Key Points
R, L, and I in relapsed/ refractory CLL do not appear to be more effective than the R + L doublet or I alone.
The regimen was associated with sustained grade 4 neutropenia, which prompted study withdrawal in many patients.
Abstract
Attempts to improve upon the activity of ibrutinib in chronic lymphocytic leukemia (CLL) include the addition of targeted therapies. The combination of lenalidomide and rituximab demonstrated an overall response rate (ORR) of 66% with a complete response (CR) of 12% in the relapsed/refractory setting. Based on these data, we conducted a phase 1 study of rituximab (R), lenalidomide (L), and ibrutinib (I) in relapsed/refractory CLL. Patients received R 375 mg/m2 cycles 1 to 6 day 1, L on cycles 1 to 12 days 1 to 21, and I until disease progression. Dose escalation used a standard 3+3 design from a dose level (DL) of L 5 mg (DL1) and increasing to 15 mg (DL3) for a total of 3 dose levels. Twelve patients were enrolled; there were 2 dose-limiting toxicities of grade 4 neutropenia at DL3; thus, DL2 was the recommended phase 2 dose. A high incidence of sustained grade 4 neutropenia occurred at all dose levels, prompting study withdrawal in 5 patients, despite growth factor support. The ORR was 67%; ORR at the RP2D was 100% (1 CR). The 12-month progression-free survival at the RP2D was 83%. Preliminary efficacy data with the triplet did not appear superior to prior reports of the rituximab-lenalidomide doublet or single-agent ibrutinib. Given these findings and the sustained neutropenia, this regimen was not pursued. The study was registered at www.clinicaltrials.gov as #NCT02200848.
Introduction
The treatment paradigm for chronic lymphocytic leukemia (CLL) has evolved dramatically with the advent of targeted therapies. Although chemoimmunotherapy is still considered a first-line option for younger patients lacking poor prognostic features, the choice for subsequent lines of therapy typically includes novel agents.1-2 A number of biologics have been developed over the past several years, varying in mechanism of action.2-4 They carry the promise of better on-target antitumor effect and less off-target toxicity.
The immunomodulatory agent, lenalidomide, was one of the first biologic therapies to be extensively explored in CLL. Preclinical studies indicate its ability to induce apoptosis, promote natural killer cell and T-cell activity, and suppress pro-survival cytokines.5 Its combination with rituximab purportedly enhances the antibody-dependent cellular cytotoxic properties of the monoclonal antibody.6 The efficacy of rituximab and lenalidomide was evaluated in two phase 2 studies of relapsed and refractory CLL.7-8 The doublet produced overall response rates (ORRs) of 45% to 66% in this heavily pretreated population. However, only 0% to 12% of patients achieved a complete response (CR) and the median time to treatment failure was 14 to 17 months, leaving considerable room for improvement.
The discovery of the first-generation Bruton tyrosine kinase inhibitor, ibrutinib, revolutionized the treatment of CLL. As monotherapy, the drug produced an impressive ORR of 63% to 86% and median progression-free survival (PFS) >4 years in the relapsed setting.2,9 As with lenalidomide, ibrutinib has an impact on the tumor microenviroment. It has been shown improve T-cell number and function in CLL patients as well as reduce the expression of PD-L1, CTLA-4, and interleukin-10.10 At conception of this novel 3-drug combination, the durability of remission with ibrutinib was unclear. Given the tolerability of ibrutinib and infrequency of complete remissions, it was an ideal candidate for combination in a multitargeted regimen. Using distinct mechanisms of action and nonoverlapping toxicity profiles, we conducted a phase 1 study of lenalidomide and ibrutinib in combination with rituximab for the treatment of relapsed and refractory CLL and small lymphocytic lymphoma (SLL).
Methods
Eligibility criteria
Patients had previously treated, pathologically confirmed CLL or SLL that required treatment per the 2008 International Workshop on CLL National Cancer Institute Working Group Guidelines.11 Other inclusion criteria included age >18 years, life expectancy >60 days, Eastern Cooperative Oncology Group performance status <2, and measurable disease by imaging or physical examination. Required initial laboratory values included an absolute neutrophil count >0.75 × 109/L and platelet count >50 × 109/L unless attributable to CLL, hemoglobin >8.0 g/dL, total bilirubin ≤1.5 times the upper limit of normal (ULN) unless the patient had disease infiltration of the liver or Gilbert syndrome, aspartate aminotransferase and alanine transaminase <3 times institutional ULN, glomerular filtration rate ≥30 mL/min per 1.73 m2 (Cockcroft-Gault), and prothrombin time/international normalized ratio and partial thromboplastin time <1.5 times ULN.
Exclusion criteria included prior systemic therapy for CLL or SLL including chemotherapy, immunotherapy, immunosuppression, radiation therapy, or surgery within 4 weeks of enrollment. Corticosteroids could not have been administered within 2 weeks before study entry, except as maintenance therapy for a nonmalignant disease. Prior lenalidomide was permitted if it had been >2 years since exposure and the patient did not experience a progression while receiving the drug. Any prior Bruton tyrosine kinase inhibitor, strong CYP3A inhibitor within 7 days of enrollment, concomitant strong CYP3A inhibitors, and concomitant vitamin K antagonists were not permitted. Other exclusion criteria were allogeneic stem cell transplantation, central nervous system involvement, Richter transformation, uncontrolled autoimmune hemolytic anemia or immune-mediated thrombocytopenia, and known bleeding disorders. Patients with uncontrolled, intercurrent illness including, but not limited to, infection, cardiac, or psychiatric disease were excluded. Patients with prior malignancies were excluded unless they were treated with curative intent >3 years prior or had adequately treated nonmelanomatous skin cancer, lentigo maligna melanoma, or cervical carcinoma in situ.
Study design
This phase 1 trial used a standard 3+3 dose escalation design. Patients received rituximab 375 mg/m2 on day 1 of cycles 1 to 6, lenalidomide as per cohort dose on days 1 to 21 of cycles 1 to 12, and ibrutinib 420 mg daily starting from day 1 and continuing until progression or intolerance. Each cycle was 28 days in length. Dose escalation began at a starting dose level (DL) of lenalidomide 5 mg (DL1) and increased to 15 mg (DL3) through 3 dose levels (Table 1).
Dosing schema
| Dose level . | Rituximab: cycles 1-6, day 1, mg/m2 . | Ibrutinib: days 1-28 until progression/intolerance, mg/d . | Lenalidomide: cycles 1-12, days 1-21/28, mg/d . |
|---|---|---|---|
| Level −2 | 375 | 280 | 2.5 |
| Level −1 | 375 | 420 | 2.5 |
| Level 1* | 375 | 420 | 5 |
| Level 2 | 375 | 420 | 10 |
| Level 3 | 375 | 420 | 15 |
| Dose level . | Rituximab: cycles 1-6, day 1, mg/m2 . | Ibrutinib: days 1-28 until progression/intolerance, mg/d . | Lenalidomide: cycles 1-12, days 1-21/28, mg/d . |
|---|---|---|---|
| Level −2 | 375 | 280 | 2.5 |
| Level −1 | 375 | 420 | 2.5 |
| Level 1* | 375 | 420 | 5 |
| Level 2 | 375 | 420 | 10 |
| Level 3 | 375 | 420 | 15 |
Dose level 1 is the starting dose level.
Patients received allopurinol 300 mg daily orally for tumor lysis prophylaxis on day −2 and continued through day 21 of cycle 3 or longer at the discretion of the treating physician. All patients were also required to take aspirin 81 mg orally daily during lenalidomide therapy to prevent thrombosis, unless it was contraindicated. Hematopoietic growth factors were used only if a patient’s absolute neutrophil count did not recover to at least grade 1 by the start of the next scheduled cycle or in the case of febrile neutropenia. Women of childbearing potential were required to use 2 forms of contraception while on lenalidomide. Pregnancy testing was performed routinely throughout study treatment.
Dose-limiting toxicity assessment
Patients enrolled at DL1 were assessed for dose-limiting toxicity (DLT) during cycle 1. Patients enrolled at DL2 or DL3 were assessed for DLT in cycles 1 and 2. Adverse events were graded using the National Cancer Institute Common Toxicity Terminology Criteria for Adverse Events, version 4.0. Nonhematologic DLT included all grade 3 or 4 adverse events and Stevens-Johnson syndrome or toxic epidermal necrolysis of any grade. Exceptions to this included fatigue, anorexia, venous thromboembolic event, nausea/vomiting/diarrhea that resolves with supportive management, fever without neutropenia, grade 3 transaminitis that resolved to <grade 2 within 7 days, and grade 3 rash that resolved to <grade 2 within 10 days with systemic corticosteroid treatment. Hematologic DLT included any grade 4 hematologic toxicity (except grade 4 neutropenia lasting <7 days), grade 2 or 3 thrombocytopenia complicated by hemorrhage, and grade 3 or 4 neutropenia complicated by fever ≥38.5°C or infection.
Dose adjustment
Dose reductions and delays were required in patients who experienced grade 4 neutropenia for >7 days, grade 3 thrombocytopenia complicated by a clinically significant bleeding event, grade 4 thrombocytopenia, grade 3 or 4 nausea/vomiting/diarrhea if persistent despite optimal therapy, or any other grade 4 or unmanageable grade 3 toxicity. Doses of lenalidomide and ibrutinib were held and resumed at 1 dose level below the previous dose if the toxicity decreased to at least grade 2. If the study drugs needed to be held >28 days, the patient was removed from the trial.
Response evaluation
Baseline imaging with computed tomography or magnetic resonance imaging with contrast and bone marrow biopsy were performed before initiation of therapy. Standard karyotyping and CLL fluorescence in situ hybridization (FISH) panel were performed on the biopsies through a commercial laboratory. Response assessments by repeat imaging were obtained during weeks 10, 24, and 52 every 6 months for years 2 and 3, followed by yearly until disease progression or for a maximum of 10 years. Bone marrow biopsies were repeated only if the baseline biopsy indicated disease involvement and follow-up imaging suggested a CR, for the purposes of confirmation. Response and progression were determined using the International Workshop Response Criteria for CLL and Malignant Lymphoma.7,12
Statistical considerations
The primary end point of the study was the maximally tolerated dose of lenalidomide, rituximab, and ibrutinib in relapsed/refractory CLL or SLL to determine the recommended phase 2 doses (R2PD). The secondary end points were to determine the safety and to describe preliminary antitumor efficacy of the regimen. Based on the 3+3 dose escalation schema, the minimum number of patients required to determine the R2PD was 12, whereas the maximum was 18. After the R2PD was reached, an expansion cohort of 10 patients was planned.
Patient characteristics were presented using contingency tables for categorical variables. The median and range were calculated for each continuous variable. Adverse events were analyzed for all treated patients using descriptive statistics. The percentages of patients who achieved stable disease (SD), partial response, or CR were reported with a 95% confidence interval. PFS was defined as the duration of time from the start of treatment to the time of progression or death, whichever occurred first.
The study was conducted at the Lombardi Comprehensive Cancer Center at Medstar Georgetown University Hospital and John Theurer Cancer Center at Hackensack University Medical Center. It was approved by the ethics committee and institutional review board at all participating cancer centers. Each patient provided written informed consent in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Lombardi Comprehensive Cancer Center. Data quality was maintained by Theradex Oncology. All analyses were based on the study database frozen on 1 October 2017.
Results
Patient characteristics
Twelve patients with CLL were enrolled between May 2014 and January 2017. The median age was 64 years (range, 50-75). (Table 2) Eighty-three percent of patients were male, 67% had an Eastern Cooperative Oncology Group performance status of 1, and 50% had Rai stage III/IV disease. The incidence of prognostic features is described in Table 2. Patients had received a median of 1 prior therapy (range, 1-7). Eleven patients had received prior rituximab-based chemoimmunotherapy with fludarabine or bendamustine. Three patients had received prior lenalidomide and none had received prior ibrutinib.
Patient characteristics
| Characteristics . | No. of patients (%) (n = 12) . |
|---|---|
| Sex | |
| Male | 10 (83) |
| Female | 2 (17) |
| Age, y | |
| Median | 64 |
| Range | 50-75 |
| ECOG performance status | |
| 0 | 4 (33) |
| 1 | 8 (67) |
| Rai stage | |
| I/II | 6 (50) |
| III/IV | 6 (50) |
| Interphase cytogenetic abnormality (FISH) | |
| Del 13q | 1 (8) |
| Trisomy 12 | 3 (25) |
| Del 11q | 3 (25) |
| Del 17p | 1 (8) |
| No CLL abnormalities by FISH | 4 (33) |
| Unmutated IGVH | 8 (67) |
| Karyotype (chromosomal banding analysis) | |
| Normal karyotype | 4 (40) |
| 1 abnormality | 1 (10) |
| 2 abnormalities | 2 (20) |
| Complex karyotype | 3 (30) |
| Data missing | 2 |
| CD38 expression >30% | 2 (17) |
| Zap-70 expression >20% | 6 (50) |
| Characteristics . | No. of patients (%) (n = 12) . |
|---|---|
| Sex | |
| Male | 10 (83) |
| Female | 2 (17) |
| Age, y | |
| Median | 64 |
| Range | 50-75 |
| ECOG performance status | |
| 0 | 4 (33) |
| 1 | 8 (67) |
| Rai stage | |
| I/II | 6 (50) |
| III/IV | 6 (50) |
| Interphase cytogenetic abnormality (FISH) | |
| Del 13q | 1 (8) |
| Trisomy 12 | 3 (25) |
| Del 11q | 3 (25) |
| Del 17p | 1 (8) |
| No CLL abnormalities by FISH | 4 (33) |
| Unmutated IGVH | 8 (67) |
| Karyotype (chromosomal banding analysis) | |
| Normal karyotype | 4 (40) |
| 1 abnormality | 1 (10) |
| 2 abnormalities | 2 (20) |
| Complex karyotype | 3 (30) |
| Data missing | 2 |
| CD38 expression >30% | 2 (17) |
| Zap-70 expression >20% | 6 (50) |
ECOG, Eastern Cooperative Oncology Group; IGVH, immunoglobulin variable-region heavy-chain.
RP2D
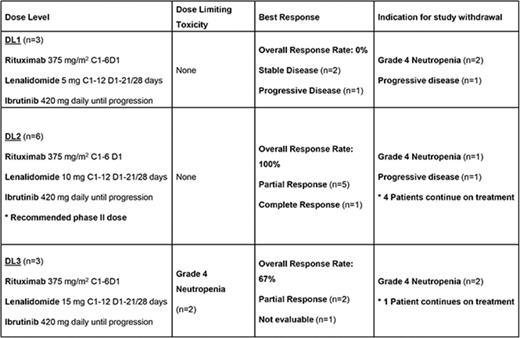
Patients were treated at DL1 (n = 3), DL2 (n = 6), and DL3 (n = 3). There were 2 DLT of grade 4 neutropenia at DL3; thus, DL2 was expanded to 6 patients. There were no DLT in DL2; therefore, DL2 was determined to be the maximally tolerated dose. RP2D was determined to be lenalidomide 10 mg days 1 to 21 for 12 28-day cycles, ibrutinib 420 mg daily until progression, and rituximab 375 mg/m2 day 1 for 6 28-day cycles.
Toxicity
The most common nonhematologic adverse events (all grades) were fatigue (67%), infection (58%), and diarrhea (58%) (Table 3). The most common nonhematologic grade 3/4 adverse event was infection (25%). The most common hematologic adverse events (all grades, grade 3/4) were neutropenia (67%, 67%) and thrombocytopenia (50%, 17%) (Table 4). Prolonged grade 4 neutropenia occurred at all dose levels. The median time to onset to grade 4 neutropenia was 2.5 months (1 week-7 months). Five patients withdrew from study because of persistent grade 4 neutropenia despite growth factor support. One patient in DL2 was able to continue on the study with dose reduction and implementation of granulocyte colony-stimulating factor support. Serious adverse events included neutropenia (n = 5), fever (n = 2), abdominal pain (n = 2), febrile neutropenia (n = 1), atrial fibrillation (n = 1), diarrhea (n = 1), hemolytic anemia (n = 1), Aspergillus pneumonia (n = 1), rash (n = 1), tumor lysis syndrome (n = 1), urinary tract infection (n = 1), cellulitis (n = 1), and heart failure (n = 1). One patient developed papillary renal cell carcinoma, which in reviewing prior imaging, appeared to be present at enrollment. There were no thrombotic events. Three patients received prophylactic aspirin.
Nonhematologic adverse events
| Adverse events . | All grades, % . | Grade 3/4, % . |
|---|---|---|
| Fatigue | 67 | 0 |
| Infection | 58 | 25 |
| Diarrhea | 58 | 8 |
| Myalgia/arthralgia | 58 | 17 |
| Rash | 42 | 8 |
| Elevated liver function tests | 42 | 0 |
| Hematoma | 42 | 0 |
| Edema | 33 | 8 |
| Headache | 33 | 0 |
| Cough | 25 | 0 |
| Dyspnea | 25 | 8 |
| Back pain | 25 | 0 |
| Abdominal pain | 25 | 17 |
| Nausea | 17 | 0 |
| Fever | 17 | 8 |
| Elevated creatinine | 17 | 0 |
| Neoplasms | 8 | 8 |
| Tumor lysis syndrome | 8 | 8 |
| Congestive heart failure exacerbation | 8 | 8 |
| Atrial fibrillation | 8 | 8 |
| Pneumonitis | 8 | 0 |
| Adverse events . | All grades, % . | Grade 3/4, % . |
|---|---|---|
| Fatigue | 67 | 0 |
| Infection | 58 | 25 |
| Diarrhea | 58 | 8 |
| Myalgia/arthralgia | 58 | 17 |
| Rash | 42 | 8 |
| Elevated liver function tests | 42 | 0 |
| Hematoma | 42 | 0 |
| Edema | 33 | 8 |
| Headache | 33 | 0 |
| Cough | 25 | 0 |
| Dyspnea | 25 | 8 |
| Back pain | 25 | 0 |
| Abdominal pain | 25 | 17 |
| Nausea | 17 | 0 |
| Fever | 17 | 8 |
| Elevated creatinine | 17 | 0 |
| Neoplasms | 8 | 8 |
| Tumor lysis syndrome | 8 | 8 |
| Congestive heart failure exacerbation | 8 | 8 |
| Atrial fibrillation | 8 | 8 |
| Pneumonitis | 8 | 0 |
Efficacy
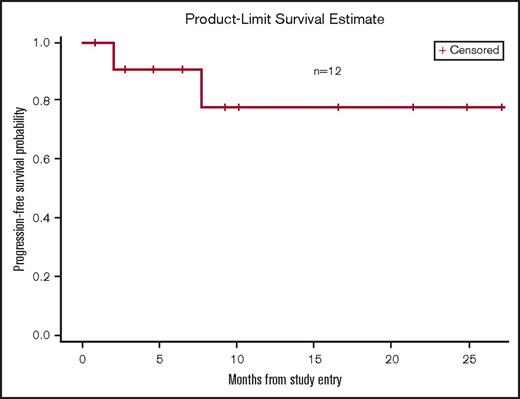
The ORR for the entire cohort of patients (n = 12) was 66.7%. Individual patient responses are delineated in Table 5. In DL1, 2 patients maintained stable disease and 1 patient experienced progression of CLL at first response assessment. The ORR at the RP2D (DL2) was 100%. One patient at this dose level achieved CR, noted at response assessment after 18 months of therapy. The median time to first response was 9 weeks (range, 8.43-13). At a median follow-up of 8.9 months, the median time on treatment was 7.6 months (range, 0.7-27.2). The 12-month PFS for the entire study population was 77.9% (range, 34.4% to 94.2%) (Figure 1). The 12-month PFS at the RP2D was 83.3% (range, 27.3% to 97.5%). One patient treated at the RP2D developed progression of CLL at 7 months of treatment.
Baseline characteristics and treatment outcomes
| Dose level . | Age/sex . | Prognostic features . | No. of prior regimens . | Prior regimens . | ANC, ×109/L . | Platelets, ×109/L . | Hgb, g/dL . | Best response . | Duration of therapy, mo . | Indication for study withdrawal . |
|---|---|---|---|---|---|---|---|---|---|---|
| DL1 | 75/M | del 13q, trisomy 12, unmutated | 1 | B+R | 9.9 | 157 | 8.3 | SD | 3 | Grade 4 neutropenia |
| DL1 | 50/M | Mutated, normal FISH | 3 | Fl+R + oblimersen; second-generation CD20 antibody; B+R | 3.8 | 113 | 12.6 | SD | 4 | Grade 4 neutropenia |
| DL1 | 63/M | IGVH unavailable, normal FISH | 1 | B+R | 7.6 | 209 | 14.4 | PD | 2 | Progressive disease |
| DL2 | 65/M | IGVH unavailable, trisomy 12 | 1 | Fl+R | 3.4 | 118 | 14 | PR | 8 | Grade 4 neutropenia |
| DL2 | 75/M | IGVH unavailable, del 13q, trisomy 12, complex karyotype | 3 | Fl+R; B+L; second-generation PI3K inhibitor | 11.3 | 101 | 9.7 | PR | 25+ | NA |
| DL2 | 53/F | Unmutated | 2 | Fl+R followed by L; R + idelalisib | 2.3 | 203 | 12.3 | CR | 23+ | NA |
| DL2 | 70/M | Unmutated, trisomy 12 | 1 | R-CVP | 8.7 | 141 | 10.4 | PR | 15+ | NA |
| DL2 | 59/M | Unmutated, del 17p, complex karyotype | 1 | B+R | 3.0 | 90 | 14.3 | PR | 19+ | NA |
| DL2 | 69/M | Unmutated, del 13q, del 11p, complex karyotype | 3 | Fl+R followed by L; R + idelalisib; venetoclax | 3.5 | 140 | 13.9 | PR | 7 | PD |
| DL3 | 69/F | Unmutated, del 11q, del 13p | 7 | Fl; Fl+R; PCR; B+R; alemtuzumab; B+R; R + idelalisib | 1.0 | 85 | 9.8 | PR | 4 | Grade 4 neutropenia |
| DL3 | 54/M | Unmutated, del 13q | 1 | B+R | 3.7 | 102 | 16.6 | PR | 20+ | NA |
| DL3 | 61/M | Unmutated, normal FISH | 1 | Fl+C+R | 2.1 | 46 | 8.9 | Not evaluable | 1 | Grade 4 neutropenia with grade 3 hemolytic anemia |
| Dose level . | Age/sex . | Prognostic features . | No. of prior regimens . | Prior regimens . | ANC, ×109/L . | Platelets, ×109/L . | Hgb, g/dL . | Best response . | Duration of therapy, mo . | Indication for study withdrawal . |
|---|---|---|---|---|---|---|---|---|---|---|
| DL1 | 75/M | del 13q, trisomy 12, unmutated | 1 | B+R | 9.9 | 157 | 8.3 | SD | 3 | Grade 4 neutropenia |
| DL1 | 50/M | Mutated, normal FISH | 3 | Fl+R + oblimersen; second-generation CD20 antibody; B+R | 3.8 | 113 | 12.6 | SD | 4 | Grade 4 neutropenia |
| DL1 | 63/M | IGVH unavailable, normal FISH | 1 | B+R | 7.6 | 209 | 14.4 | PD | 2 | Progressive disease |
| DL2 | 65/M | IGVH unavailable, trisomy 12 | 1 | Fl+R | 3.4 | 118 | 14 | PR | 8 | Grade 4 neutropenia |
| DL2 | 75/M | IGVH unavailable, del 13q, trisomy 12, complex karyotype | 3 | Fl+R; B+L; second-generation PI3K inhibitor | 11.3 | 101 | 9.7 | PR | 25+ | NA |
| DL2 | 53/F | Unmutated | 2 | Fl+R followed by L; R + idelalisib | 2.3 | 203 | 12.3 | CR | 23+ | NA |
| DL2 | 70/M | Unmutated, trisomy 12 | 1 | R-CVP | 8.7 | 141 | 10.4 | PR | 15+ | NA |
| DL2 | 59/M | Unmutated, del 17p, complex karyotype | 1 | B+R | 3.0 | 90 | 14.3 | PR | 19+ | NA |
| DL2 | 69/M | Unmutated, del 13q, del 11p, complex karyotype | 3 | Fl+R followed by L; R + idelalisib; venetoclax | 3.5 | 140 | 13.9 | PR | 7 | PD |
| DL3 | 69/F | Unmutated, del 11q, del 13p | 7 | Fl; Fl+R; PCR; B+R; alemtuzumab; B+R; R + idelalisib | 1.0 | 85 | 9.8 | PR | 4 | Grade 4 neutropenia |
| DL3 | 54/M | Unmutated, del 13q | 1 | B+R | 3.7 | 102 | 16.6 | PR | 20+ | NA |
| DL3 | 61/M | Unmutated, normal FISH | 1 | Fl+C+R | 2.1 | 46 | 8.9 | Not evaluable | 1 | Grade 4 neutropenia with grade 3 hemolytic anemia |
ANC, absolute neutrophil count; B, bendamustine; C, cyclophosphamide; F, female; Fl, fludarabine; Hgb, hemoglobin; L, lenalidomide; M, male; NA, not applicable; PCR, pentostatin, cyclophosphamide, rituximab; PD, progressive disease; PI3K, phosphatidylinositol 3-kinase; PR, partial response; R, rituximab; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone.
Dose intensity
At the time of data cutoff, 5 patients continued to receive treatment per protocol. The median number of cycles patients received rituximab was 6 (range, 1-6), lenalidomide 8 (range, 1-12), and ibrutinib 8 (range, 1-29+). Patients received 75% of the expected dose intensity of rituximab, 56% of lenalidomide, and 100% of ibrutinib. The dose intensity of rituximab and lenalidomide was calculated based on the planned duration of treatment of each agent, 6 and 12 cycles, respectively. Because ibrutinib was to be administered indefinitely, the dose intensity of ibrutinib was calculated based on the number of cycles the patient received therapy on study.
Because regimen toxicity requiring treatment withdrawal occurred in a greater than expected number of patients, the study was terminated after the R2PD was determined.
Discussion
These are the first results of the combination of rituximab, lenalidomide, and ibrutinib in any line of treatment of CLL. The concept for the regimen was based on the theory that targeting multiple aspects of CLL biology, including a key cell-surface antigen, the tumor microenvironment, and intracellular signaling, would be both efficacious and tolerable. The regimen expanded upon prior experience with the rituximab-lenalidomide doublet by incorporating a distinct novel agent, ibrutinib. Preliminary efficacy data with the triplet, however, did not appear superior to prior reports of the rituximab-lenalidomide doublet or single-agent ibrutinib in this setting.2,7-8 Furthermore, there was no apparent improvement in the complete remission rate. There was no antitumor activity seen at the first dose level (n = 3), which included the standard dose for ibrutinib in CLL (420 mg). Although this study included a small number of patients, these findings are puzzling, given that the median time to initial response with ibrutinib is 1.8 months in relapsed setting and the response assessment was performed at 10 weeks per study protocol.13 Additionally, most patients receiving lenalidomide-rituximab were able to achieve a partial remission by 3 months.7
The activity of this regimen may have been compromised by its toxicity profile. Sixty-seven percent of patients experienced grade 4 neutropenia, and 42% of patients discontinued therapy because of sustained grade 4 neutropenia. This observation corresponded with a similar rate of infections of all grades (58%), including 25% grade 3/4 infection. The incidence of severe neutropenia was higher than previous reports of single-agent ibrutinib (30%) but comparable to rituximab-lenalidomide (50% to 73%).7-8,13 Grade 4 neutropenia occurred at all dose levels and at various points in the treatment course; all but 1 patient discontinued therapy by week 13 (n = 4). The 2 patients with stable disease in the initial dose-level cohort discontinued therapy because of prolonged neutropenia shortly after the initial response assessment. We were unable to identify patient risk factors that correlated with the development of neutropenia.
In contrast to prior investigations of these agents, the majority of patients on this study withdrew because of toxicity (n = 5) as opposed to progression of disease (n = 2). The inability to continue with study drugs may have compromised the ability to achieve a complete remission. Prior studies have indicated that the majority of CR with rituximab-lenalidomide or ibrutinib occurs after at least 12 cycles of therapy. Because most patients who discontinued therapy from toxicity did so before cycle 4, they did not receive therapy for sufficient duration of time to achieve maximum clinical benefit. The only CR with this regimen was identified after cycle 18.
The most notable adverse event that has previously been reported with this triplet in other disease states is grade 3/4 rash, specifically in previously untreated follicular lymphoma and relapsed/refractory diffuse large B-cell lymphoma.14-15 The incidence of rash in this study (all grades, 42%; grade 3/4, 8%) was greater than seen with lenalidomide-rituximab (all grades, 22%; grade 3/4, 0%) or ibrutinib (all grades, 23%; grade 3/4, 0%).7,13 In contrast to prior studies, the occurrence of rash did not prompt dose modifications. These differences may be related to line of therapy, prior administration of cytotoxic therapy, or disease biology.
In this phase 1 study, 2 important conclusions can be made. First, the additional clinical benefit of a third agent was not apparent in relapsed and refractory CLL. Second, the toxicity and resulting early discontinuance rate proved the lack of feasibility of this regimen. Although the neutropenia may have been mitigated by a decrease in the duration of lenalidomide in each cycle, the clinical benefit of the triplet would be similar, if not less. Further investigation seems unwarranted in this setting, but future combinations with these individual agents and other novel therapeutics remain promising. Since conception of this study, it has been established that the achievement of a complete remission with B-cell receptor antagonists is not mandatory for maintaining a lengthy PFS.9 However, if a regimen were able to achieve a deeper response of minimal residual disease negativity with the incorporation of other biologic agents such as BCL-2 inhibitors, patients may be able to benefit from both durability of remission as well as cessation of therapy.16 Ongoing and upcoming trials continue to evaluate biologic doublets and triplets in the upfront and previously treated settings. As these newer regimens are being explored, their toxicity profiles must remain an important factor for consideration in treatment decision-making.17
Acknowledgments
The authors thank the Cancer Therapy Evaluation Program; Pharmacyclics LLC, an Abbvie Company; Celgene Corporation; and Theradex Oncology.
This study was supported by the Cancer Therapy Evaluation Program, which provided the drug, and by National Institutes of Health, National Cancer Institute grant P30 CA 051008 (principal investigator: Lou Weiner).
Authorship
Contribution: C.U., N.K., B.D.C., and H.W. conceived and designed the study; C.U., N.T., and P.R. collected and assembled data; and all authors contributed to data analysis and interpretation, manuscript writing, and final approval of the manuscript.
Conflict-of-interest disclosures: C.U. has been a consultant for and received research support from Pharmacyclics, been a consultant for Genentech, and on a speakers bureau for AbbVie. A.S. and N.K. have been on a speakers bureau for Genentech. B.D.C. has consulted for Celgene, Roche-Genentech, and Pharmacyclics, and research support was provided to this author’s institution by Roche-Genentech and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Chaitra Ujjani, Lombardi Comprehensive Cancer Center, Medstar Georgetown University Hospital, 3800 Reservoir Rd NW, Washington, DC 20007; e-mail: csu@georgetown.edu.