Key Points
The risk of recurrence in patients with VTE provoked by minor transient or persistent risk factors is uncertain.
The risk of recurrence with VTE provoked by minor transient or persistent risk factors is similar to that with unprovoked VTE.
Abstract
The optimal duration of anticoagulation for venous thromboembolism (VTE) is uncertain. In this prespecified analysis, we used data from 2 randomized trials, which compared once-daily rivaroxaban (20 mg or 10 mg) with aspirin (100 mg) or placebo for extended VTE treatment to estimate the risk of recurrence according to baseline risk factor profiles. Index VTE events were centrally classified as unprovoked, or provoked by major transient or persistent, or minor transient or persistent risk factors, and rates of recurrence at 1 year were calculated. A total of 2832 patients received rivaroxaban; 1131 received aspirin, and 590 received placebo. With unprovoked VTE, rates of recurrence in the 1173 patients given rivaroxaban, the 468 given aspirin, and the 243 given placebo were 2.0%, 5.9%, and 10.0%, respectively. There were no recurrences in patients with VTE provoked by major transient risk factors. With VTE provoked by minor persistent risk factors, recurrence rates in the 1184 patients given rivaroxaban, the 466 given aspirin, and the 248 given placebo were 2.4%, 4.5%, and 10.7%, respectively. For patients with minor transient risk factors, recurrence rates were 0.4% in the 268 patients given rivaroxaban, 4.2% in the 121 given aspirin, and 7.1% in the 56 given placebo. Recurrence rates in patients with VTE provoked by minor persistent or minor transient risk factors were not significantly lower than that with unprovoked VTE (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.56-1.16; and HR, 0.68; 95% CI, 0.32-1.30, respectively). Therefore, such patients may also benefit from extended anticoagulation therapy.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cause of death worldwide.1-2 Anticoagulation therapy is the mainstay of treatment of VTE, but the optimal duration of such treatment is uncertain.3-6 Current guidelines recommend that the decision to extend treatment beyond 3 months be based on the balance between the risk of recurrence if treatment is stopped and the risk of bleeding with continued treatment.6-7 Patient preference also needs to be considered.
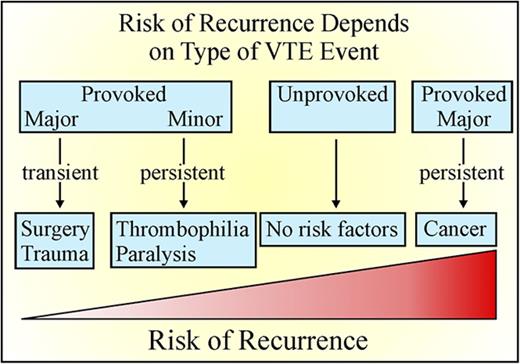
A 3- to 6-month course of anticoagulation therapy is adequate for VTE provoked by major transient risk factors such as surgery or trauma,6-7 whereas extended anticoagulation is suggested for patients with VTE that is related to cancer or unprovoked unless the risk of bleeding is high.6-7 VTE is considered to be unprovoked if it occurred in the absence of recent surgery or trauma, active cancer, or with pregnancy or estrogen use.6-7 The term unprovoked VTE is preferred over idiopathic VTE because of the recognition that nonenvironmental risk factors, such as older age8 or male sex,9 influence the risk of recurrence. Furthermore, the definition of provoked VTE has evolved with identification of additional risk factors, including prolonged travel,10 inflammatory bowel disease,11-13 lower extremity paralysis or paresis,14 congestive heart failure,15 body mass index >30 kg/m2,16 and renal impairment.17 Therefore, it is now recommended that provoked VTE be further classified depending on whether risk factors are major or minor and persistent or transient.6-7 For this classification to be useful, however, more information is needed on the risk of recurrence if anticoagulation is stopped in these categories of patients. To address this, we estimated the 1-year cumulative risk of recurrent VTE according to individual patient baseline risk factor profiles using data from the Once-daily Oral Rivaroxaban versus Placebo in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN-EXTENSION, www.clinicaltrials.gov #NCT00439725) and Reduced-dose Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism (EINSTEIN-CHOICE, www.clinicaltrials.gov #NCT02064439) trials,18-19 which compared once daily rivaroxaban (20 mg or 10 mg) with placebo or aspirin (100 mg) for extended treatment in patients with VTE who had completed 6 to 12 months of anticoagulation and with equipoise regarding the need for continued anticoagulation treatment. This prespecified analysis was possible because the same blinded committee was used in both studies to classify the index VTE as provoked by major or minor transient or persistent risk factors and to adjudicate outcome events.
Methods
Study design and oversight
EINSTEIN EXTENSION and EINSTEIN CHOICE were randomized, double-blind studies comparing the efficacy and safety of once daily rivaroxaban (20 mg) with placebo and once daily rivaroxaban (20 mg or 10 mg) with aspirin (100 mg), respectively,18-19 for extended treatment of VTE. Both trials were sponsored by Bayer Pharmaceuticals. Protocols were approved by the institutional review board at each participating center and have been published.18-19 Written informed consent was obtained from all patients. The sponsor collected and maintained the data; the academic authors had access to the data at all times through the sponsor. The same independent committee, whose members were unaware of the study group assignment, adjudicated the qualifying initial diagnosis (DVT or PE) and all suspected outcomes in both studies. Using information recorded on the case report forms, this committee also classified the index VTE as provoked by (a) major persistent risk factors (active cancer excluding basal cell or squamous cell skin cancer); (b) minor persistent risk factors (inflammatory bowel disease, lower extremity paralysis or paresis, congestive heart failure, body mass index >30 kg/m2, calculated creatinine clearance <50 mL per minute, family history of VTE, or known thrombophilia, including deficiency of antithrombin, protein C, or protein S, factor V Leiden or prothrombin gene mutation, and antiphospholipid syndrome); (c) minor transient risk factors (immobilization, travel >8 hours, pregnancy, puerperium, or use of estrogen, or lower limb trauma with transient impairment of mobility); or (d) major transient risk factors (major surgery or trauma, or cesarean section). The details of this classification are included in the supplemental Appendix. Patients without any of these risk factors were classified as having unprovoked VTE. For all patients, a prior history of VTE was also recorded. Independent data monitoring committees periodically reviewed the study outcomes.
Patients
The study details have been reported.18-20 Briefly, patients were eligible for inclusion in the study if they were 18 years of age or older and if they had objectively confirmed, symptomatic proximal DVT or PE, had been treated for 6 to 12 months with an anticoagulant, and had not interrupted therapy for >7 days prior to randomization.
Patients were ineligible if they had a contraindication to continued anticoagulant therapy or if they required extended anticoagulant therapy at therapeutic dosages. For EINSTEIN CHOICE, patients requiring antiplatelet therapy also were ineligible. Additional ineligibility criteria included a calculated creatinine clearance <30 mL per minute or hepatic disease associated with a coagulopathy. A full list of inclusion and exclusion criteria of both studies is provided in the supplemental Appendix.17,19-20
Randomization
Randomization for both studies was performed with the use of an interactive voice-response system. For EINSTEIN EXTENSION, patients were assigned in a 1:1 ratio to receive 20 mg of rivaroxaban or placebo once daily with food for 6 or 12 months at the discretion of the local investigator. For EINSTEIN CHOICE, patients were assigned, in a 1:1:1 ratio, to receive 20 mg of rivaroxaban, 10 mg of rivaroxaban, or 100 mg of aspirin, all given once daily with food. The intended duration of administration of study drug was 12 months, but patients randomized after the requisite number of primary efficacy outcomes was reached were treated for at least 6 months. Rivaroxaban (20 mg and 10 mg) and matching placebo were provided as identical-appearing, immediate-release film-coated tablets, whereas aspirin and matching placebo were provided as enteric-coated tablets.
Outcome measures
The primary efficacy and safety outcomes were identical for both studies. The primary efficacy outcome was the composite of symptomatic recurrent VTE, VTE-related death, or unexplained death for which PE could not be excluded. Recurrent VTE included fatal and nonfatal PE and DVT, and the principal safety outcome was major bleeding (see supplemental Appendix for definitions).17,19-20
Statistical analysis
The incidence of recurrent VTE and major bleeding in the 2 rivaroxaban 20 mg groups and the rivaroxaban 10 mg group were similar. Therefore, the groups were combined for comparison of the incidence of recurrent VTE and major bleeding with those in the aspirin and placebo groups.
No formal sample size calculation was performed. However, with 41.4% of the patients classified as having unprovoked VTE, we estimated that a 50% decrease in the risk of recurrent VTE could be detected with a 2-sided type I error of 0.05 and a type II error of 0.10.
The analysis included randomized patients who took at least 1 dose of study medication. Recurrent VTE was considered during the entire study period, whereas major bleeding was considered during the time from administration of the first dose of study drug to 48 hours after administration of the last dose. The primary analysis considered the efficacy and safety outcomes in the various risk profile groups using the following hierarchy: unprovoked VTE, provoked VTE with (a) major persistent, (b) minor persistent, (c) minor transient, and (d) major transient risk factors. The incidences of both outcomes were calculated in patients with or without a prior history of VTE. Differences in cumulative incidences were calculated using 1-year Kaplan-Meier estimates.
A Firth-adjusted Cox proportional hazard model was used to estimate the influence of the presence or absence of a prior history of VTE, sex, and age on the incidence of recurrent VTE or major bleeding. The model was stratified for treatment (rivaroxaban, aspirin, or placebo) and presentation of the index event.
Results
Patients
A total of 4553 patients qualified for this analysis: 1188 from the EINSTEIN EXTENSION trial and 3365 patients from the EINSTEIN CHOICE trial. Of these, 2832 received rivaroxaban (10 or 20 mg), 1131 received aspirin, and 590 received placebo. Risk profiles could be assessed in all patients (Figure 1), and the demographic and clinical characteristics according to risk profile and treatment assignment are provided in Table 1. Characteristics for each risk profile were well matched across study arms. When comparing characteristics between risk profiles, patients with VTE provoked by minor transient risk factors were more often women and younger in age.
Demographics and clinical characteristics
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 598) . | Rivaroxaban 20 mg (N = 1107) . | Rivaroxaban 10 mg (N = 1127) . | All rivaroxaban (N = 2832) . | Placebo (N = 590) . | Aspirin (N = 1131) . | |
| Unprovoked | ||||||
| Age, mean, y | 59.0 ± 14.5 | 59.2 ± 13.5 | 60.0 ± 13.9 | 59.5 ± 13.9 | 57.2 ± 15.4 | 59.2 ± 13.8 |
| Male sex, n (%) | 168 (66.7) | 280 (63.5) | 294 (61.3) | 742 (63.3) | 152 (62.6) | 314 (67.1) |
| Index DVT only, n (%) | 168 (66.7) | 246 (55.8) | 250 (52.1) | 664 (56.6) | 158 (65.0) | 253 (54.1) |
| Index PE ± DVT, n (%) | 84 (33.3) | 195 (44.2) | 230 (47.9) | 509 (43.4) | 85 (35.0) | 215 (45.9) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 177 (70.2) | 311 (70.5) | 330 (68.8) | 818 (69.7) | 178 (73.3) | 335 (71.6) |
| ≥9 mo | 75 (29.8) | 130 (29.5) | 150 (31.3) | 355 (30.3) | 65 (26.7) | 133 (28.4) |
| Provoked by major persistent risk factors | ||||||
| Age, mean, y | 71.8 ± 9.4 | 62.8 ± 12.4 | 63.3 ± 10.8 | 65.0 ± 11.7 | 65.3 ± 10.3 | 66.2 ± 11.1 |
| Male sex, n (%) | 13 (68.4) | 17 (48.6) | 14 (50.0) | 44 (53.7) | 13 (50.0) | 19 (48.7) |
| Index DVT only, n (%) | 6 (31.6) | 17 (48.6) | 12 (42.9) | 35 (42.7) | 15 (57.7) | 21 (53.8) |
| Index PE ± DVT, n (%) | 13 (68.4) | 18 (51.4) | 16 (57.1) | 47 (57.3) | 11 (42.3) | 18 (46.2) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 11 (57.9) | 27 (77.1) | 22 (78.6) | 60 (73.2) | 20 (76.9) | 28 (71.8) |
| ≥9 mo | 8 (42.1) | 8 (22.9) | 6 (21.4) | 22 (26.8) | 6 (23. 1) | 11 (28.2) |
| Provoked by minor persistent risk factors | ||||||
| Age, mean, y | 57.5 ± 16.4 | 58.3 ± 14.9 | 59.2 ± 15.1 | 58.4 ± 15.3 | 60.8 ± 16.0 | 59.4 ± 15.2 |
| Male sex, n (%) | 122 (49.6) | 244 (51.3) | 233 (50.4) | 599 (50.6) | 141 (56.9) | 242 (51.9) |
| Index DVT only, n (%) | 164 (66.7) | 235 (49.4) | 224 (48.6) | 623 (52.6) | 145 (58.5) | 223 (47.9) |
| Index PE ± DVT, n (%) | 82 (33.3) | 241 (50.6) | 238 (51.5) | 561 (47.4) | 103 (41.5) | 243 (52.1) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 180 (73.2) | 324 (68.1) | 312 (67.5) | 816 (68.9) | 167 (67.3) | 380 (66.1) |
| ≥9 mo | 66 (26.8) | 152 (31.8) | 150 (32.5) | 368 (31.1) | 81 (32.7) | 158 (33.9) |
| Provoked by minor transient risk factors | ||||||
| Age, mean, y | 53.2 ± 15.5 | 49.3 ± 16.7 | 50.9 ± 16.6 | 50.8 ± 16.4 | 48.3 ± 15.9 | 51.0 ± 15.4 |
| Male sex, n (%) | 36 (57.1) | 38 (36.5) | 49 (47.5) | 122 (45.5) | 21 (37.5) | 46 (38.0) |
| Index DVT only, n (%) | 38 (60.3) | 46 (44.2) | 52 (51.5) | 136 (50.7) | 32 (57.1) | 64 (52.9) |
| Index PE ± DVT, n (%) | 25 (39.7) | 58 (55.8) | 49 (48.5) | 132 (49.3) | 24 (42.9) | 57 (47.1) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 50 (79.4) | 71 (68.3) | 77 (76.2) | 198 (73.9) | 44 (78.6) | 94 (77.7) |
| ≥9 mo | 13 (20.6) | 33 (31.7) | 24 (23.8) | 70 (26.1) | 12 (21.4) | 37 (22.3) |
| Provoked by major transient risk factors | ||||||
| Age, mean, y | 59.1 ± 16.1 | 56.7 ± 14.7 | 58.1 ± 12.8 | 57.7 ± 14.0 | 63.4 ± 17.0 | 60.6 ± 11.9 |
| Male sex, n (%) | 12 (66.7) | 23 (45.1) | 31 (55.4) | 66 (52.8) | 11 (64.7) | 22 (59.5) |
| Index DVT only, n (%) | 10 (55.6) | 26 (51.0) | 29 (51.8) | 65 (52.0) | 7 (41.2) | 21 (56.8) |
| Index PE ± DVT, n (%) | 8 (44.4) | 25 (49.0) | 27 (48.2) | 60 (48.0) | 10 (58.8) | 16 (43.2) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 14 (77.8) | 41 (80.4) | 41 (73.2) | 96 (76.8) | 13 (76.5) | 28 (75.7) |
| ≥9 mo | 4 (22.2) | 10 (19.6) | 15 (26.8) | 29 (23.2) | 4 (23.5) | 9 (24.3) |
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 598) . | Rivaroxaban 20 mg (N = 1107) . | Rivaroxaban 10 mg (N = 1127) . | All rivaroxaban (N = 2832) . | Placebo (N = 590) . | Aspirin (N = 1131) . | |
| Unprovoked | ||||||
| Age, mean, y | 59.0 ± 14.5 | 59.2 ± 13.5 | 60.0 ± 13.9 | 59.5 ± 13.9 | 57.2 ± 15.4 | 59.2 ± 13.8 |
| Male sex, n (%) | 168 (66.7) | 280 (63.5) | 294 (61.3) | 742 (63.3) | 152 (62.6) | 314 (67.1) |
| Index DVT only, n (%) | 168 (66.7) | 246 (55.8) | 250 (52.1) | 664 (56.6) | 158 (65.0) | 253 (54.1) |
| Index PE ± DVT, n (%) | 84 (33.3) | 195 (44.2) | 230 (47.9) | 509 (43.4) | 85 (35.0) | 215 (45.9) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 177 (70.2) | 311 (70.5) | 330 (68.8) | 818 (69.7) | 178 (73.3) | 335 (71.6) |
| ≥9 mo | 75 (29.8) | 130 (29.5) | 150 (31.3) | 355 (30.3) | 65 (26.7) | 133 (28.4) |
| Provoked by major persistent risk factors | ||||||
| Age, mean, y | 71.8 ± 9.4 | 62.8 ± 12.4 | 63.3 ± 10.8 | 65.0 ± 11.7 | 65.3 ± 10.3 | 66.2 ± 11.1 |
| Male sex, n (%) | 13 (68.4) | 17 (48.6) | 14 (50.0) | 44 (53.7) | 13 (50.0) | 19 (48.7) |
| Index DVT only, n (%) | 6 (31.6) | 17 (48.6) | 12 (42.9) | 35 (42.7) | 15 (57.7) | 21 (53.8) |
| Index PE ± DVT, n (%) | 13 (68.4) | 18 (51.4) | 16 (57.1) | 47 (57.3) | 11 (42.3) | 18 (46.2) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 11 (57.9) | 27 (77.1) | 22 (78.6) | 60 (73.2) | 20 (76.9) | 28 (71.8) |
| ≥9 mo | 8 (42.1) | 8 (22.9) | 6 (21.4) | 22 (26.8) | 6 (23. 1) | 11 (28.2) |
| Provoked by minor persistent risk factors | ||||||
| Age, mean, y | 57.5 ± 16.4 | 58.3 ± 14.9 | 59.2 ± 15.1 | 58.4 ± 15.3 | 60.8 ± 16.0 | 59.4 ± 15.2 |
| Male sex, n (%) | 122 (49.6) | 244 (51.3) | 233 (50.4) | 599 (50.6) | 141 (56.9) | 242 (51.9) |
| Index DVT only, n (%) | 164 (66.7) | 235 (49.4) | 224 (48.6) | 623 (52.6) | 145 (58.5) | 223 (47.9) |
| Index PE ± DVT, n (%) | 82 (33.3) | 241 (50.6) | 238 (51.5) | 561 (47.4) | 103 (41.5) | 243 (52.1) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 180 (73.2) | 324 (68.1) | 312 (67.5) | 816 (68.9) | 167 (67.3) | 380 (66.1) |
| ≥9 mo | 66 (26.8) | 152 (31.8) | 150 (32.5) | 368 (31.1) | 81 (32.7) | 158 (33.9) |
| Provoked by minor transient risk factors | ||||||
| Age, mean, y | 53.2 ± 15.5 | 49.3 ± 16.7 | 50.9 ± 16.6 | 50.8 ± 16.4 | 48.3 ± 15.9 | 51.0 ± 15.4 |
| Male sex, n (%) | 36 (57.1) | 38 (36.5) | 49 (47.5) | 122 (45.5) | 21 (37.5) | 46 (38.0) |
| Index DVT only, n (%) | 38 (60.3) | 46 (44.2) | 52 (51.5) | 136 (50.7) | 32 (57.1) | 64 (52.9) |
| Index PE ± DVT, n (%) | 25 (39.7) | 58 (55.8) | 49 (48.5) | 132 (49.3) | 24 (42.9) | 57 (47.1) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 50 (79.4) | 71 (68.3) | 77 (76.2) | 198 (73.9) | 44 (78.6) | 94 (77.7) |
| ≥9 mo | 13 (20.6) | 33 (31.7) | 24 (23.8) | 70 (26.1) | 12 (21.4) | 37 (22.3) |
| Provoked by major transient risk factors | ||||||
| Age, mean, y | 59.1 ± 16.1 | 56.7 ± 14.7 | 58.1 ± 12.8 | 57.7 ± 14.0 | 63.4 ± 17.0 | 60.6 ± 11.9 |
| Male sex, n (%) | 12 (66.7) | 23 (45.1) | 31 (55.4) | 66 (52.8) | 11 (64.7) | 22 (59.5) |
| Index DVT only, n (%) | 10 (55.6) | 26 (51.0) | 29 (51.8) | 65 (52.0) | 7 (41.2) | 21 (56.8) |
| Index PE ± DVT, n (%) | 8 (44.4) | 25 (49.0) | 27 (48.2) | 60 (48.0) | 10 (58.8) | 16 (43.2) |
| Duration of previous anticoagulation, n (%) | ||||||
| <9 mo | 14 (77.8) | 41 (80.4) | 41 (73.2) | 96 (76.8) | 13 (76.5) | 28 (75.7) |
| ≥9 mo | 4 (22.2) | 10 (19.6) | 15 (26.8) | 29 (23.2) | 4 (23.5) | 9 (24.3) |
Plus-minus values are means ± SD.
Patients with unprovoked VTE or VTE provoked by a major persistent risk factor
In patients without any recognized risk factors, recurrent VTE occurred in 19 of the 1173 (1.6%) patients who received rivaroxaban, in 26 of the 468 (5.5%) patients who received aspirin, and in 20 of the 243 (8.2%) patients who received placebo. The corresponding cumulative 1-year incidences were 2.0%, 5.9%, and 10.0%, respectively. The differences in cumulative incidences in favor of rivaroxaban were 3.9% (95% confidence interval [CI], 1.5%-6.3%) vs aspirin and 8.0% (95% CI, 3.1%-12.9%) vs placebo. The incidences in each study arm are provided in Table 2. Major bleeding in patients with unprovoked VTE occurred in 8 patients (0.7%) who received rivaroxaban, in 1 patient (0.2%) who received aspirin, and in none who received placebo (Table 2). The differences in cumulative 1-year incidences were 0.5% (95% CI, −0.2% to 1.2%) in favor of aspirin, and 0.8% (95% CI, 0.2%-1.3%) in favor of placebo.
Crude incidences of recurrent VTE and major bleeding in patients receiving rivaroxaban, placebo, or aspirin according to baseline risk factor profiles
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (n = 598) . | Rivaroxaban 20 mg (n = 1107) . | Rivaroxaban 10 mg (n = 1127) . | All rivaroxaban (n = 2832) . | Placebo (n = 590) . | Aspirin (n = 1131) . | |
| Unprovoked, n (%) | 252 (42.1) | 441 (39.8) | 480 (42.6) | 1173 (41.4) | 243 (41.1) | 468 (41.4) |
| Recurrent VTE | 4 (1.6) | 8 (1.8) | 7 (1.5) | 19 (1.6) | 20 (8.2) | 26 (5.6) |
| Major bleeding | 2 (0.8) | 4 (0.9) | 2 (0.4) | 8 (0.7) | 0 (0.0) | 1 (0.2) |
| Provoked by major persistent risk factor, n (%) | 19 (3.2) | 35 (3.2) | 28 (2.5) | 82 (2.9) | 26 (4.4) | 39 (3.4) |
| Recurrent VTE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.8) | 2 (5.1) |
| Major bleeding | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (5.1) |
| Provoked by minor persistent risk factors, n (%) | 246 (41.1) | 476 (43.0) | 462 (41.0) | 1184 (41.8) | 248 (42.0) | 466 (41.2) |
| Recurrent VTE | 4 (1.6) | 8 (1.7) | 6 (1.3) | 18 (1.5) | 17 (6.9) | 18 (3.9) |
| Major bleeding | 1 (0.4) | 1 (0.2) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| Provoked by minor transient risk factors, n (%) | 63 (10.5) | 104 (9.4) | 101 (9.0) | 268 (9.5) | 56 (9.5) | 121 (10.7) |
| Recurrent VTE | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (0.4) | 4 (7.1) | 4 (3.3) |
| Major bleeding | 0 (0.0) | 1 (1.0) | 2 (2.0) | 3 (1.1) | 0 (0.0) | 0 (0.0) |
| Provoked by major transient risk factor, n (%) | 18 (3.0) | 51 (4.6) | 56 (5.0) | 125 (4.4) | 17 (2.9) | 37 (3.3) |
| Recurrent VTE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Major bleeding | 1 (5.6) | 0 (0.0) | 1 (1.8) | 2 (1.6) | 0 (0.0) | 0 (0.0) |
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (n = 598) . | Rivaroxaban 20 mg (n = 1107) . | Rivaroxaban 10 mg (n = 1127) . | All rivaroxaban (n = 2832) . | Placebo (n = 590) . | Aspirin (n = 1131) . | |
| Unprovoked, n (%) | 252 (42.1) | 441 (39.8) | 480 (42.6) | 1173 (41.4) | 243 (41.1) | 468 (41.4) |
| Recurrent VTE | 4 (1.6) | 8 (1.8) | 7 (1.5) | 19 (1.6) | 20 (8.2) | 26 (5.6) |
| Major bleeding | 2 (0.8) | 4 (0.9) | 2 (0.4) | 8 (0.7) | 0 (0.0) | 1 (0.2) |
| Provoked by major persistent risk factor, n (%) | 19 (3.2) | 35 (3.2) | 28 (2.5) | 82 (2.9) | 26 (4.4) | 39 (3.4) |
| Recurrent VTE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.8) | 2 (5.1) |
| Major bleeding | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (5.1) |
| Provoked by minor persistent risk factors, n (%) | 246 (41.1) | 476 (43.0) | 462 (41.0) | 1184 (41.8) | 248 (42.0) | 466 (41.2) |
| Recurrent VTE | 4 (1.6) | 8 (1.7) | 6 (1.3) | 18 (1.5) | 17 (6.9) | 18 (3.9) |
| Major bleeding | 1 (0.4) | 1 (0.2) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| Provoked by minor transient risk factors, n (%) | 63 (10.5) | 104 (9.4) | 101 (9.0) | 268 (9.5) | 56 (9.5) | 121 (10.7) |
| Recurrent VTE | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (0.4) | 4 (7.1) | 4 (3.3) |
| Major bleeding | 0 (0.0) | 1 (1.0) | 2 (2.0) | 3 (1.1) | 0 (0.0) | 0 (0.0) |
| Provoked by major transient risk factor, n (%) | 18 (3.0) | 51 (4.6) | 56 (5.0) | 125 (4.4) | 17 (2.9) | 37 (3.3) |
| Recurrent VTE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Major bleeding | 1 (5.6) | 0 (0.0) | 1 (1.8) | 2 (1.6) | 0 (0.0) | 0 (0.0) |
In patients with VTE provoked by a major persistent risk factor (ie, cancer), recurrent VTE occurred in none of the 82 (0%) patients who received rivaroxaban, in 2 of the 39 (5.1%) patients who received aspirin, and in 1 of the 26 (3.8%) patients who received placebo (Table 2), whereas major bleeding occurred in none, 2 (5.1%), and none, respectively.
Patients with minor persistent risk factors
Recurrent VTE in patients with minor persistent risk factors occurred in 18 of the 1184 (1.5%) patients who received rivaroxaban, in 18 of the 466 (3.9%) patients who received aspirin, and in 17 of the 248 (6.9%) patients who received placebo (Table 2). The corresponding cumulative incidences at 1 year with rivaroxaban, aspirin, and placebo were 2.4%, 4.5%, and 10.7%, respectively. The differences in cumulative incidences in favor of rivaroxaban were 2.2% (95% CI, −0.3% to 4.7%) vs aspirin and 8.4% (95% CI, 2.4%-14.3%) vs placebo. The incidences of recurrent VTE for each individual risk factor are provided in Table 3.
Crude incidences of recurrent VTE and major bleeding according to individual minor persistent or transient risk factors
| . | Recurrent VTE, n (%) . | |
|---|---|---|
| Risk factor . | Rivaroxaban 10 and 20 mg . | Placebo/aspirin . |
| Provoked by minor persistent risk factors, n (%) | ||
| Inflammatory bowel disease | 0/26 (0.0) | 0/14 (0.0) |
| Lower extremity paralysis or paresis | 0/12 (0.0) | 0/4 (0.0) |
| Congestive heart failure | 2/23 (8.7) | 0/10 (0.0) |
| Body mass index >30 kg/m2 | 13/907 (1.4) | 25/536 (4.7) |
| Creatinine clearance <50 mL/min | 2/122 (1.6) | 8/104 (7.7) |
| Family history of VTE | 2/31 (6.5) | 0/13 (0.0) |
| Hereditary thrombophilia | 3/173 (1.7) | 8/102 (7.8) |
| Acquired thrombophilia | 1/20 (5.0) | 0/5 (0.0) |
| Provoked by minor transient risk factors, n (%) | ||
| Immobilization | 1/99 (1.0) | 5/68 (7.4) |
| Travel >8 h | 0/11 (0.0) | 0/9 (0.0) |
| Use of estrogen therapy | 0/75 (0.0) | 1/64 (1.6) |
| Pregnancy or puerperium | 0/17 (0.0) | 0/2 (0.0) |
| Leg injury with impaired mobility | 0/76 (0.0) | 2/40 (5.0) |
| . | Recurrent VTE, n (%) . | |
|---|---|---|
| Risk factor . | Rivaroxaban 10 and 20 mg . | Placebo/aspirin . |
| Provoked by minor persistent risk factors, n (%) | ||
| Inflammatory bowel disease | 0/26 (0.0) | 0/14 (0.0) |
| Lower extremity paralysis or paresis | 0/12 (0.0) | 0/4 (0.0) |
| Congestive heart failure | 2/23 (8.7) | 0/10 (0.0) |
| Body mass index >30 kg/m2 | 13/907 (1.4) | 25/536 (4.7) |
| Creatinine clearance <50 mL/min | 2/122 (1.6) | 8/104 (7.7) |
| Family history of VTE | 2/31 (6.5) | 0/13 (0.0) |
| Hereditary thrombophilia | 3/173 (1.7) | 8/102 (7.8) |
| Acquired thrombophilia | 1/20 (5.0) | 0/5 (0.0) |
| Provoked by minor transient risk factors, n (%) | ||
| Immobilization | 1/99 (1.0) | 5/68 (7.4) |
| Travel >8 h | 0/11 (0.0) | 0/9 (0.0) |
| Use of estrogen therapy | 0/75 (0.0) | 1/64 (1.6) |
| Pregnancy or puerperium | 0/17 (0.0) | 0/2 (0.0) |
| Leg injury with impaired mobility | 0/76 (0.0) | 2/40 (5.0) |
Minor persistent risk factors are listed for the patient who did not have a major persistent risk factor; minor transient risk factors are listed for patients who did not have persistent risk factors. Hereditary thrombophilia includes deficiency of antithrombin, protein C, or protein S, and factor V Leiden or the prothrombin gene mutation. Acquired thrombophilia includes antiphospholipid syndrome. A patient can contribute to multiple rows.
Major bleeding in patients with VTE provoked by a minor persistent risk factor occurred in 2 patients (0.2%) who received rivaroxaban, and in none who received aspirin or placebo. The differences in cumulative incidences were 0.2% (95% CI, −0.1% to 0.4%) in favor of aspirin and 0.2% (95% CI, −0.1 to 0.4%) in favor of placebo. The incidences in each study arm are provided in Table 2.
Patients with minor transient risk factors
Recurrent VTE in patients with minor transient risk factors occurred in 1 of the 268 (0.4%) patients who received rivaroxaban, in 4 of the 121 (3.3%) patients who received aspirin, and in 4 of the 56 (7.1%) patients who received placebo (Table 2). The corresponding cumulative incidences at 1 year were 0.4%, 4.2%, and 4.2%, respectively. The differences in cumulative incidences in favor of rivaroxaban were 3.8% (95% CI, −0.4% to 8.0%) vs aspirin and 6.8% (95% CI, 0.0%-13.5%) vs placebo. The incidences of recurrent VTE for each individual risk factor are given in Table 3.
Major bleeding in patients with VTE provoked by a minor transient risk factor occurred in 3 patients (1.1%) who received rivaroxaban and in none who received aspirin or placebo. The differences in cumulative incidences were 1.6% (95% CI, −0.3% to 3.6%) in favor of aspirin and 1.6% (95% CI, −0.3% to 3.6%) in favor of placebo. The incidences in each study arm are given in Table 2.
Additional observations
The incidence of recurrent VTE was nonsignificantly higher in men than in women and in patients with a prior history of VTE than in those without (hazard ratio [HR], 1.14 [95% CI, 0.79-1.61], and HR, 1.33 [95% CI, 0.85-1.99], respectively). The incidences of recurrent VTE for patients with or without a prior history of VTE are presented in Tables 4 and 5. None of the patients with major transient risk factors who received rivaroxaban (n = 125), aspirin (n = 37), or placebo (n = 17) had recurrent VTE (Table 2).
Crude incidences of recurrent nonfatal or fatal VTE in patients without a prior history of VTE
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 488) . | Rivaroxaban 20 mg (N = 909) . | Rivaroxaban 10 mg (N = 930) . | All rivaroxaban (N = 2327) . | Placebo (N = 502) . | Aspirin (N = 937) . | |
| Unprovoked, n/N (%) | 4/198 (2.0) | 5/363 (1.4) | 5/407 (1.2) | 14/968 (1.5) | 18/203 (8.9) | 16/392 (4.1) |
| Provoked by major persistent risk factors, n/N (%) | 0/17 (0.0) | 0/30 (0.0) | 0/21 (0.0) | 0/68 (0.0) | 1/26 (3.9) | 1/29 (3.5) |
| Provoked by minor persistent risk factors, n/N (%) | 3/201 (1.5) | 8/383 (2.1) | 6/367 (1.6) | 17/951 (1.8) | 15/206 (7.3) | 12/382 (3.1) |
| Provoked by minor transient risk factors, n/N (%) | 0/56 (0.0) | 1/84 (1.2) | 0/86 (0.0) | 1/226 (0.4) | 4/52 (7.7) | 4/106 (3.8) |
| Provoked by major transient risk factors, n/N (%) | 0/16 (0.0) | 0/49 (0.0) | 0/49 (0.0) | 0/114 (0.0) | 0/15 (0.0) | 0/28 (0.0) |
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 488) . | Rivaroxaban 20 mg (N = 909) . | Rivaroxaban 10 mg (N = 930) . | All rivaroxaban (N = 2327) . | Placebo (N = 502) . | Aspirin (N = 937) . | |
| Unprovoked, n/N (%) | 4/198 (2.0) | 5/363 (1.4) | 5/407 (1.2) | 14/968 (1.5) | 18/203 (8.9) | 16/392 (4.1) |
| Provoked by major persistent risk factors, n/N (%) | 0/17 (0.0) | 0/30 (0.0) | 0/21 (0.0) | 0/68 (0.0) | 1/26 (3.9) | 1/29 (3.5) |
| Provoked by minor persistent risk factors, n/N (%) | 3/201 (1.5) | 8/383 (2.1) | 6/367 (1.6) | 17/951 (1.8) | 15/206 (7.3) | 12/382 (3.1) |
| Provoked by minor transient risk factors, n/N (%) | 0/56 (0.0) | 1/84 (1.2) | 0/86 (0.0) | 1/226 (0.4) | 4/52 (7.7) | 4/106 (3.8) |
| Provoked by major transient risk factors, n/N (%) | 0/16 (0.0) | 0/49 (0.0) | 0/49 (0.0) | 0/114 (0.0) | 0/15 (0.0) | 0/28 (0.0) |
Crude incidences of recurrent VTE in patients with a prior history of VTE
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 110) . | Rivaroxaban 20 mg (N = 198) . | Rivaroxaban 10 mg (N = 197) . | All rivaroxaban (N = 505) . | Placebo (N = 88) . | Aspirin (N = 194) . | |
| Unprovoked, n/N (%) | 0/54 (0.0) | 3/78 (3.9) | 2/73 (2.8) | 5/205 (2.4) | 2/40 (5.0) | 10/76 (13.2) |
| Provoked by major persistent risk factors, n/N (%) | 0/2 (0.0) | 0/5 (0.0) | 0/7 (0.0) | 0/14 (0.0) | 0 | 1/10 (10.0) |
| Provoked by minor persistent risk factors, n/N (%) | 1/45 (2.2) | 0/93 (0.0) | 0/95 (0.0) | 1/233 (0.4) | 2/42 (4.8) | 6/84 (7.1) |
| Provoked by minor transient risk factors, n/N (%) | 0/7 (0.0) | 0/20 (0.0) | 0/15 (0.0) | 0/42 (0.0) | 0/4 (0.0) | 0/15 (0.0) |
| Provoked by major transient risk factors, n/N (%) | 0/2 (0.0) | 0/2 (0.0) | 0/7 (0.0) | 0/11 (0.0) | 0/2 (0.0) | 0/9 (0.0) |
| Risk factor profile . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . | EINSTEIN CHOICE . | EINSTEIN EXTENSION and EINSTEIN CHOICE . | EINSTEIN EXTENSION . | EINSTEIN CHOICE . |
|---|---|---|---|---|---|---|
| Rivaroxaban 20 mg (N = 110) . | Rivaroxaban 20 mg (N = 198) . | Rivaroxaban 10 mg (N = 197) . | All rivaroxaban (N = 505) . | Placebo (N = 88) . | Aspirin (N = 194) . | |
| Unprovoked, n/N (%) | 0/54 (0.0) | 3/78 (3.9) | 2/73 (2.8) | 5/205 (2.4) | 2/40 (5.0) | 10/76 (13.2) |
| Provoked by major persistent risk factors, n/N (%) | 0/2 (0.0) | 0/5 (0.0) | 0/7 (0.0) | 0/14 (0.0) | 0 | 1/10 (10.0) |
| Provoked by minor persistent risk factors, n/N (%) | 1/45 (2.2) | 0/93 (0.0) | 0/95 (0.0) | 1/233 (0.4) | 2/42 (4.8) | 6/84 (7.1) |
| Provoked by minor transient risk factors, n/N (%) | 0/7 (0.0) | 0/20 (0.0) | 0/15 (0.0) | 0/42 (0.0) | 0/4 (0.0) | 0/15 (0.0) |
| Provoked by major transient risk factors, n/N (%) | 0/2 (0.0) | 0/2 (0.0) | 0/7 (0.0) | 0/11 (0.0) | 0/2 (0.0) | 0/9 (0.0) |
Discussion
Patients with unprovoked VTE often receive extended anticoagulation, but many of those with provoked VTE do not.5 The results of this study confirm that the risk of recurrent VTE is highest if anticoagulation therapy is stopped in patients with unprovoked VTE, and low if anticoagulation is stopped in patients with VTE provoked by a major transient risk factor such as major surgery or trauma. These findings justify current guidelines that suggest extended anticoagulation in patients with unprovoked VTE, but not in those with VTE provoked by a major transient risk factor. The novel finding of this study is that the annual risk of recurrent VTE is at least 7% if anticoagulation therapy is stopped in patients with VTE provoked by minor persistent or transient risk factors. In these groups, rivaroxaban reduced the risk of recurrent VTE by >75% compared with placebo.
Some strengths and limitations of our study deserve attention. To ensure uniformity and to reduce bias, risk factor profiles and efficacy and safety outcomes in both studies were centrally adjudicated by the same committee whose members were blinded to treatment allocation and outcome status. Although the sample size was large and patients with various risk factor profiles were included, those at highest risk for recurrence may be underrepresented because EINSTEIN EXTENSION and EINSTEIN CHOICE only enrolled patients with equipoise regarding the need for extended anticoagulant treatment. Therefore, the rates of recurrence in such patients may be higher than those reported in this study, but are unlikely to be lower. Only 147 patients with VTE provoked by a major persistent risk factor were included, likely reflecting reluctance to enroll patients with active cancer into studies where they may be randomized to aspirin or placebo. Although the small numbers limit the strength of any conclusions in this group, there were no recurrences in patients randomized to rivaroxaban. Likewise, few patients at high risk for bleeding were included so our findings may not apply to such patients. Finally, only well-documented risk factors for VTE were considered in our classification scheme, and other risk factors may also contribute to the risk of recurrence.
The results of this study have the potential to influence practice. First, they confirm that patients with unprovoked VTE benefit from extended anticoagulation therapy, whereas those whose VTE was provoked by major transient risk factors do not.21 Second, they suggest that some patients with VTE provoked by minor persistent or transient risk factors may also benefit from extended anticoagulation therapy. Finally, regardless of whether VTE was unprovoked or provoked, rivaroxaban reduced the risk of recurrence and was associated with a major bleeding rate similar to those with aspirin or placebo. Therefore, with ease of use and the option to lower the dose from 20 mg to 10 mg once daily,19 rivaroxaban is an attractive option for extended treatment of VTE.
In conclusion, our study shows that patients with VTE provoked by minor persistent or transient risk factors have a substantial risk of recurrence, and like those with unprovoked VTE, are likely to benefit from extended anticoagulation. Regardless of the risk profile, rivaroxaban is an effective and safe option for extended treatment.
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by Bayer AG. J.I.W. holds the Canada Research Chair in Thrombosis and the Hearet and Stroke Foundation J. F. Mustard Chair in Cardiovascular Research.
Authorship
Contribution: M.H.P., A.W.A.L., and J.I.W. provided the study concept and design; M.H.P., A.W.A.L., J.I.W., P.P., P.S.W., P.V., and J.B.-W. were in charge of acquisition, analysis, or interpretation of data; M.H.P., A.W.A.L., P.P., P.S.W., and J.I.W. drafted the manuscript: R.B., H.B., T.A.B., A.T.C., B.L.D., H.D., A.K.K., and B.v.B. critically revised the manuscript for important intellectual content; and A.F.P., M.H., and M.T. oversaw the statistical analysis.
Conflict-of-interest disclosure: M.H.P. receives consulting fees from Pfizer and Daiichi Sankyo. A.W.A.L., A.F.P., M.H., and M.T. are employees of Bayer. P.P. receives consulting and lecture fees from Bayer, Sanofi, Daiichi Sankyo, and Pfizer. P.S.W. receives grant support, lecture fees, and fees for serving on advisory boards from Bayer; fees for serving on a writing committee from Itreas; consulting fees from Janssen Scientific Affairs; grant support from Bristol-Myers Squibb and Pfizer; and lecture fees from Daiichi Sankyo. P.V. receives grant support, lecture fees, and fees for serving on advisory boards from Boehringer Ingelheim and LEO Pharma; lecture fees from Pfizer and Bristol-Myers Squibb; grant support from Sanofi; lecture fees and fees for serving on advisory boards from Daiichi Sankyo; and fees for serving on an advisory board from Portola Pharmaceuticals. J.B.-W. receives grant support, lecture fees, and fees for serving on advisory boards from Boehringer Ingelheim, Daiichi Sankyo, and Pfizer. R.B. receives consulting and lecture fees from Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo. H.B. receives grant support and fees for serving on the Thrombosis Research Institute Garfield Registry steering committee; consulting fees from Amgen; and fees for serving on advisory boards from Bayer, Pfizer, and Sanofi Aventis. T.A.B. receives lecture fees from Bayer, Novo Nordisk, and GlaxoSmithKline. A.T.C. receives fees for serving on a committee for Boehringer Ingelheim; grant support and fees for serving on committees from Bristol-Myers Squibb and Daiichi Sankyo; consulting fees and fees for serving on steering committees from Johnson & Johnson and Portola; grant support, consulting fees, and fees for serving on committees from Pfizer; and consulting fees from Sanofi, Janssen, and Ono Pharmaceuticals. B.L.D. receives consulting fees from Janssen and Portola. H.D. receives fees for attending symposia from Aspen; fees for serving on advisory boards from Pfizer and Bristol-Myers Squibb; and grant support and fees for board membership from Daiichi Sankyo and Bayer. A.K.K. receives grant support, consulting fees, and lecture fees from Bayer; and consulting and lecture fees from Sanofi, Janssen, Boehringer Ingelheim, and Daiichi Sankyo. B.v.B. receives lecture fees and fees for serving on an advisory board from Bayer and Daiichi Sankyo; and for serving on an advisory board from Bristol-Myers Squibb. J.I.W. receives consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Ionis Pharmaceuticals, Janssen, Johnson & Johnson, Novartis, Portola, Pfizer, and Servier.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St East, Hamilton, ON L8L 2X2, Canada; e-mail: weitzj@taari.ca.