Background
Sickle cell disease (SCD) accounts for up to 15% of mortality in children younger than age 5 years in developing countries that have a high prevalence of SCD. Diagnosis of SCD can improve survival, but high costs and complex operational requirements of standard genetic or protein separation diagnostic methods preclude their wide application in low-resource settings. In addition to their technical limitations, current screening methods do not provide point-of-care diagnoses necessary to capture patients who live in the vast rural hinterlands and who come in contact with health facilities only sporadically. Another challenge of diagnosing SCD is that populations in developing countries in which SCD is prevalent are not aware of it.
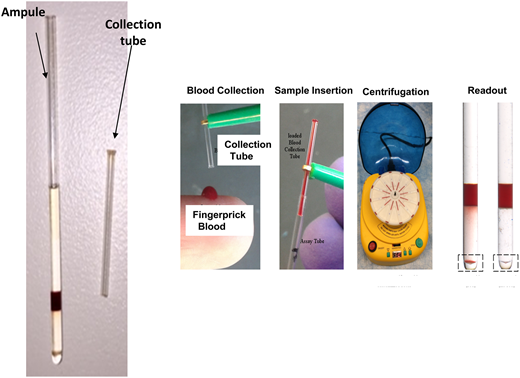
We have developed a simple, low-cost and innovative test to screen for SCD using aqueous multiphase systems (AMPSs) that form immiscible self-assembling step-gradients. An AMPS designed to target the density signature of sickle cell anemia forms the basis of this novel multi-phase analyzer (named MPANA) diagnostic technique. MPANA consists of an AMPS-filled capillary tube. A drop of blood obtained from an individual by finger prick and introduced into the tube is subjected to centrifugation for 15 minutes in a small rotor powered by an automobile battery. The centrifugal force causes blood cells to separate according to their density. Because deformed SCD red blood cells are very dense, after centrifugation they form a red button visible to the naked eye at the bottom of the tube. The estimated cost per test is 50 cents.1
To address the challenges of large-scale SCD screening in rural settings, we exploited a partnership between the nonprofit Options for Children in Zambia and the National Zambian Dental Training School. Over 14 years, Options for Children in Zambia has demonstrated that providing free toothbrushes, toothpaste, and other minimal health services is a powerful way to attract residents of rural regions in Zambia who have high rates of tooth decay to health care centers. Once collected, these individuals can receive other health care interventions.
Objectives
Three objectives in rural Zambia
Field test a novel device for point-of care screening for SCD.
Field test a public health strategy that combines dental and SCD screening.
Provide health education on dental care and on recognition and detection of SCD.
Hypotheses
A simple screening device operable in settings with few amenities can provide point-of-care diagnosis of SCD in rural areas.
Community awareness of the availability of rudimentary dental prevention services can attract large numbers of individuals for SCD screening.
Methods
Study design
We conducted a cross-sectional study in August and September of 2017 in 2 rural districts of northern Zambia. A local drama group and radio and mobile broadcasts informed the target communities about the dental intervention. The study team provided dental care and SCD education and recruited participants for MPANA testing and collection of a dried blood sample.
Laboratory diagnosis
Dried blood samples were analyzed by high-performance liquid chromatography at the Massachusetts State Screening Laboratory. MPANA tests performed on site were read by eye by a trained reader.
Results
Over a period of 2 weeks in August-September 2017, the team provided dental prevention for 700 individuals and SCD screening for 503 children age 3 months to 18 years. Of the screened individuals, analysis of the dried blood samples revealed that 78 had sickle cell trait and 17 had SCD, a prevalence of 15.5% and 3.4%, respectively. The combined clinical histories and MPANA results had a sensitivity of 70.6% (95% confidence interval [CI], 47%-88%), a specificity of 99.2% (95% CI, 98%-100%), and an overall diagnostic accuracy of 99%.
Conclusion
A multidisciplinary consortium has achieved the first validation of a low-cost simple point-of-care test to diagnose SCD in a rural area of a developing nation and has demonstrated the ability of dental services to facilitate such screening. Scaling up of this approach has great potential for capacity building.
Authorship
Conflict-of-interest disclosure: A.A.K., T.P.S., and Harvard University jointly hold patents related to the technology. T.H. is employed by Nano Terra, Inc. The remaining authors declare no competing financial interests.
Correspondence: Catherine Chunda-Liyoka, University Teaching Hospitals –Children's Hospital, Lusaka, Zambia; e-mail: catherinechunda@yahoo.co.uk.