Key Points
Selectin and chemokine signals use both Rap1a and PIP5Kγ90 to activate β2 integrins in neutrophils.
Suboptimal chemokine signals synergize with selectin signals to activate β2 integrins in neutrophils.
Abstract
Rolling neutrophils receive signals while engaging P- and E-selectin and chemokines on inflamed endothelium. Selectin signaling activates β2 integrins to slow rolling velocities. Chemokine signaling activates β2 integrins to cause arrest. Despite extensive study, key aspects of these signaling cascades remain unresolved. Using complementary in vitro and in vivo assays, we found that selectin and chemokine signals in neutrophils triggered Rap1a-dependent and phosphatidylinositol-4-phosphate 5-kinase γ (PIP5Kγ90)–dependent pathways that induce integrin-dependent slow rolling and arrest. Interruption of both pathways, but not either pathway alone, blocked talin-1 recruitment to and activation of integrins. An isoform of PIP5Kγ90 lacking the talin-binding domain (PIP5Kγ87) could not activate integrins. Chemokines, but not selectins, used phosphatidylinositol-4,5-bisphosphate 3-kinase γ (PI3Kγ) in cooperation with Rap1a to mediate integrin-dependent slow rolling (at low chemokine concentrations), as well as arrest (at high chemokine concentrations). High levels of chemokines activated β2 integrins without selectin signals. When chemokines were limiting, they synergized with selectins to activate β2 integrins.
Introduction
During inflammation, neutrophils roll in venules, decelerate (slow rolling), arrest, spread, crawl to endothelial cell junctions, and migrate into underlying tissues.1 Selectin–ligand interactions mediate rolling, whereas β2 integrin–ligand interactions mediate slow rolling, arrest, and crawling.1-4 Signaling in neutrophils regulates this multistep adhesive process. Engagement of P- or E-selectin on endothelial cells with P-selectin glycoprotein ligand-1 on neutrophils triggers signals that convert the neutrophil integrin αLβ2 from a bent to an extended intermediate-affinity conformation, which slows rolling through rapidly reversible interactions with intercellular adhesion molecule-1 (ICAM-1) on endothelial cells. Binding of endothelium-presented chemokines such as CXCL1 to the receptor CXCR2 on neutrophils triggers signals that convert integrin αLβ2 to an extended high-affinity conformation, which mediates neutrophil arrest on ICAM-1.1-4 Activated integrin αMβ2 then mediates crawling and migration.1-4
Integrins are heterodimers of α and β transmembrane subunits.5 Integrin activation requires that the large protein talin bind to the cytoplasmic tail of the β subunit.6-7 The talin head domain binds to membrane-distal and membrane-proximal sites on the integrin β tail.8-9 Talin-1 is the major isoform in hematopoietic cells.10 In mice expressing a talin-1 mutant that binds only to the membrane-distal site, chemokines activate neutrophil β2 integrins to a conformation that mediates slow rolling, but not arrest or migration.11
Talin must move from the cytoplasm to the membrane to interact with integrin β tails.6-7 Two signaling proteins enable recruitment of talin to the membrane: the small GTPase Rap1 and the enzyme phosphatidylinositol-4-phosphate 5-kinase γ (PIP5Kγ).10,12-14 Because each protein has been studied separately, it is not known whether they cooperate to activate integrins or whether other proteins can recruit talin in their absence. Through 2 Rap1a-dependent signaling cascades in neutrophils, selectins initiate integrin-dependent slow rolling and chemokines initiate integrin-dependent arrest.4,15 In both of these signaling events, Rap1a acts through the effector Rap1-GTP-interacting adaptor molecule to unfold talin-1 and bring it to the membrane.16-20 In neutrophils and lymphocytes, chemokines activate PIP5Kγ that facilitates integrin-dependent adhesion and migration.21-22 Loss of PIP5Kγ in lymphocytes does not block chemokine-triggered extension of αLβ2 to the intermediate-affinity conformation, but does block conversion to the high-affinity conformation.21 PIP5Kγ has 2 isoforms, PIP5Kγ87 and PIP5Kγ90, resulting from alternative splicing of mRNA.23-24 Only the PIP5Kγ90 isoform includes the talin-binding domain encoded in exon 17 of its gene. Biochemical studies suggest that chemokine-induced signaling events promote binding of PIP5Kγ90 to talin. In turn, PIP5Kγ90-generated phosphatidylinositol 4,5 bisphosphate recruits talin to the membrane.14 It is not known whether PIP5Kγ87, which retains enzymatic activity but does not bind talin, can activate integrins.
Chemokines also activate another lipid kinase, phosphatidylinositol-4,5-bisphosphate 3-kinase γ (PI3Kγ), in neutrophils and lymphocytes. In mice lacking PI3Kγ in hematopoietic cells, neutrophils roll and arrest in venules but detach quickly, suggesting a defect in the strength of neutrophil adhesion.25 Chemical inhibition of PI3K in lymphocytes does not prevent chemokine-enhanced affinity of integrin αLβ2 for ICAM-1. Instead, it impairs the chemokine-induced lateral mobility of αLβ2 that increases adhesion avidity.26 The specific roles of PI3Kγ and Rap1a are controversial. One group reports that selectin signaling in neutrophils triggers parallel PI3Kγ- and Rap1a-dependent pathways that cooperate to convert αLβ2 to a conformation that mediates slow rolling.27 In contrast, another group finds no evidence for selectin-triggered PI3Kγ activation in neutrophils or for PI3Kγ-dependent slow rolling.28 Thus, it is still unresolved whether PI3Kγ cooperates with other mediators to activate β2 integrins.
Here we used genetically engineered mice, reporter antibodies, biochemical assays, flow-chamber studies, intravital microscopy, and inflammation models to comprehensively examine the signals that activate β2 integrins in neutrophils. We found that selectins and chemokines triggered parallel Rap1a- and PIP5Kγ90-dependent pathways to recruit talin-1 and to induce integrin-dependent slow rolling and arrest; PIP5Kγ87 lacking the talin-binding domain could not activate integrins; chemokines, but not selectins, used PI3Kγ in cooperation with Rap1a to mediate slow rolling (at low chemokine concentrations) and arrest (at high chemokine concentrations); high levels of chemokines activated β2 integrins without selectin signals; and when chemokines were limiting, they synergized with selectins to activate β2 integrins. Together, our findings clarify and expand the existing model of how external stimuli induce integrin-mediated neutrophil rolling and arrest.
Methods
Additional information on reagents and protocol details are provided in the supplemental Methods.
Mice
All mice were backcrossed at least 10 times in the C57BL/6J background. C57BL/6J and Cxcr2−/− mice were from The Jackson Laboratory (Bar Harbor, ME). The following mice have been described: Rap1a−/−,29 Rap1b−/−,30 Pik3cg−/−,31-32 Pip5k1c+/+MLC-2vCre−, Pip5k1c−/−MLC-2vCre+ (indicated as Pip5k1c−/−), Pip5k1cE17f/fCMVCre−, and Pip5k1cE17f/f CMVCre+ (indicated as Pip5k1c∆E17) mice.33 Pip5k1cE17f/f CMVCre+ mice were bred with Rap1a−/− mice to generate Rap1a−/−Pip5k1cE17f/fCMVCre+ (indicated as Rap1a−/−Pip5k1c∆E17) mice. Rap1a−/−Pik3cg−/− mice were generated by breeding Rap1a−/− with Pik3cg−/− mice.
All mouse protocols were approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Cells
Mouse bone marrow or peripheral blood leukocytes were isolated as described previously.34-35 Human neutrophils were isolated as described previously.36 Blood was obtained from healthy volunteers using a protocol approved by the Institutional Review Board of the Oklahoma Medical Research Foundation and in accordance with the Declaration of Helsinki. In some experiments, mouse neutrophils were isolated from bone marrow leukocytes by a density gradient method.37
Flow cytometry
Flow cytometry was performed as described previously.38
Flow chamber assay
For human neutrophils, 10 μg/mL recombinant human ICAM-1, KIM127, MEM148, or isotype control mouse immunoglobulin G (IgG) was co-immobilized with 1 μg/mL human P-selectin with or without 1 μg/mL or 10 μg/mL interleukin 8 (IL-8) in 35-mm polystyrene dishes. For mouse leukocytes, 10 μg/mL goat anti-human IgM Fc antibody was absorbed in 35-mm polystyrene dishes. In some experiments, 20 μg/mL mouse ICAM-1-Fc was co-immobilized with or without 0.1 μg/mL or 1 μg/mL mouse CXCL1. After incubation at 4°C overnight, the dishes were blocked with 1% human serum albumin, and then E-selectin-IgM was captured on the dishes. Human neutrophils or mouse leukocytes were perfused over dishes mounted in a parallel-plate flow chamber at a wall shear stress of 1.0 dyn/cm2. After 5 to 10 minutes, rolling and arrested cells were analyzed using a video microscopy system coupled to Element digital image-analysis software (Nikon). Arrested cells were scored as “round” (round and bright) or “spread” (irregular and dark).11
Immunoprecipitation and western blot
Bone marrow leukocytes (2 × 107) were incubated with or without 100 ng/mL CXCL1 for 10 minutes at 37°C and centrifuged. The pellets were dissolved in lysis buffer: 1% Triton X-100, 125 mM NaCl, 50 mM Tris at pH 7.4, 10 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 0.1% sodium dodecyl sulfate, with a protease inhibitor cocktail (1:50; Thermo Fisher Scientific). The lysates were incubated with control rat IgG or rat anti-mouse β2 integrin monoclonal antibody (mAb) GAME-46 and with protein A/G agarose beads. After centrifugation, the beads were washed, and bound proteins were eluted by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer with β-mercaptoethanol. The eluted proteins were probed by western blotting, using anti-talin mAb or rabbit anti-β2 integrin mAb, followed by HRP-conjugated goat anti-mouse IgG or goat anti-rabbit IgG.
Spinning-disk intravital microscopy of cremaster muscle
Spinning-disk intravital microscopy of the cremaster muscle was performed as described previously.11,36
Thioglycollate-induced peritonitis
Wild-type (WT) or Cxcr2−/− mice were injected with 1 mL 4% thioglycollate intraperitoneally, as described.28 After 4 hours, peritoneal cells were collected with 10 mL phosphate-buffered saline containing 0.1% bovine serum albumin and 5 mm EDTA. Neutrophils in the peritoneal exudate were counted on the basis of scatter properties and expression of Ly6G.
Competitive neutrophil recruitment into the peritoneum was measured as described.28,36
Statistical analysis
Statistical differences between groups were analyzed using unpaired and 2-tailed Student t test. Values were considered significant at P < .05.
Results
Selectin-interacting mouse leukocytes from bone marrow or peripheral blood are mature neutrophils
Previous studies of selectin- or chemokine-induced β2 integrin activation in mice have used unfractionated bone marrow leukocytes, density gradient-purified bone marrow neutrophils, or peripheral blood leukocytes.11,27-28,38-44 To directly compare these cell types, we used flow cytometry to analyze scatter profiles of unfractionated bone marrow leukocytes (Figure 1A), bone marrow leukocytes bound to immobilized E-selectin (Figure 1B), purified bone marrow neutrophils (Figure 1C), peripheral blood leukocytes (Figure 1D), or peripheral blood leukocytes bound to immobilized E-selectin (Figure 1E). A gate (red circle in each panel) was set around cells expressing high levels of Ly6G, a marker for neutrophils (Figure 1F). Analysis of the scatter profiles indicated that bone marrow or peripheral blood leukocytes bound to E-selectin were enriched in Ly6G-positive neutrophils. Ly6G-positive neutrophils from all sources expressed comparable levels of P-selectin glycoprotein ligand-1 (Figure 1G), CD44 (Figure 1H), and CD11a (Figure 1I). Ly6G-positive neutrophils in unfractionated bone marrow expressed broader levels of CD11b (Figure 1J) and CXCR2 (Figure 1K), consistent with developmental heterogeneity. However, E-selectin-bound leukocytes from both bone marrow and peripheral blood expressed the same high levels of these proteins as purified neutrophils. These results confirm that selectin-interacting cells are mature neutrophils.
Selectin-interacting mouse leukocytes from bone marrow or peripheral blood are mature neutrophils. (A-E) Scatter profiles of the indicated mouse leukocyte populations. A gate (red circle in each panel) was set around cells expressing high levels of Ly6G. (F-K) Expression levels of the indicated surface protein in the gated populations. The colored histograms numbered 1 to 5 in each panel correspond to the cells in A-E. (L) Rolling velocities of cells from the indicated population on E-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb, the Src family kinase inhibitor PP2, or its inactive analog PP3. (M) Percentages of cells from the indicated population rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and CXCL1 in the presence of the chemokine receptor inhibitor PTx or its solvent control dimethyl sulfoxide (DMSO). The histograms in A-K are representative of 5 independent experiments. The data in L and M represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
Selectin-interacting mouse leukocytes from bone marrow or peripheral blood are mature neutrophils. (A-E) Scatter profiles of the indicated mouse leukocyte populations. A gate (red circle in each panel) was set around cells expressing high levels of Ly6G. (F-K) Expression levels of the indicated surface protein in the gated populations. The colored histograms numbered 1 to 5 in each panel correspond to the cells in A-E. (L) Rolling velocities of cells from the indicated population on E-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb, the Src family kinase inhibitor PP2, or its inactive analog PP3. (M) Percentages of cells from the indicated population rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and CXCL1 in the presence of the chemokine receptor inhibitor PTx or its solvent control dimethyl sulfoxide (DMSO). The histograms in A-K are representative of 5 independent experiments. The data in L and M represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
To directly compare signaling in bone marrow and peripheral blood neutrophils, we perfused unfractionated bone marrow leukocytes, purified bone marrow neutrophils, or peripheral blood leukocytes over immobilized E-selectin with or without co-immobilized ICAM-1 (Figure 1L). Rolling on selectins triggers signals that enable β2 integrin-dependent slow rolling on ICAM-1. The most proximal signal is activation of Src family kinases.28,40 Leukocytes from all sources rolled with comparable velocities on E-selectin and rolled slower when ICAM-1 was co-immobilized. Slow rolling was blocked by anti-ICAM-1 mAb and by PP2, an inhibitor of Src family kinases, but not by PP3, an inactive analog. We next perfused each cell population over co-immobilized E-selectin, ICAM-1, and CXCL1 (Figure 1M). Leukocytes from all sources rapidly converted from rolling to arrest. Many arrested cells also spread, consistent with adhesion strengthening. Pertussis toxin (PTx) inhibits signaling through CXCR2 and other Gαi-coupled chemokine receptors.39 PTx, but not control dimethyl sulfoxide, prevented rolling cells from arresting and spreading. These results demonstrate that selectin-interacting leukocytes from bone marrow or peripheral blood similarly activate β2 integrins in response to either selectin or chemokine signals. In subsequent experiments in mice, we used unfractionated bone marrow leukocytes. We used genetically modified mice to examine the contributions of signaling molecules to β2 integrin activation in neutrophils. All genotypes had normal blood counts, except for moderate neutrophilia in 3 genotypes with more severe defects in neutrophil trafficking. All genotypes expressed normal levels of integrin αLβ2 (CD11a) (supplemental Table 1; supplemental Figure 1).
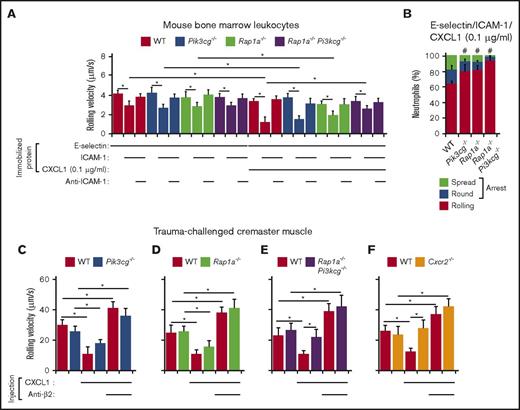
Rap1a and PIP5Kγ90 cooperate to induce neutrophil slow rolling and arrest
Pip5k1c−/−MLC-2vCre+ mice (indicated as Pip5k1c−/−) lack PIP5Kγ in all cells except cardiomyocytes.33 Pip5k1cE17f/fCMVCre+ mice (Pip5k1c∆E17) have a global deletion of exon 17 encoding the talin-binding domain in PIP5Kγ90, and thus express only PIP5Kγ87.33 We perfused bone marrow leukocytes over E-selectin with or without co-immobilized ICAM-1. Neutrophils of both genotypes and their controls exhibited equivalent slow rolling that was blocked by anti-ICAM-1 mAb (Figure 2A,C). When CXCL1 (1.0 μg/mL) was co-immobilized, the rolling neutrophils of both genotypes and controls arrested and spread equally (Figure 2B,D). These results demonstrate that loss of PIP5Kγ alone does not affect neutrophil rolling or arrest.
Rap1a and PIP5Kγ90 cooperate to induce neutrophil slow rolling and arrest. (A,C,E,G,I) Rolling velocities of neutrophils of the indicated genotype on E-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B,D,F,H,J) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and CXCL1. (K) Isolated bone marrow neutrophils of the indicated genotype were incubated with or without CXCL1, lysed, and immunoprecipitated (IP) with control or anti-β2 integrin mAb. Immunoprecipitates were analyzed by immunoblotting (IB) with anti-talin or anti-β2 integrin antibodies. (L-N) Numbers of differentially labeled adherent bone marrow leukocytes from the indicated genotype in TNF-stimulated venules of cremaster muscle. In some experiments, labeled leukocytes were pretreated with PTx and then injected into TNF-challenged mice that were previously injected with PTx. (O-Q) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in TNF-stimulated venules of cremaster muscle, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2 integrins. The labeled leukocytes were pretreated with PTx and then injected into TNF-challenged WT mice that were previously injected with PTx. The data in K are representative of 3 experiments. Other data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
Rap1a and PIP5Kγ90 cooperate to induce neutrophil slow rolling and arrest. (A,C,E,G,I) Rolling velocities of neutrophils of the indicated genotype on E-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B,D,F,H,J) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and CXCL1. (K) Isolated bone marrow neutrophils of the indicated genotype were incubated with or without CXCL1, lysed, and immunoprecipitated (IP) with control or anti-β2 integrin mAb. Immunoprecipitates were analyzed by immunoblotting (IB) with anti-talin or anti-β2 integrin antibodies. (L-N) Numbers of differentially labeled adherent bone marrow leukocytes from the indicated genotype in TNF-stimulated venules of cremaster muscle. In some experiments, labeled leukocytes were pretreated with PTx and then injected into TNF-challenged mice that were previously injected with PTx. (O-Q) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in TNF-stimulated venules of cremaster muscle, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2 integrins. The labeled leukocytes were pretreated with PTx and then injected into TNF-challenged WT mice that were previously injected with PTx. The data in K are representative of 3 experiments. Other data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
We next examined the contributions of Rap1a to integrin activation. In contrast to previous reports that neutrophils from Rap1a−/− mice are partially defective in slow rolling on E-selectin and ICAM-1,41 we could not detect any rolling defect (Figure 2E). These results indicate that either Rap1a or PIP5Kγ is sufficient to support E-selectin-mediated β2 integrin function in neutrophils. Unlike Pip5k1c−/− neutrophils, fewer rolling Rap1a−/− neutrophils arrested when CXCL1 was co-immobilized (Figure 2F), whereas Rap1b−/− neutrophils rolled and arrested normally (Figure 2G-H).
We analyzed mice lacking Rap1a and expressing only the PIP5Kγ87 isoform (Rap1a−/−Pip5k1c∆E17) to determine whether Rap1a and PIP5Kγ cooperate to activate integrins. Neutrophils from the mice had markedly impaired selectin-triggered, integrin-dependent slow rolling and CXCL1-triggered, integrin-dependent arrest on ICAM-1 (Figure 2I-J). Furthermore, anti-β2 integrin antibody coprecipitated talin-1 from lysates of CXCL1-stimulated neutrophils from Rap1a−/− or Pip5k1c∆E17 mice, but not from Rap1a−/−Pip5k1c∆E17 mice (Figure 2K).
To extend these results in vivo, we used spinning-disk intravital microscopy to visualize neutrophil rolling and arrest in venules of the cremaster muscle stimulated with tumor necrosis factor (TNF), which induces expression of P-selectin, E-selectin, ICAM-1, and CXCL1 on endothelial cells.1 We injected a 1:1 mixture of control leukocytes labeled with red dye and test leukocytes labeled with far-red dye into WT mice. This allowed rolling and arrest of neutrophils of each control and test genotype to be visualized simultaneously within the same venules. Furthermore, the gene modification was restricted to injected test leukocytes, which is important for genes such as Pip5k1c that are expressed in other cells that could influence leukocyte adhesion. Control and Pip5k1c∆E17 neutrophils converted from rolling to arrest at similar frequencies (Figure 2L). In contrast, fewer Rap1a−/− neutrophils arrested (Figure 2M), and almost no Rap1a−/−Pip5k1c∆E17 neutrophils arrested (Figure 2N).
Pretreating leukocytes with PTx blocked arrest (Figure 2L-N) and enabled measurement of selectin-triggered slow rolling without chemokine-induced integrin activation (Figure 2O-Q). Injecting blocking anti-P-selectin mAb did not affect rolling velocities, consistent with the dominance of E-selectin for controlling rolling (Figure 2O-Q). Injecting blocking anti-β2 integrin mAb similarly increased rolling velocities of control, Pip5k1c∆E17, and Rap1a−/− neutrophils, confirming selectin-induced, integrin-dependent slow rolling (Figure 2O-P). In contrast, Rap1a−/−Pip5k1c∆E17 neutrophils rolled at significantly higher velocities that were not further increased by anti-β2 integrin mAb (Figure 2Q). Collectively, these results demonstrate that Rap1a and PIP5Kγ90 in neutrophils cooperate to recruit talin-1 to β2 integrins; mediate selectin-triggered, integrin-dependent slow rolling; and facilitate chemokine-triggered, integrin-dependent arrest in vitro and in vivo. These data also demonstrate that PIP5Kγ90 requires its talin-binding domain to activate β2 integrins.
Rap1a and PI3Kγ cooperate to mediate chemokine- but not selectin-triggered neutrophil slow-rolling and chemokine-triggered arrest
Selectin-induced signaling has been proposed to proceed through 2 parallel pathways downstream of the adaptor SLP-76 (Src homology domain-containing leukocyte phosphoprotein of 76 kDa).27,41,45 The first pathway activates p38 MAPK and leads to activation of Rap1a. The second pathway activates PI3Kγ and leads to activation of Rac1/Rac2. The relative role of each pathway is unclear. For example, it has been reported that deleting either Rap1a or PI3Kγ in neutrophils partially impairs slow rolling on E-selectin and ICAM-1, and that deleting both Rap1a and PI3Kγ eliminates slow rolling.41 In contrast, we observed that neutrophils lacking Rap1a alone or both Rap1a and PI3Kγ have normal slow rolling on immobilized E-selectin and ICAM-1 (Figure 3A,C), and instead have defective CXCL1-induced arrest (Figure 3B,D).
Rap1a and PI3Kγ in neutrophils cooperate to mediate chemokine-triggered, but not selectin-triggered, slow rolling and chemokine-triggered arrest. (A,C) Rolling velocities of neutrophils of the indicated genotype on E-selectin or P-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B,D) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin or P-selectin, ICAM-1, and CXCL1. (E-G) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in TNF-stimulated venules of cremaster muscle, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2 integrins. The labeled leukocytes were pretreated with PTx and then injected into TNF-challenged WT mice that were previously injected with PTx. (H-J) Numbers of differentially labeled adherent bone marrow leukocytes from the indicated genotype in TNF-stimulated venules of cremaster muscle. The labeled bone marrow leukocytes were injected into TNF-challenged WT mice. The data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
Rap1a and PI3Kγ in neutrophils cooperate to mediate chemokine-triggered, but not selectin-triggered, slow rolling and chemokine-triggered arrest. (A,C) Rolling velocities of neutrophils of the indicated genotype on E-selectin or P-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B,D) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin or P-selectin, ICAM-1, and CXCL1. (E-G) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in TNF-stimulated venules of cremaster muscle, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2 integrins. The labeled leukocytes were pretreated with PTx and then injected into TNF-challenged WT mice that were previously injected with PTx. (H-J) Numbers of differentially labeled adherent bone marrow leukocytes from the indicated genotype in TNF-stimulated venules of cremaster muscle. The labeled bone marrow leukocytes were injected into TNF-challenged WT mice. The data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
We observed parallel results in vivo. PTx-treated neutrophils from Pi3kcg−/− or Rap1a−/−Pi3kcg−/− mice injected into TNF-challenged WT mice had normal integrin-dependent slow rolling (Figure 3E-F). Neutrophils from Cxcr2−/− mice had similar slow rolling (Figure 3G). When leukocytes not pretreated with PTx were injected, we observed partial reduction of arrested Pi3kcg−/− neutrophils (Figure 3H). Arrest of Rap1a−/−Pi3kcg−/− neutrophils was virtually eliminated (Figure 3I), to the low level observed with Cxcr2−/− neutrophils (Figure 3J). Together, and in contrast to the previous report,41 these results exclude a role for PI3Kγ in selectin-induced, integrin-dependent slow rolling, but support its importance in chemokine-mediated, integrin-dependent arrest.
Several previous studies used ex vivo autoperfusion to visualize mouse leukocyte rolling and arrest in flow chambers.27,41,45 In this system, circulating blood contains low concentrations of CXCL1 that could contribute to neutrophil activation.39 Therefore, CXCL1 signaling might mediate part of the observed integrin-dependent slow rolling of neutrophils. To test whether CXCL1 signaling can contribute to slow rolling, we adsorbed a 10-fold lower concentration of CXCL1 (0.1 μg/mL) to flow chambers. At this lower CXCL1 density, WT, Pi3kcg−/−, or Rap1a−/− neutrophils rolled longer before arresting. Notably, they rolled more slowly on co-immobilized E-selectin and ICAM-1 (Figure 4A). However, the co-immobilized CXCL1 did not further slow rolling velocities of Rap1a−/−Pi3kcg−/− neutrophils (Figure 4A). Deleting either Rap1a or PI3K partially decreased arrest, whereas deleting both Rap1a and PI3K blocked arrest (Figure 4B).
Rap1a and PI3Kγ in neutrophils cooperate to mediate chemokine-triggered, but not selectin-triggered, slow rolling and chemokine-triggered arrest. (A) Rolling velocities of neutrophils of the indicated genotype on E-selectin co-immobilized with ICAM-1 and low-dose CXCL1 (0.1 μg/mL) in the presence or absence of anti-ICAM-1 mAb. (B) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and low-dose CXCL1. (C-F) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in trauma-challenged venules of cremaster muscle of WT mice, measured before and after injection of CXCL1 (50 ng) and then a blocking mAb to β2 integrins. The data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
Rap1a and PI3Kγ in neutrophils cooperate to mediate chemokine-triggered, but not selectin-triggered, slow rolling and chemokine-triggered arrest. (A) Rolling velocities of neutrophils of the indicated genotype on E-selectin co-immobilized with ICAM-1 and low-dose CXCL1 (0.1 μg/mL) in the presence or absence of anti-ICAM-1 mAb. (B) Percentages of neutrophils of the indicated genotype rolling, arrested and round, or arrested and spread on co-immobilized E-selectin, ICAM-1, and low-dose CXCL1. (C-F) Velocities of differentially labeled bone marrow leukocytes from mice of the indicated genotype rolling in trauma-challenged venules of cremaster muscle of WT mice, measured before and after injection of CXCL1 (50 ng) and then a blocking mAb to β2 integrins. The data represent the mean ± SEM from 5 experiments, with 5 mice in each experimental group. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
We next injected CXCL1 intravenously in mice after exposing the cremaster muscle, which mobilizes P-selectin to the endothelial cell surface in venules.35 We then introduced a 1:1 mixture of differentially labeled WT leukocytes and leukocytes of another genotype. Injection of 600 ng of CXCL1 causes virtually all neutrophils rolling on P-selectin to arrest within 1 min.39 We injected 12-fold less CXCL1 (50 ng), which allowed more than half of the neutrophils to continue rolling 5 min after injection. At this dose, CXCL1 significantly reduced integrin-dependent rolling velocities of WT, Pi3kcg−/−, or Rap1a−/− neutrophils (Figure 4C-D). Injecting anti-β2 integrin mAb increased rolling velocities above pre-CXCL1 levels, consistent with basal selectin-induced, integrin-dependent slow rolling. Thus, selectin and chemokine signaling cooperatively activated β2 integrins to reduce rolling velocities. In contrast, neither Rap1a−/−Pi3kcg−/− nor Cxcr2−/− neutrophils rolled slower after CXCL1 injection (Figure 4E-F). Together, these results demonstrate that Rap1a and PI3Kγ cooperate to mediate chemokine-triggered slow rolling and arrest, but not selectin-triggered slow rolling.
Chemokines, but not selectins, cooperatively use Rap1 and PI3Kγ to trigger β2 integrin activation
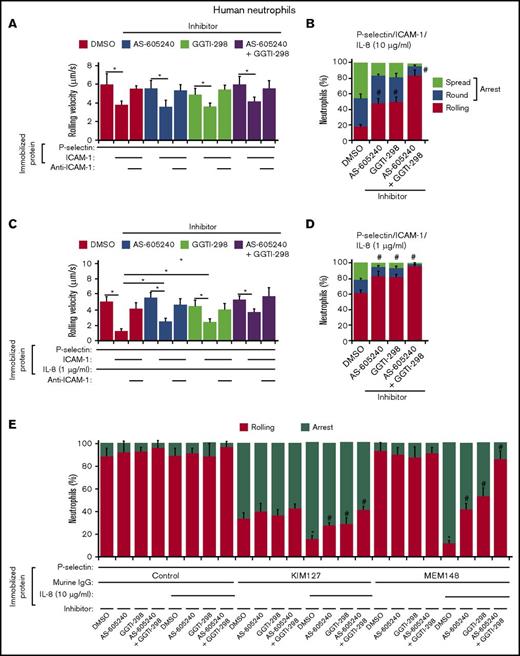
Some mAbs to the human integrin β2 subunit identify conformational changes associated with activation. Experiments with these mAbs demonstrated that selectin or chemokine signaling induces β2 integrins to extend from their bent (resting) conformation, and this structural change is sufficient for slow rolling on ICAM-1. However, only chemokine signaling induces the extended integrin to swing out the hybrid domain in the β2 subunit, creating the high-affinity conformation that mediates arrest on ICAM-1.42 The mAb KIM127 reports the conformational change associated with integrin extension,46 whereas the mAb MEM148 reports the conformational change associated with swing-out of the hybrid domain.47 Similar reporter mAbs for mouse β2 integrins are not available. We therefore used KIM127 and MEM148 to examine the contributions of Rap1 and PI3Kγ to β2 integrin extension and hybrid-domain swing-out in human neutrophils. We used chemical inhibitors of Rap1 (GGTI-298) and PI3Kγ (AS-605240) because genetically altered human neutrophils lacking Rap1 or PI3Kγ are not available. The respective inhibitors did not affect rolling or arrest of mouse neutrophils lacking Rap1a or PI3Kγ, supporting their specific actions on their target molecules (supplemental Figure 2). Control human neutrophils manifested integrin-dependent slow rolling on P-selectin and ICAM-1. Inhibitors of PI3Kγ and Rap1, alone or in combination, did not affect slow rolling (Figure 5A), but together they eliminated arrest when the chemokine IL-8 (10 μg/mL) was co-immobilized (Figure 5B). To examine chemokine-induced slow rolling, we immobilized a 10-fold-lower concentration of IL-8 (1 μg/mL). This slowed integrin-dependent rolling velocities and decreased arrest frequency (Figure 5C-D). Inhibitors of PI3Kγ and Rap1 blocked chemokine-induced slow rolling and arrest. These results recapitulate those in neutrophils from Rap1a−/−, Pi3kc−/−, and Rap1a−/−Pi3kc−/− mice.
Chemokines, but not selectins, cooperatively use Rap1 and PI3Kγ to trigger β2 integrin activation. (A) Rolling velocities of human neutrophils treated with the Rap1 inhibitor GGTI-298 and/or the PI3Kγ inhibitor AS-605240 on P-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B) Percentages of human neutrophils treated with the indicated inhibitor rolling, arrested and round, or arrested and spread on co-immobilized P-selectin, ICAM-1, and IL-8 (10 μg/mL). (C) Rolling velocities of human neutrophils treated with the indicated inhibitor on P-selectin with co-immobilized ICAM-1 and low-dose IL-8 (1 μg/mL) in the presence or absence of anti-ICAM-1 mAb. (D) Percentages of human neutrophils treated with the indicated inhibitor rolling, arrested and round, or arrested and spread on co-immobilized P-selectin, ICAM-1, and low dose IL-8 (1 μg/mL). (E) Percentages of human neutrophils treated with the indicated inhibitor rolling or arrested on P-selectin co-immobilized with control IgG, KIM127, or MEM148 with or without IL-8 (10 μg/mL). The data represent the mean ± SEM from 5 experiments. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
Chemokines, but not selectins, cooperatively use Rap1 and PI3Kγ to trigger β2 integrin activation. (A) Rolling velocities of human neutrophils treated with the Rap1 inhibitor GGTI-298 and/or the PI3Kγ inhibitor AS-605240 on P-selectin with or without co-immobilized ICAM-1 in the presence or absence of anti-ICAM-1 mAb. (B) Percentages of human neutrophils treated with the indicated inhibitor rolling, arrested and round, or arrested and spread on co-immobilized P-selectin, ICAM-1, and IL-8 (10 μg/mL). (C) Rolling velocities of human neutrophils treated with the indicated inhibitor on P-selectin with co-immobilized ICAM-1 and low-dose IL-8 (1 μg/mL) in the presence or absence of anti-ICAM-1 mAb. (D) Percentages of human neutrophils treated with the indicated inhibitor rolling, arrested and round, or arrested and spread on co-immobilized P-selectin, ICAM-1, and low dose IL-8 (1 μg/mL). (E) Percentages of human neutrophils treated with the indicated inhibitor rolling or arrested on P-selectin co-immobilized with control IgG, KIM127, or MEM148 with or without IL-8 (10 μg/mL). The data represent the mean ± SEM from 5 experiments. *P < .05 for rolling velocity; #P < .05 for percentage of rolling cells compared with that in WT, as determined by unpaired Student t test.
We then co-immobilized P-selectin with control mouse IgG, KIM127, or MEM148, with or without co-immobilized IL-8 (10 μg/mL). Without IL-8, control neutrophils rolling on P-selectin arrested on KIM127, but not on control IgG or MEM148 (Figure 5E). With IL-8, control neutrophils arrested even more frequently on KIM127 and also arrested on MEM148 (Figure 5E). Thus, P-selectin triggered β2 integrin extension, but not hybrid-domain swing-out, whereas IL-8 further increased extension and also induced swing-out. Inhibitors of PI3Kγ and Rap1 blocked the IL-8-induced component of integrin extension and prevented swing-out (Figure 5E). These results demonstrate that chemokines, but not selectins, cooperatively use Rap1 and PI3Kγ to trigger β2 integrin extension and hybrid-domain swing-out.
Selectins and chemokines cooperatively activate β2 integrins
We observed even slower rolling of neutrophils on P/E-selectin and ICAM-1 when low-density chemokine was co-immobilized (Figures 4A and 5C). This suggests that selectin signaling augments low-level chemokine signaling. To further test this hypothesis, we used flow cytometry to quantify binding of integrin reporter mAbs to human neutrophils treated with P-selectin and/or IL-8. This assay employs soluble reagents to preclude signaling by external forces applied to immobilized agonists or antibodies bound to surface receptors.38 Because monomeric soluble P-selectin does not signal,48 we used oligomeric P-selectin isolated from human platelets. As control for mAbs KIM127 and MEM148, we used mAb IB4, which binds equivalently to nonactivated and activated β2 integrins. We treated neutrophils with buffer only (control), P-selectin alone, low-dose (0.1 nM) IL-8 or high-dose (1 nM) IL-8 alone, or P-selectin plus low- or high-dose IL-8 (Table 1). These stimuli had little effect on binding of IB4. P-selectin increased binding of KIM127, but not MEM148. Low-dose IL-8 did not alter KIM127 or MEM148 binding. However, P-selectin plus low-dose IL-8 further increased binding of KIM127 and also increased binding of MEM148. These data demonstrate that selectin and chemokine signals synergize to activate β2 integrins at low chemokine concentrations. High-dose IL-8 markedly increased binding of both KIM127 and MEM148. P-selectin plus high-dose IL-8 further increased binding, indicating synergy in activating β2 integrins even at high chemokine concentrations. Inhibitors of PI3Kγ and Rap1 blocked IL-8-induced binding of KIM127 or MEM148, but did not affect P-selectin-induced binding of KIM127.
P-selectin and IL-8 cooperatively trigger β2 integrin extension and hybrid-domain swing-out in human neutrophils
| . | Inhibitor . | |||
|---|---|---|---|---|
| None . | AS-605240 . | GGTI-298 . | AS-605240 + GGTI-298 . | |
| β2 integrin, total (mAb IB4) | ||||
| Stimulation | ||||
| Control | 155 ± 25 | 165 ± 18 | 150 ± 22 | 170 ± 25 |
| P-sel | 160 ± 20 | 148 ± 20 | 140 ± 25 | 150 ± 30 |
| IL-8 (0.1 nM) | 150 ± 22 | 170 ± 25 | 155 ± 20 | 180 ± 25 |
| IL-8 (1 nM) | 200 ± 35 | 190 ± 15 | 200 ± 18 | 210 ± 40 |
| P-sel/IL-8 (0.1 nM) | 185 ± 30 | 185 ± 18 | 178 ± 20 | 200 ± 30 |
| P-sel/IL-8 (1 nM) | 210 ± 35 | 200 ± 40 | 190 ± 35 | 220 ± 50 |
| β2 integrin, extended (mAb KIM127) | ||||
| Stimulation | ||||
| Control | 8 ± 2 | 11 ± 3 | 10 ± 2 | 12 ± 5 |
| P-sel | 45 ± 8* | 38 ± 10 | 40 ± 7 | 42 ± 5 |
| IL-8 (0.1 nM) | 10 ± 2 | 13 ± 4 | 15 ± 8 | 12 ± 5 |
| IL-8 (1 nM) | 120 ± 18* | 78 ± 10† | 70 ± 10† | 30 ± 10†‡ |
| P-sel/IL-8 (0.1 nM) | 90 ± 10§ | 68 ± 8† | 62 ± 5† | 48 ± 5†‡ |
| P-sel/IL-8 (1 nM) | 165 ± 22§ | 110 ± 15† | 105 ± 10† | 65 ± 15†‡ |
| β2 integrin, extended with hybrid-domain swing-out (mAb MEM148) | ||||
| Stimulation | ||||
| Control | 15 ± 5 | 17 ± 5 | 20 ± 8 | 20 ± 5 |
| P-sel | 18 ± 5 | 20 ± 4 | 15 ± 5 | 22 ± 5 |
| IL-8 (0.1 nM) | 20 ± 5 | 16 ± 5 | 22 ± 6 | 20 ± 5 |
| IL-8 (1 nM) | 178 ± 20* | 88 ± 10† | 75 ± 10† | 25 ± 5†‡ |
| P-sel/IL-8 (0.1 nM) | 65 ± 8§ | 35 ± 6† | 40 ± 5† | 17 ± 10†‡ |
| P-sel/IL-8 (1 nM) | 220 ± 25§ | 145 ± 30† | 115 ± 20† | 35 ± 10†‡ |
| . | Inhibitor . | |||
|---|---|---|---|---|
| None . | AS-605240 . | GGTI-298 . | AS-605240 + GGTI-298 . | |
| β2 integrin, total (mAb IB4) | ||||
| Stimulation | ||||
| Control | 155 ± 25 | 165 ± 18 | 150 ± 22 | 170 ± 25 |
| P-sel | 160 ± 20 | 148 ± 20 | 140 ± 25 | 150 ± 30 |
| IL-8 (0.1 nM) | 150 ± 22 | 170 ± 25 | 155 ± 20 | 180 ± 25 |
| IL-8 (1 nM) | 200 ± 35 | 190 ± 15 | 200 ± 18 | 210 ± 40 |
| P-sel/IL-8 (0.1 nM) | 185 ± 30 | 185 ± 18 | 178 ± 20 | 200 ± 30 |
| P-sel/IL-8 (1 nM) | 210 ± 35 | 200 ± 40 | 190 ± 35 | 220 ± 50 |
| β2 integrin, extended (mAb KIM127) | ||||
| Stimulation | ||||
| Control | 8 ± 2 | 11 ± 3 | 10 ± 2 | 12 ± 5 |
| P-sel | 45 ± 8* | 38 ± 10 | 40 ± 7 | 42 ± 5 |
| IL-8 (0.1 nM) | 10 ± 2 | 13 ± 4 | 15 ± 8 | 12 ± 5 |
| IL-8 (1 nM) | 120 ± 18* | 78 ± 10† | 70 ± 10† | 30 ± 10†‡ |
| P-sel/IL-8 (0.1 nM) | 90 ± 10§ | 68 ± 8† | 62 ± 5† | 48 ± 5†‡ |
| P-sel/IL-8 (1 nM) | 165 ± 22§ | 110 ± 15† | 105 ± 10† | 65 ± 15†‡ |
| β2 integrin, extended with hybrid-domain swing-out (mAb MEM148) | ||||
| Stimulation | ||||
| Control | 15 ± 5 | 17 ± 5 | 20 ± 8 | 20 ± 5 |
| P-sel | 18 ± 5 | 20 ± 4 | 15 ± 5 | 22 ± 5 |
| IL-8 (0.1 nM) | 20 ± 5 | 16 ± 5 | 22 ± 6 | 20 ± 5 |
| IL-8 (1 nM) | 178 ± 20* | 88 ± 10† | 75 ± 10† | 25 ± 5†‡ |
| P-sel/IL-8 (0.1 nM) | 65 ± 8§ | 35 ± 6† | 40 ± 5† | 17 ± 10†‡ |
| P-sel/IL-8 (1 nM) | 220 ± 25§ | 145 ± 30† | 115 ± 20† | 35 ± 10†‡ |
The data represent the average mean fluorescence intensity (MFI) ± SD, n = 3. AS-605240 is a PI3Kγ inhibitor. GGTI-298 is a Rap1 inhibitor. P < .05 for each comparison.
P-sel, P-selectin.
Compared with control group.
Compared with non–inhibitor-treated group.
Compared with single inhibitor–treated group in each stimulation group.
Compared with any single stimulation group.
Neutrophils require chemokine but not selectin signaling to migrate into the peritoneum after thioglycollate challenge
We used competitive homing of differentially labeled neutrophils to examine signal-mediated migration into the inflamed peritoneum. Equal numbers of green control leukocytes and far-red control or mutant leukocytes were injected intravenously into WT mice 2 h after injecting thioglycollate into the peritoneum. After another 2 hours, blood and peritoneal cells were collected. Recruitment of endogenous neutrophils in Cxcr2−/− mice (Figure 6A) and of labeled Cxcr2−/− neutrophils injected into WT mice (Figure 6B) were virtually eliminated, confirming the importance of chemokine signaling and validating the use of labeled leukocytes in this model. Comparable control and mutant neutrophils remained in blood, confirming equal injection efficiency (Figure 6B-G). Comparable co-injected green and far-red control cells entered the peritoneum, indicating that labeling did not affect recruitment. Recruitment of Pip5k1c∆E17 neutrophils was normal (Figure 6C). Recruitment of Rap1a−/− and Pi3kcg−/− neutrophils was partially reduced (Figure 6D-E). Recruitment of Rap1a−/−Pip5k1c∆E17 and Rap1a−/−Pi3kcg−/− neutrophils was nearly eliminated (Figure 6F-G). These results further illustrate how PIP5Kγ90, Rap1a, and PI3Kγ cooperatively activate β2 integrins when chemokines are abundant.
Neutrophils require chemokine but not selectin signaling to migrate into the peritoneum after thioglycollate challenge. (A) WT or Cxcr2−/− mice were injected intraperitoneally with thioglycollate. After 2 hours, peritoneal cells were collected and the number of neutrophils was measured by flow cytometry. Neutrophils were identified by their scatter properties and by staining with anti-Ly6G mAb. (B-G) WT mice were injected intraperitoneally with thioglycollate. After 2 hours, they were injected intravenously with a 1:1 mixture of PKH-67-labeled WT bone marrow leukocytes and PKH-26-labeled bone marrow leukocytes of the indicated genotype. After another 2 hours, blood and peritoneal cells were collected, and the number of neutrophils labeled with each dye was measured by flow cytometry. Neutrophils were identified by their scatter properties and by staining with anti-Ly6G mAb. Results are plotted as the ratio of PKH-26-labeled neutrophils from the indicated genotype to PKH-67-labeled WT neutrophils. The data represent the mean ± SEM from 5 to 8 mice in each experimental group. *P < .05, as determined by unpaired Student test.
Neutrophils require chemokine but not selectin signaling to migrate into the peritoneum after thioglycollate challenge. (A) WT or Cxcr2−/− mice were injected intraperitoneally with thioglycollate. After 2 hours, peritoneal cells were collected and the number of neutrophils was measured by flow cytometry. Neutrophils were identified by their scatter properties and by staining with anti-Ly6G mAb. (B-G) WT mice were injected intraperitoneally with thioglycollate. After 2 hours, they were injected intravenously with a 1:1 mixture of PKH-67-labeled WT bone marrow leukocytes and PKH-26-labeled bone marrow leukocytes of the indicated genotype. After another 2 hours, blood and peritoneal cells were collected, and the number of neutrophils labeled with each dye was measured by flow cytometry. Neutrophils were identified by their scatter properties and by staining with anti-Ly6G mAb. Results are plotted as the ratio of PKH-26-labeled neutrophils from the indicated genotype to PKH-67-labeled WT neutrophils. The data represent the mean ± SEM from 5 to 8 mice in each experimental group. *P < .05, as determined by unpaired Student test.
Discussion
Both selectins and chemokines transduce signals into rolling neutrophils that augment β2 integrin function. Using complementary in vitro and in vivo assays, we demonstrated that selectins and chemokines employ 2 signaling proteins, Rap1a and PIP5Kγ90, to recruit talin-1 to β2 integrins and trigger neutrophil slow rolling and arrest on ICAM-1 under flow (Figure 7). Chemokines, but not selectins, activated PI3Kγ, which enhances the ability of Rap1a to mediate slow rolling and ultimately arrest. Selectin signaling was dispensable when chemokines were abundant. When chemokines were limiting, selectin and chemokine signals cooperated to activate β2 integrins and maximize neutrophil recruitment.
Selectin and chemokine signaling pathways in neutrophils. Each arrow indicates a signaling outcome. Signaling intermediates are omitted for clarity. See “Discussion” for details.
Selectin and chemokine signaling pathways in neutrophils. Each arrow indicates a signaling outcome. Signaling intermediates are omitted for clarity. See “Discussion” for details.
Previous studies of talin-mediated integrin activation focused on Rap1 or PIP5Kγ90, but not on their potential complementary roles. We found that either Rap1a or PIP5Kγ90 enables selectins or chemokines to activate neutrophil β2 integrins. Deleting both proteins prevented activation, establishing their joint contributions to permit integrins to bind to their ligands (Figure 7). Rap1-GTP and phosphatidylinositol 4,5 bisphosphate on membranes cooperatively recruit Rap1-GTP-interacting adaptor molecule, which interacts with talin, thereby positioning it to bind to integrin β tails.16,18-19 PIP5Kγ facilitates this process by generating phosphatidylinositol 4,5 bisphosphate in the cell membrane. We observed that PIP5Kγ90 also uses its talin-binding domain in a nonenzymatic process to position talin-1 to bind to integrin β2 tails. Platelets lacking PIP5Kγ or expressing only PIP5Kγ87 still activate β3 integrins, presumably because they also express Rap1b.33 Platelets lacking both PIP5Kγ90 and Rap1b might have more severe defects in activating β3 integrins. Knockdown of PIP5Kγ90 in lymphocytes does not prevent chemokine-triggered extension of β2 integrins but does prevent their conversion to the high-affinity conformation.21 In contrast, we found that PIP5Kγ90 contributes to selectin-induced slow rolling as well as chemokine-induced arrest on ICAM-1 (Figure 7). These observations suggest that PIP5Kγ90 facilitates both extension and hybrid-domain swing-out of β2 integrins, at least in neutrophils. Further studies are required to determine how PIP5Kγ90 affects inside-out signaling and activation of β2 integrins.
It has been proposed that selectin signaling in neutrophils bifurcates downstream of the adaptor SLP-76 into 2 parallel pathways, which cooperate to induce integrin-dependent slow rolling on ICAM-1.27,41,43,45 The first pathway serially activates p38 MAPK and Rap1a. The second pathway serially activates PI3Kγ and Rac1/Rac2. However, we previously observed normal selectin-triggered, integrin-dependent slow rolling of neutrophils lacking PI3Kγ or treated with PI3K inhibitors.28 Here, we observed normal selectin-induced slow rolling of neutrophils lacking both PI3Kγ and Rap1a. Instead, chemokines triggered PI3Kγ-dependent neutrophil arrest. At lower concentrations, chemokines cooperated with selectins to further slow rolling velocities. Both chemokine-induced slow rolling and arrest were eliminated in neutrophils lacking both Rap1a and PI3Kγ. We hypothesize that PI3Kγ-dependent slow rolling previously attributed to selectin signaling was instead caused by low-level chemokine signaling. The previous studies perfused blood from aorta-cannulated mice through flow chambers. The perfused blood contained detectable CXCL1 that could have influenced the results.39 Previous models of TNF- or thioglycollate-induced inflammation may have overestimated the ability of PTx to block chemokine signaling in endogenous neutrophils for prolonged periods.11,27-28,37,39,41,45,49 This could explain why some signaling pathways attributed to selectins, notably activation of PI3Kγ, could instead be caused by chemokines. Although we cannot exclude some contribution of selectins to activating PI3Kγ in neutrophils, our data indicate that chemokine-induced signals are dominant. Chemokine activation of PI3Kδ, another isoform in neutrophils, also contributes to arrest.28
How does PI3Kγ cooperate with Rap1a to facilitate chemokine-induced slow rolling and arrest of neutrophils? In chemokine-stimulated lymphocytes, PI3K does not increase the affinity of integrin αLβ2 for ICAM-1.26 Instead, PI3K increases the lateral mobility of αLβ2 to strengthen adhesion avidity. We found that Rap1 and PI3Kγ cooperatively increase both hybrid-domain swing-out and extension of β2 integrins in IL-8-stimulated human neutrophils in suspension, which prevents outside-in signaling through integrins bound to immobilized ligands. This suggests that PI3K also increases the affinity of αLβ2 for ICAM-1 (Figure 7). Unlike Rap1a, PI3K is not known to activate an effector that positions talin-1 to bind to β2 tails. However, both Rap1a and PI3K induce actin polymerization,50-52 and neutrophils require actomyosin tension for chemokines to induce hybrid-domain swing-out of β2 integrins and arrest on ICAM-1.38 This supports a model in which transition of αLβ2 from an extended, intermediate-affinity conformation to a high-affinity conformation requires alterations in the cytoskeleton that exert lateral forces to fully separate the αL and β2 tails.53 Recruitment of kindlin-3 to β2 tails may contribute to this process.54
Mice expressing a talin-1 mutant that converts β2 integrins to an intermediate-affinity conformation, but not a high-affinity conformation, cannot mobilize neutrophils in response to potent inflammatory stimuli.11 Thus, selectin signaling is not sufficient for neutrophil recruitment. Here we showed that neutrophils in Cxcr2−/− mice failed to arrest in the venules of the cremaster muscle after TNF challenge and did not migrate into the peritoneum after thioglycollate challenge. Thus, chemokine signaling is essential for neutrophil recruitment. It remained unclear whether signaling through selectins and chemokines in neutrophils could synergize when chemokines are limiting. Here we demonstrated that selectins do synergize with low-level chemokines to convert β2 integrins to intermediate- and high-affinity conformations that enhance slow rolling and arrest. Signaling through toll-like, adenosine, and transforming growth factor-β receptors also influences neutrophil function.36,55-56 Insights into how these pathways intersect may offer new approaches to treating inflammatory and thrombotic disorders.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Lawrence Quilliam, Gilbert White, Joseph Penninger, and Paul Kubes for mice and other materials, and Cindy Carter for technical assistance.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL034363, HL120846, and HL040387, and National Institute of General Medical Sciences grant GM114731.
Authorship
Contribution: T.Y. and N.Z. performed experiments; L.Z. and C.S.A. provided valuable mice and interpreted data; and T.Y. and R.P.M. designed and interpreted experiments and wrote the manuscript, with input from all authors.
Conflict-of-interest disclosure: R.P.M. is a cofounder of Selexys Pharmaceuticals, now part of Novartis AG, and of Tetherex Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Rodger P. McEver, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 N.E. 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.