Background
Sickle cell anemia neurologic complications
The collaboration aims at exploring the relationship between the cerebral vasculature and neurologic complications in Tanzanian children with sickle cell anemia (SCA). Cerebral vasculopathy in internal carotid, middle, anterior cerebral, and posterior cerebral arteries is diagnosed by transcranial Doppler (TCD) utrasonography1 and magnetic resonance angiography (MRA). The neurologic complications include clinical stroke (ischemic and hemorrhagic) and silent brain infarction (SCI).2,3 Although clinical stroke (overt cerebral infarction) is a catastrophic complication in SCA, it is relatively rare. However SCI, diagnosed by magnetic resonance imaging (MRI), is a very common neurologic injury in these patients, with approximately 50% affected by middle age in the United States. SCI is an independent predictor of stroke and is associated with neurocognitive impairment.4
Three-quarters of the SCA disease burden is in Africa, and Tanzania is ranked fourth globally.5 We have been screening for stroke risk using TCD ultrasonography at Muhimbili hospital since 2004. In a study of 200 patients undergoing TCD ultrasonography, of whom 60 with TCD abnormalities had MRI scans, the prevalence of stroke was 11.5% and the presence of SCI was 37% (verbal communication). However, the prevalence of SCI and other brain abnormalities6-8 in unselected African patients with SCA is unknown.
Despite the high prevalence of SCA with neurologic complications, there is a lack of expertise in early detection and diagnosis using neuroimaging. We have therefore investigated the use of MRI as well as TCD ultrasonography.
Collaboration and capacity building
Collaboration between Muhimbili University of Health and Allied Sciences (MUHAS) and University College London (UCL) on SCA research has been ongoing since 2004. We have focussed on the following:
Neurologic complications
MRI and MRA of the brain at 1.5T
Advanced neuroimaging, including diffusion tensor imaging for white matter disease and gradient-echo and susceptibility-weighted imaging for brain iron
Experts from UCL have been working with medical personnel and scientists in Tanzania to reduce morbidity and mortality as a result of neurologic complications by training Tanzanians to perform the following tasks:
Record neurologic history and perform thorough examination.
Use TCD ultrasonography to detect risk of stroke (Fenella Kirkham; United Kingdom) (Figure 1).
Interpret conventional brain MRI scans and magnetic resonance angiography (MRA) images in SCD (Dawn Saunders, United Kingdom/United States [Figure 2C]) (Figures 3 and 4).
Advanced neuroimaging sequences were installed in 2011 into the Muhimbili National Hospital MRI machine (Angela Darekar, India/United Kingdom; Christopher Clark, United Kingdom [Figure 2A]).
Analyze diffusion imaging data (Jamie Kawadler, United States/United Kingdom [Figure 2D]; Winok Lapidaire, The Netherlands/United Kingdom [Figure 2B]; and Christopher Clark, United Kingdom [Figure 2A]).
Perform neurocognitive testing (Hanne Stotesbury, Norway/United Kingdom; Melanie Koelbel, Germany/United Kingdom; Jamie Kawadler, Winok Lapidaire, and Fenella Kirkham, United Kingdom).
Train health care providers on brain volume measurement techniques (Jamie Kawadler, Winok Lapidaire, and Christopher Clark, United Kingdom).
Train health care providers on brain iron analysis techniques (Russell Murdoch and Karin Shmueli, United Kingdom) (Figure 5).
(A) TCD training by Fenella Kirkham, MD. (B) Healthy African children on their way to the clinic.
(A) TCD training by Fenella Kirkham, MD. (B) Healthy African children on their way to the clinic.
(A-E) Photos of neuroimaging capacity team during MUHAS-Tanzania and UK-UCL visits.
(A-E) Photos of neuroimaging capacity team during MUHAS-Tanzania and UK-UCL visits.
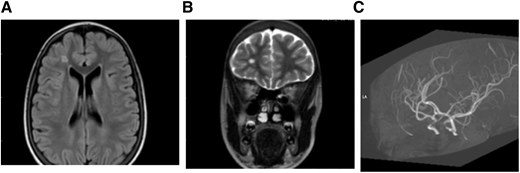
MRI (A) FLAIR (axial) and (B) T2W (coronal). Brain images from a 15-year-old female with SCA without neurologic complications according to history from parents and child. SCI seen in 2 planes measuring .3 mm in right frontal lobe deep white matter. (C) MRA: right and left cavernous ICA stenosis.
MRI (A) FLAIR (axial) and (B) T2W (coronal). Brain images from a 15-year-old female with SCA without neurologic complications according to history from parents and child. SCI seen in 2 planes measuring .3 mm in right frontal lobe deep white matter. (C) MRA: right and left cavernous ICA stenosis.
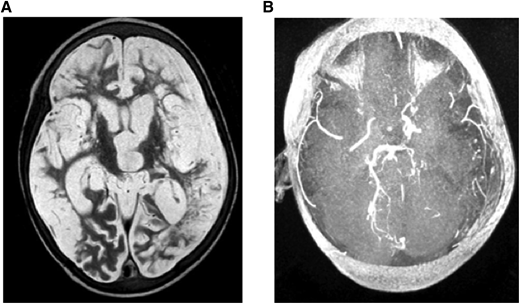
(A) MRI: T2W (axial) and (B) MRA. Brain images from a 7-year-old quadriplegic boy showing (A) global cerebral infarction and (B) occlusion of both terminal internal carotid arteries and small left posterior cerebral artery but no moyamoya collaterals.
(A) MRI: T2W (axial) and (B) MRA. Brain images from a 7-year-old quadriplegic boy showing (A) global cerebral infarction and (B) occlusion of both terminal internal carotid arteries and small left posterior cerebral artery but no moyamoya collaterals.
(A) Susceptibility mapping on MRI for brain iron measurement, (B) brain iron measurements techniques team, and (C) an imaging machine and the head of the MUHAS Radiology Department.
(A) Susceptibility mapping on MRI for brain iron measurement, (B) brain iron measurements techniques team, and (C) an imaging machine and the head of the MUHAS Radiology Department.
Capacity building
Interpretation of brain abnormalities.
Measurement of brain iron.
In the deep iron-rich regions of the brain, magnetic susceptibility values are proportional to tissue iron content.7 Susceptibility mapping can be used to noninvasively measure tissue iron concentration.
MUHAS-Tanzania and UCL visits for neuroimaging.
Mboka Jacob (Tanzania) visited UCL for 5 weeks beginning in January 2017 and 3 weeks in July 2018. She now has a Commonwealth scholarship to complete 1 year of study at UCL.
Jamie Kawadler, Dawn Saunders, Winok Lapidaire, and Christopher Clark (United Kingdom) and Angela Darekar (India and United Kingdom) have visited Tanzania.
Fenella Kirkham (United Kingdom) has visited Tanzania many times.
Conclusions
Neuroimaging capacity building in Africa may reduce morbidity and mortality that results from neurologic complications in SCA. Along with other Tanzanian scientists, we will be able to explore the effects of genetic and environmental exposures, including anemia and nutrition.
Acknowledgments
This study is partly supported by Swedish International Development Cooperation Agency small grant MUHAS and Fenella Kirkham.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mboka Jacob, Department of Radiology and Imaging, Muhimbili University of Health and Allied Sciences, Tanania; e-mail: mbokajacob@gmail.com.