukemia and myelodysplastic syndrome who underwent HSCT within the ALLIVE trial.
Methods
Study design
Serum samples of a selected cohort of patients who participated in the ALLIVE trial have been analyzed. All patients gave written informed consent to participate in the trial, which was approved by the ethics committee of the Technical University Dresden (registration number EK338102012). This trial was registered at www.clinicaltrials.gov as #NCT01746147.
Fungal in vitro assay and procedures
The scheme of fungal growth assay is shown in supplemental Figure 1. All further details are given in the supplemental Data.
Statistics
Statistical analysis was performed using R. Data were analyzed using generalized linear models with a logit link function. P < .05 is considered significant. All further details are given in the supplemental Data.
Results and discussion
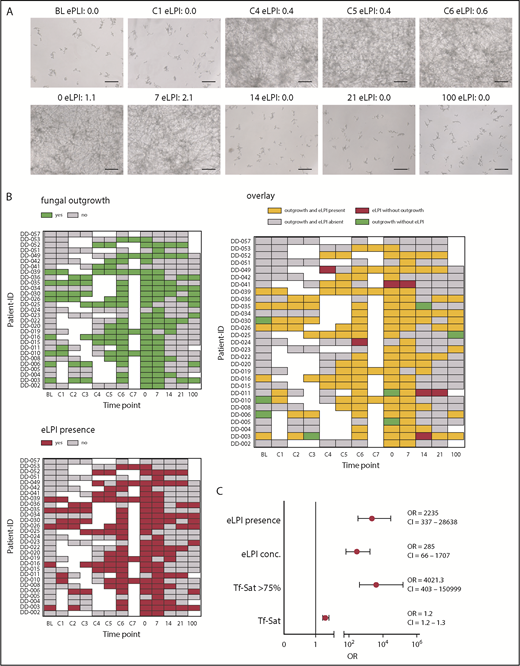
To study the relationship between AFU infection and iron parameters in HSCT patients, we developed an in vitro assay to investigate the development of fungal outgrowth in sera after inoculation with AFU (supplemental Figure 1A-B). Longitudinally collected serum samples during the consecutive phases of HSCT of 29 representative ALLIVE participants were screened (details given in supplemental Table 2).3 Of importance, eLPI development of our cohort resembled the one seen in the whole ALLIVE cohort: Mean eLPI concentration and percentage of patients showing measurable eLPI levels increased during conditioning, being highest on day 7 (C7; 1.1 ± 0.7; 100%), at transplantation (0; 0.9 ± 0.8; 75.9%), and 7 days after transplantation (7; 0.8 ± 0.6; 79.3%; supplemental Figure 2A-B).
Strikingly, we found that AFU outgrowth was almost exclusively observed when eLPI was present (Figures 1A-B). Therefore, the probability of fungal outgrowth was dependent on the presence of eLPI per se (defined as eLPI ≥ 0.2 arbitrary units; odds ratio [OR], 2235; 95% confidence interval [CI], [337-28 638; P = 4×10−12), rather than on the absolute eLPI amount (OR, 285; 95% CI, 66-1707; P = 7×10−12; Figure 1C). Importantly, when considering other clinical confounders including sex, disease type, liver iron content, and transfusion burden, eLPI positivity remained a highly significant predictor for fungal outgrowth (OR, 778; 95% CI, 197-4676; P = 6.2×10−17; supplemental Figure 2C).
Presence of enhanced labile plasma iron in sera of patients undergoing allogeneic hematopoietic stem cell transplantation determines AFU outgrowth. Human serum samples of 29 representative ALLIVE participants, which were collected during the consecutive phases of allogeneic hematopoietic stem cell transplantation (HSCT) were screened for AFU outgrowth. AFU spores (5 × 104/mL) were seeded in RPMI containing 10% complement-depleted patient serum. Outgrowth was determined after 48 hours by microscopic examination. (A) Microscopy images showing fungal cultures of a representative patient (DD-15) with eLPI serum concentrations as indicated (original magnification ×20; bars represent 30 µm). (B) Heat map representation of fungal outgrowth (upper left), eLPI presence (lower), and their overlay (right) of patients analyzed here at all times (green, fungal outgrowth; red, eLPI present; yellow, both fungal outgrowth and eLPI present; gray, fungal growth and eLPI absent; white, sample not available). (C) Relative probability of AFU outgrowth in sera of patients depending on iron-related parameters (eLPI concentration, eLPI presence, Tf-Sat, and Tf-Sat > 75%. The graph depicts ORs ± 95% CIs for probability of fungal outgrowth. Statistical significance was assessed using a multiparameter logistic regression model for the outgrowth response variable (no fungal growth vs fungal outgrowth). 0, day of transplantation; 7-100, days 7-100 after transplantation; BL, baseline; C1-7, conditioning days 1-7.
Presence of enhanced labile plasma iron in sera of patients undergoing allogeneic hematopoietic stem cell transplantation determines AFU outgrowth. Human serum samples of 29 representative ALLIVE participants, which were collected during the consecutive phases of allogeneic hematopoietic stem cell transplantation (HSCT) were screened for AFU outgrowth. AFU spores (5 × 104/mL) were seeded in RPMI containing 10% complement-depleted patient serum. Outgrowth was determined after 48 hours by microscopic examination. (A) Microscopy images showing fungal cultures of a representative patient (DD-15) with eLPI serum concentrations as indicated (original magnification ×20; bars represent 30 µm). (B) Heat map representation of fungal outgrowth (upper left), eLPI presence (lower), and their overlay (right) of patients analyzed here at all times (green, fungal outgrowth; red, eLPI present; yellow, both fungal outgrowth and eLPI present; gray, fungal growth and eLPI absent; white, sample not available). (C) Relative probability of AFU outgrowth in sera of patients depending on iron-related parameters (eLPI concentration, eLPI presence, Tf-Sat, and Tf-Sat > 75%. The graph depicts ORs ± 95% CIs for probability of fungal outgrowth. Statistical significance was assessed using a multiparameter logistic regression model for the outgrowth response variable (no fungal growth vs fungal outgrowth). 0, day of transplantation; 7-100, days 7-100 after transplantation; BL, baseline; C1-7, conditioning days 1-7.
As mentioned earlier, Tf-saturation (Tf-Sat) and eLPI are mutually interconnected parameters. In our cohort, eLPI and fungal outgrowth were clearly associated with Tf-Sat exceeding 75% (supplemental Figure 2D). Comparing receiver-operator curves, eLPI with an optimal threshold of 0.2 arbitrary units and Tf-Sat with an optimal cutoff of 75% revealed excellent sensitivity and specificity for prediction of in vitro fungal outgrowth (area under the curve [AUC]eLPI, 0.94; AUCTf-Sat, 0.96; supplemental Figure 2E). Overall, Tf-Sat only marginally influenced outgrowth probability (OR, 1.2; 95% CI, 1.2-1.3; P = 2 × 10−10). However, Tf-Sat higher than 75% dramatically increased outgrowth risk (OR, 4021; 95% CI, 403-150 999, P = 8 × 10−9; Figure 1C).
To see whether AFU has evolved mechanisms to overcome the growth dependence related to eLPI, we analyzed 9 different AFU isolates in ferric iron-spiked human plasma samples (supplemental Figure 3A-C). Outgrowth again only occurred in the presence of eLPI, thus making it very likely that eLPI serves as the relevant iron source for AFU in general (supplemental Figure 3D).
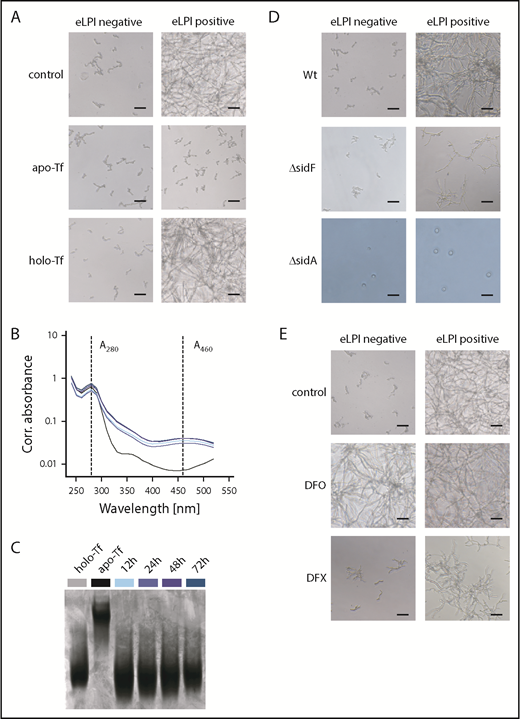
Published evidence indicates that AFU can acquire iron from holo-Tf via its siderophore system, being the most important iron acquisition pathway for AFU.11,12 According to our results, only eLPI or highly saturated Tf are able to support outgrowth. To clarify, which is more critical, we scavenged labile iron in eLPI-positive samples on addition of apo-Tf, which abrogated fungal outgrowth. Correspondingly, the addition of holo-Tf to eLPI-negative specimens could not promote AFU outgrowth (Figure 2A). In agreement, monitoring of apo- and holo-Tf in culture supernatants via absorbance measurements at 280 and 460 nm and urea–polyacrylamide gel electrophoresis did not illustrate conversion of holo- to apo-Tf, suggesting that holo-Tf is not a natural iron source for AFU (Figure 2B-C).13 Interestingly, it has recently been published that serum levels of N,N′,N″-triacetylfusarinine C, being AFU’s main extracellular siderophore, in patients suffering from IA are less than 10 ng/mL. Thus, it seems probable that N,N′,N″-triacetylfusarinine C concentrations are too low to efficiently retrieve iron from Tf.12,14
AFU cannot retrieve iron from holo-transferrin, and enhanced labile plasma iron uptake depends on fungal siderophores. (A) Role of holo-Tf as an iron source for AFU growth. Representative microscopy images of AFU cultures taken 48 hours after inoculation (5 × 104 spores/mL) of RPMI containing 10% ferric iron-spiked human plasma (0-50 µM Fe3+) supplemented with 2,5 mg/mL apo-Tf or holo-Tf, respectively (original magnification ×20; bars represent 30 µm). (B-C) Removal of iron from holo-Tf by AFU was studied. AFU spores (5 × 104/mL) were inoculated into RPMI containing 5µM Fe3+ and 2 mg/mL apo- or holo-Tf in 6-well plates. Holo-Tf:apo-Tf conversion in culture supernatant was monitored by absorbance measurements at 280 nm (A280corr) and 460 nm (A460corr) (B) and urea–polyacrylamide gel electrophoresis (C) at indicated times. Uninoculated RPMI media with apo- and holo-Tf were used as controls in both assays. (D) eLPI use by AFU mutant strains with defects in iron acquisition systems. Representative microscopy images showing the growth pattern of wild-type (Wt), ΔsidF, and ΔsidA conidia in RPMI containing 10% ferric iron-spiked human plasma after 48-hour culture (original magnification ×20; bars represent 30 µm). (E) Influence of clinically applicable iron chelators on AFU outgrowth in eLPI-deficient and eLPI-positive serum cultures. AFU spores (5 × 104/mL) were cultured in RPMI containing 10% ferric iron-spiked human plasma supplemented with 100 µM deferoxamine (DFO) or 200 µM deferasirox (DFX). Control cultures were DMSO treated. Photographs were taken 48 hours after inoculation. Representative images of fungal cultures are shown (original magnification ×20; bars represent 30 µm). Experiments shown in panels A, D, and E were performed at least in 6 replicates, all showing consistent results.
AFU cannot retrieve iron from holo-transferrin, and enhanced labile plasma iron uptake depends on fungal siderophores. (A) Role of holo-Tf as an iron source for AFU growth. Representative microscopy images of AFU cultures taken 48 hours after inoculation (5 × 104 spores/mL) of RPMI containing 10% ferric iron-spiked human plasma (0-50 µM Fe3+) supplemented with 2,5 mg/mL apo-Tf or holo-Tf, respectively (original magnification ×20; bars represent 30 µm). (B-C) Removal of iron from holo-Tf by AFU was studied. AFU spores (5 × 104/mL) were inoculated into RPMI containing 5µM Fe3+ and 2 mg/mL apo- or holo-Tf in 6-well plates. Holo-Tf:apo-Tf conversion in culture supernatant was monitored by absorbance measurements at 280 nm (A280corr) and 460 nm (A460corr) (B) and urea–polyacrylamide gel electrophoresis (C) at indicated times. Uninoculated RPMI media with apo- and holo-Tf were used as controls in both assays. (D) eLPI use by AFU mutant strains with defects in iron acquisition systems. Representative microscopy images showing the growth pattern of wild-type (Wt), ΔsidF, and ΔsidA conidia in RPMI containing 10% ferric iron-spiked human plasma after 48-hour culture (original magnification ×20; bars represent 30 µm). (E) Influence of clinically applicable iron chelators on AFU outgrowth in eLPI-deficient and eLPI-positive serum cultures. AFU spores (5 × 104/mL) were cultured in RPMI containing 10% ferric iron-spiked human plasma supplemented with 100 µM deferoxamine (DFO) or 200 µM deferasirox (DFX). Control cultures were DMSO treated. Photographs were taken 48 hours after inoculation. Representative images of fungal cultures are shown (original magnification ×20; bars represent 30 µm). Experiments shown in panels A, D, and E were performed at least in 6 replicates, all showing consistent results.
Next, we tried to elucidate the mechanism by which AFU accesses eLPI. Notably, deficiency in extracellular siderophores (ΔsidF mutant) led to an impaired outgrowth in the presence of eLPI, whereas lack of extra- and intracellular siderophores (ΔsidA mutant) blocked even spore germination, independent of the presence of eLPI (Figure 2D). Together, these results indicate that iron in the form of eLPI can promote AFU outgrowth without extracellular siderophores, albeit at a considerably slower rate, unless the intracellular iron stores (represented by intracellular siderophores) are depleted. These results are in accordance with ΔsidF displaying attenuated virulence and ΔsidA being avirulent in murine aspergillosis models.11,15
Considering that IA still represents a major hindrance to a favorable outcome in patients undergoing HSCT, and wide use of antifungal regimes elicit resistant strains, new therapeutic targets are warranted. Although apo-Tf has already been effectively tested in conditions with eLPI presence, its medical use is limited.16 Moreover, eLPI can be targeted by iron chelators. Yet, application of these drugs in the setting of HSCT is still controversial.17-19 In agreement with previous reports, deferoxamine served as xenosiderophore for AFU, thus promoting fungal outgrowth even in samples, which were negative for eLPI.20 In contrast, outgrowth was absent in cultures containing deferasirox, proposing that iron chelated by deferasirox does not serve as a xenosiderophore, at least for AFU (Figure 2E). Deferasirox application has previously been shown to have an activity against AFU in vitro and in vivo models.18,21 Notably, application of deferasirox for mucormycosis in mice and humans yielded inconsistent outcomes, so that further work up is warranted and data must be interpreted with caution.22 Moreover, pharmacologic increase of hepcidin levels, being the master regulator of systemic iron homeostasis, may also turn out to be a possible treatment option for the prevention of eLPI.23 Preclinical studies have shown the potential of minihepcidin therapy in reversing bacterial infections.7
In summary, our study provides evidence on the putative role of eLPI for promoting IA, being the most common invasive fungal infection in patients undergoing allogeneic HSCT. To further validate the value of eLPI as a biomarker for IA, eLPI measurements in patients undergoing HSCT suffering from IA vs noninfected patients are warranted, maybe including quantitative measurements instead of semiquantitative, as used in this study and in the ALLIVE trail. Our studies recommend eLPI measurement as a predictor for the risk for IA and will potentially pave the way for therapeutic interventional trials aiming at scavenging eLPI, and thereby improving the outcome of HSCT patients at risk.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the doctoral college project W 1253 HOROS (V.P., G.W., and H.H.), the Austrian Research Funds project P 28302 (I.T.), the “Verein zur Förderung von Forschung und Weiterbildung in Infektiologie und Immunologie, Innsbruck” (G.W.), the Christian Doppler Society, Austria (G.W.), and Krebshilfe Tirol project 15024 (P.T.).
Authorship
Contribution: V.P., M.W., P.T., H.H., U.P., and I.T. conceived the project, designed experiments, and analyzed and interpreted data; V.P., P.T., H.H., and I.T. wrote the manuscript; V.P., P.T., M.S., R.O., S.B., and L.L. performed experiments; G.W. provided intellectual input and edited the manuscript; D.O.-H. provided A fumigatus wild-type isolates and intellectual input; M.W., D.W., and U.P., designed and supervised the ALLIVE trial, provided patients’ material and details, and provided intellectual input; and all authors read and corrected the manuscript.
Conflict-of-interest disclosure: M.W. and U.P. received research funding and honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Igor Theurl, Department of Internal Medicine II, Medical University of Innsbruck, Anichstr 35, A-6020 Innsbruck, Austria; e-mail: igor.theurl@i-med.ac.at; and Hubertus Haas, Division of Molecular Biology-Biocenter, Medical University of Innsbruck, Innrain 80, 6020 Innsbruck, Austria; e-mail: hubertus.haas@i-med.ac.at.
References
Author notes
U.P. and I.T. contributed equally to this study.