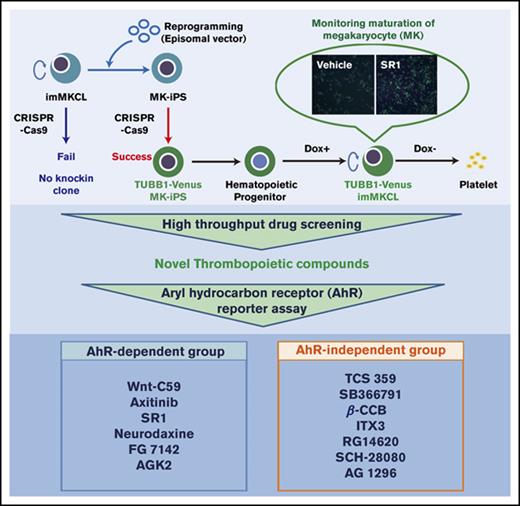
During maturation, megakaryocytes (MKs) express β1-tubulin (TUBB1) and rearrange their microtubule components to enlarge, form proplatelets, and eventually release platelets. The development of a platform to identify in vitro conditions that would efficiently promote MK development could potentially enable large-scale platelet production. Here, we show that an immortalized MK cell line (imMKCL) genetically modified to express the β1-tubulin–Venus reporter provides a practical system to efficiently monitor the in vitro production of platelet-like particles (PLPs). The Venus transgene was inserted downstream of the TUBB1 locus in imMKCLs using CRISPR/Cas9, and the expression was visualized by Venus fluorescence intensity. This imMKCL reporter line was then used for high-throughput drug screening. We identified several compounds that significantly improved the efficiency of PLP production in vitro under feeder-free conditions and showed a significant tendency to recover platelets in vivo in a mouse thrombocytopenia model induced by anti-GPIbα antibody administration. Interestingly, most of these compounds, including a WNT signaling pathway inhibitor, Wnt-C59, antagonized the aryl hydrocarbon receptor (AhR) to increase PLP production, confirming the crucial role of AhR inhibition in MK maturation. Consistently, small interfering RNA treatment against AhR increased the Venus intensity and PLP production. TCS 359, an FLT3 inhibitor, significantly increased PLP production independently of FLT3 or AhR. This study highlights the usefulness of the β1-tubulin reporter MK line as a useful tool to study the mechanisms underlying thrombopoiesis and to identify novel inducers of ex vivo platelet production.
Visual Abstract
Introduction
Platelets are anucleate blood cells derived from megakaryocytes (MKs) and play crucial roles in the blood clotting system, innate immunity, and the maintenance of blood vessel integrity.1,2 The current donor-derived platelet system is confronting several challenges, including difficulty in balancing supply and demand due to the short shelf life of platelets, the risk for viral or bacterial contamination, alloimmune-based platelet-transfusion refractoriness, and aging populations in developed countries that lower donor population size.2 As such, innovative systems that increase platelet supply independent of donors should be established.
Human induced pluripotent stem cells (iPSCs)3 or embryonic stem cells4 could serve as potential sources of MKs and platelets to meet clinical transfusion demands. Expandable MK cell lines made from these cells are considered suitable as master cells for producing platelets, and 2 groups have reported the generation of expandable MKs by the overexpression of specific sets of genes.5,6 Our group generated immortalized MK progenitor cell lines (imMKCLs) from iPSCs using a doxycycline (DOX)-inducible promoter to precisely control the expression of c-MYC, BMI1, and BCL-XL.6 imMKCLs are cryopreservable master cells that can be robustly expanded and then mature into MKs that release platelet-like particles (PLPs) upon switching off the expression of the aforementioned transgenes by removing DOX from the culture medium. However, imMKCLs exhibit a low maturity rate and require mouse mesenchymal feeder cells.2,7 The maturation of imMKCLs under feeder-free conditions is required to achieve clinically relevant large-scale platelet production. Accordingly, a platform that allows for the evaluation and optimization of MK development and in vitro PLP production in a high-throughput manner should be valuable.
Our conceptual strategy to address these requirements was to establish a reporter system to identify compounds that could facilitate the maturation of imMKCLs, which is exemplified by considerably enlarged diameter (∼100 μm), polyploidy, formation of a demarcation membrane system (DMS), elongation of proplatelets,8 and, finally, PLP release.9,10 The maturation phase of MKs accompanies the increased expression of the transcription factors GATA1,11 FOG1,12 FLI1,13 and NF-E2,14 the cell surface molecule CD42b (GPIbα),15 and the microtubule component molecule β1-tubulin (TUBB1).8 Microtubules, which are composed of heterodimers of mostly α4-tubulin and β1-tubulin, are the main cytoskeletal component of the enlarged and elongated cytoplasm in MKs.16,17 The expression of β1-tubulin is restricted to the MK/platelet lineage18 and is indispensable for the cytoskeletal rearrangement in MKs necessary for platelet formation.16
In the current study, we generated β1-tubulin–Venus reporter imMKCLs to visualize and evaluate the maturation process in real time using fluorescence intensity. This reporter line enabled high-throughput screening (HTS) through which we identified several compounds that robustly facilitate in vitro PLP production. The candidate compounds were classified into 2 groups that depend on their different types of action: inhibition of the aryl hydrocarbon receptor (AhR) pathway by the small molecule Wnt-C59 and activation of PLP production through an AhR-independent pathway by TCS 359. The reporter system was also compatible with small interfering RNA (siRNA) technology to identify key genes that regulate megakaryopoiesis and thrombopoiesis. This imMKCL-based reporter platform can be used to study the mechanisms underlying MK maturation and PLP production and applied to the high-throughput identification and validation of novel inducers of large-scale ex vivo platelet production.
Methods
Cell culture
imMKCLs (Cl-7 clone) were established from human iPSCs as previously reported.6,19 imMKCLs harbor 3 DOX-inducible transgenes: c-MYC, BMI1, and BCL-XL. Cl-7 clone was cultured in Iscove modified Dulbecco medium (Sigma) in the presence of recombinant human thrombopoietin (PeproTech) and recombinant human stem cell factor (R&D Systems) under feeder-free conditions. The addition of DOX turns on the expression of these transgenes (DOX-On), leading to imMKCL proliferation, whereas depletion of DOX (DOX-Off) induces their maturation. Candidate drugs were added at the beginning of the DOX-Off stage and were not replenished. MK differentiation from cord blood (CB) cells20 and direct MK differentiation from iPSCs21 were accomplished as described previously.
Reagents
The commercial drug libraries used are listed in supplemental Table 1. Information about the candidate compounds is provided in supplemental Materials and methods.
In vitro MK analysis
Cell surface marker analysis of imMKCLs and electron microscopy observations were performed as described previously.22 β1-tubulin–Venus reporter cells were seeded (30 000 cells per well) in 96-well microplates (Greiner Bio-One) and examined using a Cellomics ArrayScan VTI HCS Reader (Thermo Fisher Scientific, Waltham, MA) for automated image acquisition and morphometric analysis. Image analysis was performed using Cellomics scan software (Thermo Fisher Scientific).
Confocal quantitative imaging
After the depletion of DOX in medium, imMKCLs were cultured in a confocal quantitative image cytometer (CQ1; Yokogawa, Tokyo, Japan) for 7 days in the presence of thrombopoietin, stem cell factor, and the indicated drugs. A total of 1000 cells per well was seeded on an Elplasia micro-space cell culture 96-well plate (Kuraray), in which each well is divided into 200 squares to prevent cell aggregation. The morphological features of the cells were quantified sequentially. Data were obtained and analyzed using CellPathfinder (Yokogawa) and TIBCO Spotfire (TIBCO Software, Boston, MA). In brief, we set cutoff values of the cell surface area, Venus intensity, and other factors to select true cells among the cell-like particles recognized by the software. Afterward, we analyzed the average cell surface area time-course and the numbers of proplatelet-like cells in each condition. Proplatelet-bearing MKs were defined as cells with high circumference (>300 μm) and relatively low circularity (<0.5), which were validated visually.
Luciferase reporter gene assay
Reporter gene assays were performed as described previously with minor modifications.23 Huh7 cells were seeded into a 96-well plate at 1.5 × 104 cells per well and incubated for 24 hours in Dulbecco's modified Eagle medium supplemented with fetal bovine serum (Gibco). The cells were then cotransfected with hCYP1A1-pGL4.27 and pRL-TK-pGL4.74 overnight using Lipofectamine 3000 (Thermo Fisher Scientific), according to the manufacturer's instructions. The medium was then replaced with fresh Dulbecco's modified Eagle medium supplemented with charcoal-stripped fetal bovine serum (Gibco) containing the test compounds, followed by incubation for 24 hours, after which the cells were treated with 1 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (Cambridge Isotope Laboratories) overnight. Firefly and Renilla luciferase activities were evaluated using a Wallac Arvo Sx 1420 (PerkinElmer) with a Dual-Glo Luciferase Assay System (Promega).
Mice
Twelve-week-old male C57BL/6JRj mice were purchased from the Central Institute for Experimental Animals (Kawasaki, Japan). Thrombocytopenia was induced in the mice by anti-GPIbα at a dose of 200 ng/g body weight (IV) before drug administration. Peripheral platelet counts were determined using an XS-500i (Sysmex) automated hematology analyzer. All animal experiments were performed according to the Guidelines for Animal Experiments of Kyoto University and the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA). Data are expressed as the mean ± standard deviation (SD). One-way or 2-way analysis of variance (ANOVA), followed by multiple comparisons, was used to evaluate statistical differences. Other statistical evaluations were performed using the Student t test. Values of P < .05 were considered significant.
Results
Establishment of a β1-tubulin–Venus reporter line that allows for the direct visualization of MK maturation and PLP release
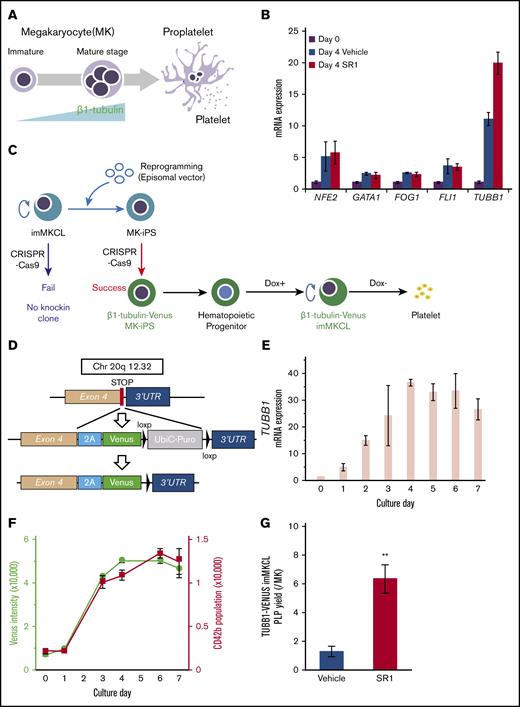
Immature MKs undergo a series of maturation events before they are able to shed platelets,8 and the expression of β1-tubulin increases gradually with maturation (Figure 1A). The transcriptional expression of markers for MK maturation, such as NF-E2, GATA1, FOG1, and FLI1, increase throughout MK development, and the addition of a small molecule AhR antagonist, StemRegenin 1 (SR1), greatly enhanced the expression of β1-tubulin during the DOX-Off stage in the imMKCL system (Figure 1B). Thus, SR1 was useful as a positive control for the upregulated β1-tubulin expression associated with MK maturation, which we also observed for feeder-independent maturation of imMKCLs through random screening of drugs.19
Establishment of a β1-tubulin–Venus imMKCL reporter line to fluorescently monitor MK maturation. (A) Schematic representation of MK maturation. The increased expression level of TUBB1 is induced in the terminal stage of MK maturation. (B) The mRNA expression of several markers of MK maturation. Samples were obtained from imMKCLs (Cl-7 clone; 2 × 105 cells) incubated in the absence or presence of SR1 (0.75 μM) on day 4 (DOX-Off stage). Gene expression levels were measured using quantitative real-time reverse transcription polymerase chain reaction and normalized to GAPDH expression. (C) Schematic illustration of the establishment of a β1-tubulin–Venus reporter line from secondary imMKCL-derived iPSCs. The imMKCL-derived secondary iPSCs were used to establish the reporter cell line with a green (Venus) fluorescent transgene, followed by redifferentiation into the imMKCL state. (D) CRISPR/Cas9-mediated knock-in strategy to generate β1-tubulin–Venus imMKCLs. A self-cleaving 2A peptide with a green (Venus) transgene was inserted downstream of exon 4. (E) The mRNA expression of TUBB1 increased upon MK maturation in β1-tubulin–Venus imMKCLs. Samples were obtained from β1-tubulin–Venus imMKCLs (2 × 105 cells) on the indicated days. (F) Venus intensity and CD42b+ population were increased upon the maturation of β1-tubulin–Venus imMKCLs. The Venus fluorescence intensity was assessed using an ArrayScan over 7 days. The CD42b+ population was detected using flow cytometry. (G) Effect of SR1 on PLP yield from β1-tubulin–Venus imMKCLs. Cells (2 × 105) were incubated in the absence or presence of SR1 (0.75 μM) for 7 days, and CD41+CD42b+ PLPs were detected and counted using flow cytometry. Data are expressed as the mean ± SD from 3 independent experiments. **P < .01 vs Vehicle, Student t test.
Establishment of a β1-tubulin–Venus imMKCL reporter line to fluorescently monitor MK maturation. (A) Schematic representation of MK maturation. The increased expression level of TUBB1 is induced in the terminal stage of MK maturation. (B) The mRNA expression of several markers of MK maturation. Samples were obtained from imMKCLs (Cl-7 clone; 2 × 105 cells) incubated in the absence or presence of SR1 (0.75 μM) on day 4 (DOX-Off stage). Gene expression levels were measured using quantitative real-time reverse transcription polymerase chain reaction and normalized to GAPDH expression. (C) Schematic illustration of the establishment of a β1-tubulin–Venus reporter line from secondary imMKCL-derived iPSCs. The imMKCL-derived secondary iPSCs were used to establish the reporter cell line with a green (Venus) fluorescent transgene, followed by redifferentiation into the imMKCL state. (D) CRISPR/Cas9-mediated knock-in strategy to generate β1-tubulin–Venus imMKCLs. A self-cleaving 2A peptide with a green (Venus) transgene was inserted downstream of exon 4. (E) The mRNA expression of TUBB1 increased upon MK maturation in β1-tubulin–Venus imMKCLs. Samples were obtained from β1-tubulin–Venus imMKCLs (2 × 105 cells) on the indicated days. (F) Venus intensity and CD42b+ population were increased upon the maturation of β1-tubulin–Venus imMKCLs. The Venus fluorescence intensity was assessed using an ArrayScan over 7 days. The CD42b+ population was detected using flow cytometry. (G) Effect of SR1 on PLP yield from β1-tubulin–Venus imMKCLs. Cells (2 × 105) were incubated in the absence or presence of SR1 (0.75 μM) for 7 days, and CD41+CD42b+ PLPs were detected and counted using flow cytometry. Data are expressed as the mean ± SD from 3 independent experiments. **P < .01 vs Vehicle, Student t test.
To visualize the MK maturation process using a fluorescent indicator, we used CRISPR-Cas9 editing to establish a β1-tubulin reporter line with imMKCL-derived iPSCs (Figure 1C-D). Given that iPSC-derived imMKCLs are not capable of genetic manipulation, imMKCLs were first “rereprogrammed” to iPSCs (secondary imMKCL-derived iPSCs). Next, cells were transfected first with Cas9 and a guide RNA (supplemental Figure 1A) carrying a self-cleaving 2A peptide, followed by a transgene encoding a green fluorescent protein (Venus) to the N terminus of the TUBB1 gene, after which the cells were redifferentiated into the imMKCL state (Figure 1C).
We then chose clone 12-23 for further study. This clone has 1 copy of the Venus construct relative to GAPDH (supplemental Figure 1B) and exhibited a continuous expansion under the control of the DOX-inducible promoter consistent with the original Cl-7 imMKCLs (supplemental Figure 1C). During the differentiation stage, the expression of TUBB1 messenger RNA (mRNA) gradually increased (Figure 1E; supplemental Figure 1D). Moreover, the intensity of the Venus fluorescence was synchronized with the increase in TUBB1 expression (supplemental Figure 1E) and was accompanied by an increase in the expression of CD42b, a surface marker of MK maturation (Figure 1F).
Inhibition of the AhR pathway reportedly promotes in vitro expansion of human hematopoietic stem cells and hematopoietic progenitor cells23,24 and MK progenitor growth.25 In addition, AhR-knockout mice exhibit an increased number of bone marrow hematopoietic stem cells.26 Accordingly, PLP generation from imMKCLs was significantly improved by SR1 addition (supplemental Figure 1F). SR1 also promoted PLP generation from β1-tubulin–Venus reporter cells (Figure 1G). These results indicate that the intensity of fluorescence from the β1-tubulin–Venus reporter line parallels MK maturation and could be used to screen for conditions that facilitate MK maturation.
Development of an HTS screening system to identify compounds that promote MK maturation
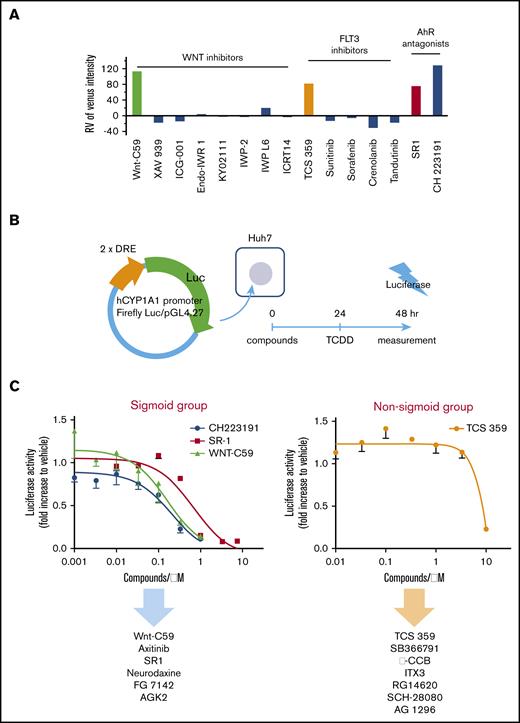
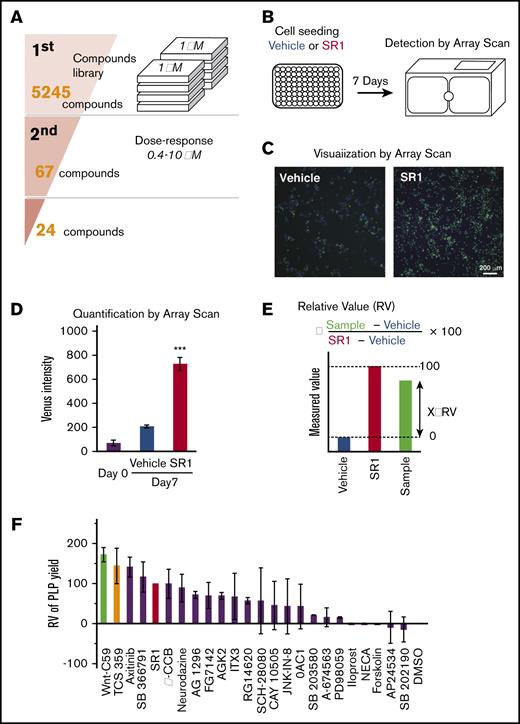
In this study, we used a β1-tubulin–Venus reporter line assay system to evaluate 5245 compounds from commercial libraries (supplemental Table 1) composed of oncology-focused agents that were prioritized for possible clinical relevance (Figure 2A). All data were normalized to the plate negative control (vehicle dosed) and the positive control (SR1 dosed). β1-tubulin–Venus reporter cells were cultured under static conditions for 7 days in the presence of vehicle, SR1, or the drug of interest. The fluorescence images were acquired using an ArrayScan reader (Figure 2B-C), and the Venus fluorescence intensity was analyzed and quantified using Cellomics Scan software (Figure 2D). The Z′ factor, which is an estimate of statistical effect and an index for assay quality control,27,28 was 0.62 for this system. Because a Z′ factor > 0.5 indicates an acceptable condition in robust screening assays,27 the reporter imMKCL-based screening was deemed competent for HTS (supplemental Figure 2A).
Development of an HTS system to discover compounds that facilitate MK maturation. (A) Schematic illustration of the screening procedure. From an initial pool of 5245 compounds, 67 compounds were selected based on the Venus fluorescence intensity in the first screening and were subjected to a second screening for further validation. Twenty-eight compounds improved MK maturation and subsequent PLP production. (B) Strategy for screening compounds that promote MK maturation based on the visualization of β1-tubulin–Venus expression. β1-tubulin–Venus imMKCLs (30 000 cells per well) were cultured in 96-well plates for 7 days in the presence of drugs. Venus fluorescence intensities were detected using an ArrayScan. (C) Representative fluorescent images of β1-tubulin–Venus imMKCL maturation in the absence or presence of SR1. The green fluorescent cells represent Venus-expressing cells. (D) Quantified Venus intensity of β1-tubulin–Venus imMKCLs cultured in the absence or presence of SR1. β1-tubulin–Venus imMKCLs were cultured in 96-well plates for 7 days in the absence or presence of SR1 (0.75 μM). (E) Formula for calculating relative values from measured ones. Data were normalized to the plate negative (Vehicle) and positive (SR1) controls. (F) Relative PLP production from imMKCLs treated with candidate compounds. β1-tubulin–Venus imMKCLs (2 × 105) were incubated for 7 days in the absence or presence of candidate drugs at their optimal concentrations (0.4-10 μM), after which CD41+CD42b+ PLPs were detected and counted using flow cytometry. Values were normalized to PLP production in the presence of SR1. Data are expressed as the mean ± SD from 3 to 5 independent experiments. ***P < .001 vs Vehicle, Student t test.
Development of an HTS system to discover compounds that facilitate MK maturation. (A) Schematic illustration of the screening procedure. From an initial pool of 5245 compounds, 67 compounds were selected based on the Venus fluorescence intensity in the first screening and were subjected to a second screening for further validation. Twenty-eight compounds improved MK maturation and subsequent PLP production. (B) Strategy for screening compounds that promote MK maturation based on the visualization of β1-tubulin–Venus expression. β1-tubulin–Venus imMKCLs (30 000 cells per well) were cultured in 96-well plates for 7 days in the presence of drugs. Venus fluorescence intensities were detected using an ArrayScan. (C) Representative fluorescent images of β1-tubulin–Venus imMKCL maturation in the absence or presence of SR1. The green fluorescent cells represent Venus-expressing cells. (D) Quantified Venus intensity of β1-tubulin–Venus imMKCLs cultured in the absence or presence of SR1. β1-tubulin–Venus imMKCLs were cultured in 96-well plates for 7 days in the absence or presence of SR1 (0.75 μM). (E) Formula for calculating relative values from measured ones. Data were normalized to the plate negative (Vehicle) and positive (SR1) controls. (F) Relative PLP production from imMKCLs treated with candidate compounds. β1-tubulin–Venus imMKCLs (2 × 105) were incubated for 7 days in the absence or presence of candidate drugs at their optimal concentrations (0.4-10 μM), after which CD41+CD42b+ PLPs were detected and counted using flow cytometry. Values were normalized to PLP production in the presence of SR1. Data are expressed as the mean ± SD from 3 to 5 independent experiments. ***P < .001 vs Vehicle, Student t test.
We next set up a β1-tubulin–Venus reporter line system to screen for compounds that facilitate MK maturation (Figure 2A). In a primary screening (1st), a series of libraries (supplemental Table 1) were screened in a 96-well format. Some of these libraries (ie, LOPAC 1280) are widely used, contain all major drug target classes, and have high pharmacological diversity. β1-tubulin–Venus reporter cells were cultured for 7 days in the presence of compounds from the libraries at a concentration of 1 μM. The effect of each drug was represented by an arbitrary value calculated relative to the values obtained with SR1 and vehicle (Figure 2E; supplemental Figure 2). Sixty-seven compounds with Venus intensity values >30 were selected for the second screening (2nd), in which 4 different doses within the range of 0.4 μM to 10 μM were applied. The 24 compounds that elicited dose-dependent increased β1-tubulin expression were considered candidates for further investigation.
The effects of the 24 candidate compounds on PLP production were then evaluated in a 6-well format. β1-tubulin–Venus reporter cells were cultured for 7 days in the presence of each candidate compound at an optimal concentration (supplemental Table 4) and then PLP production was evaluated using flow cytometry. Following normalization to the positive and negative controls discussed above, most of the candidates elicited a greater production of PLPs than did vehicle (dimethyl sulfoxide [DMSO] alone) (Figure 2F). As for the top 4 candidates, Wnt-C59 and SB 366791 seemed to have the same optimal concentration for Venus intensity and PLP production, whereas TCS 359 and axitinib at 10 μM resulted in the highest Venus intensity but less PLP production, suggesting an overdose for the PLP production (supplemental Figure 3A-B).
Identification and characterization of novel drugs that promote MK maturation and subsequent PLP production
We next focused on the top 2 drugs, Wnt-C59 and TCS 359, which are inhibitors of WNT and FLT3, respectively. The reporter cell line permits the visualization and evaluation of the degree and behavior of MK maturation in real time by a confocal quantitative image cytometer. Morphological changes assessed by sequential imaging analysis revealed that the surface areas of imMKCLs were enlarged by the addition of these drugs (Figure 3A). Furthermore, electronic micrographs of imMKCLs treated with the drugs showed significant development of the DMS, a hallmark of MK maturation (Figure 3B). These drugs showed marked effects on eliciting imMKCL maturation, as judged by upregulated TUBB1 levels (supplemental Figure 3C), enlarged surface area (Figure 3A), enhanced number of proplatelet-bearing MKs (supplemental Figure 4), and increased PLP yield (Figure 2F; supplemental Figure 5). The results suggested that increased PLP production results from a higher number of MKs that extend proplatelets rather than a higher degree of MK maturation. We also confirmed the structure of PLPs produced under the addition of the individual drugs using transmission electron microscopy (supplemental Figure 6) and that the levels of PAC-1 binding were comparable with human donor–derived platelets (Figure 3C).
Candidate compounds promote MK maturation and subsequent PLP production in vitro and in vivo. (A) Enlargement of imMKCL surface area upon exposure to candidate compounds. imMKCLs were incubated with SR1 (0.75 μM), TCS 359 (3.3 μM), Wnt-C59 (1.1 μM), or vehicle. (B) Representative electronic micrographs of imMKCLs showing extensive DMS development in the presence of the candidate compounds. imMKCLs were incubated for 6 days with each candidate compound or vehicle. Scale bars, 2μm. (C) PAC-1 binding to human donor platelets and imMKCL-derived PLPs was quantified in the absence or presence of 100 μM ADP and 40 μM TRAP-6 using flow cytometry. The change in mean fluorescence intensity (ΔMFI) was calculated as agonist (+) − agonist (−). (D) Candidate compounds promote PLP production from CB MNC-derived MKs. MNCs were isolated from human CB and differentiated into MK lineage. The CB MNC–derived MKs were incubated for 7 days with each candidate compound or vehicle. PLP yield per initial CD41a+ MK in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (E) Candidate compounds promote PLP production from MKs differentiated directly from iPSCs (direct iPSC-derived MKs). iPSCs (TkDN 3-4 clone) were first differentiated into hematopoietic progenitor cells in the presence of feeder cells, followed by MK lineage differentiation without intervention. Direct iPSC-derived MKs were incubated for 10 days with each candidate compound or vehicle. PLP yield per initial cell in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (F) Schema of mice experiments to evaluate the in vivo thrombopoietic effect of the candidate compounds. Platelets were depleted by the intravenous (i.v.) administration of anti-GPIbα (200 ng/g body weight) to male C57BL/6JRj mice. The mice were then divided into 4 groups and administered 100 μg/kg CH-223191, Wnt-C59, or TCS 359, or PBS (intraperitoneally [i.p.]) on the days indicated. Blood samples were collected before and after anti-GPIbα injection. (G) Promotion of in vivo platelet recovery in an anti-GPIbα–induced thrombocytopenic mouse model by the candidate compounds. Data are expressed as the mean ± S.D. from 3 to 5 independent experiments. *P < .05, **P < .01, ***P < .001 vs vehicle or PBS, 2-way ANOVA, followed by multiple comparisons (G) or 1-way analysis (C-E). N.S., not significant.
Candidate compounds promote MK maturation and subsequent PLP production in vitro and in vivo. (A) Enlargement of imMKCL surface area upon exposure to candidate compounds. imMKCLs were incubated with SR1 (0.75 μM), TCS 359 (3.3 μM), Wnt-C59 (1.1 μM), or vehicle. (B) Representative electronic micrographs of imMKCLs showing extensive DMS development in the presence of the candidate compounds. imMKCLs were incubated for 6 days with each candidate compound or vehicle. Scale bars, 2μm. (C) PAC-1 binding to human donor platelets and imMKCL-derived PLPs was quantified in the absence or presence of 100 μM ADP and 40 μM TRAP-6 using flow cytometry. The change in mean fluorescence intensity (ΔMFI) was calculated as agonist (+) − agonist (−). (D) Candidate compounds promote PLP production from CB MNC-derived MKs. MNCs were isolated from human CB and differentiated into MK lineage. The CB MNC–derived MKs were incubated for 7 days with each candidate compound or vehicle. PLP yield per initial CD41a+ MK in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (E) Candidate compounds promote PLP production from MKs differentiated directly from iPSCs (direct iPSC-derived MKs). iPSCs (TkDN 3-4 clone) were first differentiated into hematopoietic progenitor cells in the presence of feeder cells, followed by MK lineage differentiation without intervention. Direct iPSC-derived MKs were incubated for 10 days with each candidate compound or vehicle. PLP yield per initial cell in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (F) Schema of mice experiments to evaluate the in vivo thrombopoietic effect of the candidate compounds. Platelets were depleted by the intravenous (i.v.) administration of anti-GPIbα (200 ng/g body weight) to male C57BL/6JRj mice. The mice were then divided into 4 groups and administered 100 μg/kg CH-223191, Wnt-C59, or TCS 359, or PBS (intraperitoneally [i.p.]) on the days indicated. Blood samples were collected before and after anti-GPIbα injection. (G) Promotion of in vivo platelet recovery in an anti-GPIbα–induced thrombocytopenic mouse model by the candidate compounds. Data are expressed as the mean ± S.D. from 3 to 5 independent experiments. *P < .05, **P < .01, ***P < .001 vs vehicle or PBS, 2-way ANOVA, followed by multiple comparisons (G) or 1-way analysis (C-E). N.S., not significant.
To determine whether the positive effects of the noted drugs would also be seen with MK lineages produced by other methods, we assessed mononuclear cells (MNCs) isolated from human CB that were induced to differentiate into the MK lineage using the cytokine cocktail, including IL-6, shown in supplemental Figure 7A. In addition, we performed parallel experiments using MKs derived directly from iPSCs (supplemental Figure 7C). The results demonstrated that the candidate compounds also facilitated PLP production in CB MNC–derived and iPSC-derived MKs (Figure 3D-E; supplemental Figure 7B,D), indicating that the effect of these drugs is a consistent phenomenon in MK lineage, irrespective of the source of differentiated MKs or cytokines applied.
Given that WNT and FLT3 inhibitors are used extensively in clinical therapies, we next studied whether Wnt-C59 or TCS 359 could improve in vivo thrombopoiesis using a mouse thrombocytopenia model induced by the administration of anti-GPIbα antibody (200 ng/g body weight; Figure 3F). Because SR1 is a human specific AhR antagonist,23 we investigated the actions of Wnt-C59, TCS 359, and CH223191,24,29 an AhR antagonist nonselective for species. The 3 drugs showed a significant tendency to recover platelet levels compared with the control (phosphate-buffered saline; PBS) group (Figure 3G; supplemental Figure 8), suggesting that thrombocytopenia in mice was better restored by the 3 drugs.
Novel compounds induce MK maturation and PLP production through AhR-dependent and -independent mechanisms
We next sought to identify the mechanism of enhanced PLP production by these drugs. Among various WNT and FLT3 inhibitors included in the drug screening, only Wnt-C59 and TCS 359 enhanced Venus fluorescence intensity, suggesting that general WNT and FLT3 signaling pathways are not relevant (Figure 4A). We then evaluated their AhR-antagonizing effects using an AhR reporter gene assay (Figure 4B). Initially, a human hepatoma cell line (Huh 7) carrying a vector incorporating the firefly luciferase gene downstream of the AhR binding region (dioxin responsive elements) was generated. These cells were then treated with the noted drugs, and the luciferase activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin, an AhR agonist,23 was quantified by measuring the evoked luminescence.30 The dose-dependent inhibition of luciferase activity by CH223191 (blue circle), SR1 (red square), and Wnt-C59 (green triangle) approximates a sigmoid curve that is characteristic of competitive pharmacological antagonism (Figure 4C, left panel). In contrast, the dose-inhibition curve for TCS 359 showed its effect only at the highest concentrations in a nonsigmoid pattern that differed from a specific antagonist (Figure 4C, right panel). Based on their different patterns of inhibition, the compounds depicted in Figure 2F were classified into sigmoid or nonsigmoid groups (Figure 4C). Whereas the sigmoid group (ie, Wnt-C59, axitinib, SR1, neurodazine, FG 7142, and AGK2) facilitates PLP production via sufficient inhibition of AhR in a concentration-dependent manner, the nonsigmoid group (ie, TCS 359, SB 366791, ITX3, RG14620, SCH-28080, and AG 1296) is AhR independent at physiological concentrations.
AhR-dependent and -independent pathways to MK maturation identified using luciferase AhR reporter gene assay. (A) WNT and FLT3 signaling was not relevant to the enhancement of imMKCL maturation by Wnt-C59 and TCS 359. Shown are the effects of inhibitors of WNT and FLT3 signaling on Venus fluorescence intensity in β1-tubulin–Venus reporter imMKCLs. (B) Construction of a luciferase AhR reporter gene assay. The human CYP1A1 promoter region containing 2 AhR-recognition sequences was inserted into the XhoI-BglII site of pGL4.27. Huh7 cells were cotransfected with hCYP1A1-pGL4.27 and pRL-TK-pGL4.74 vectors overnight using Lipofectamine 3000, after which they were incubated with compounds for 24 hours, followed by treatment with 1 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin overnight. Firefly and Renilla luciferase activity was evaluated using a Wallac Arvo Sx 1420 (PerkinElmer) with a Dual-Glo Luciferase Assay kit. (C) Dose-response curves of the candidate compounds obtained with the luciferase AhR reporter gene assay. The candidate compounds were categorized into 2 groups based on whether their dose-response curves were sigmoid or nonsigmoid.
AhR-dependent and -independent pathways to MK maturation identified using luciferase AhR reporter gene assay. (A) WNT and FLT3 signaling was not relevant to the enhancement of imMKCL maturation by Wnt-C59 and TCS 359. Shown are the effects of inhibitors of WNT and FLT3 signaling on Venus fluorescence intensity in β1-tubulin–Venus reporter imMKCLs. (B) Construction of a luciferase AhR reporter gene assay. The human CYP1A1 promoter region containing 2 AhR-recognition sequences was inserted into the XhoI-BglII site of pGL4.27. Huh7 cells were cotransfected with hCYP1A1-pGL4.27 and pRL-TK-pGL4.74 vectors overnight using Lipofectamine 3000, after which they were incubated with compounds for 24 hours, followed by treatment with 1 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin overnight. Firefly and Renilla luciferase activity was evaluated using a Wallac Arvo Sx 1420 (PerkinElmer) with a Dual-Glo Luciferase Assay kit. (C) Dose-response curves of the candidate compounds obtained with the luciferase AhR reporter gene assay. The candidate compounds were categorized into 2 groups based on whether their dose-response curves were sigmoid or nonsigmoid.
Application of β1-tubulin–Venus reporter imMKCLs to siRNA-based assays
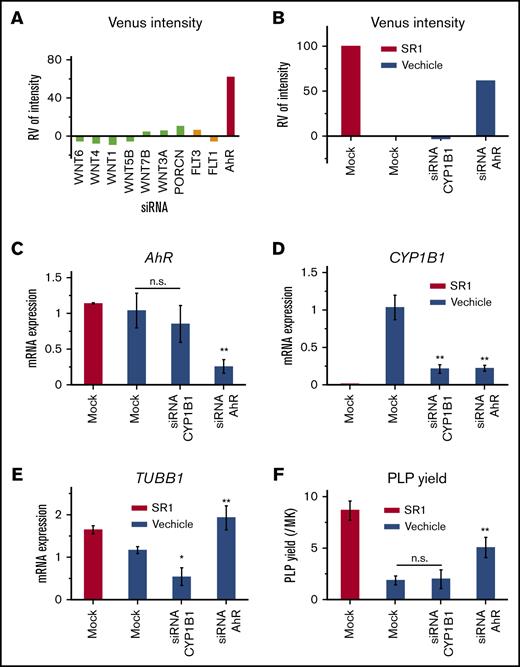
Finally, we sought to test whether the reporter system can be used for gene-knockdown experiments. The reporter cells were treated in a 96-well plate with siRNAs that target WNT family molecules (FLT3, FLT1 or AhR), matured, and analyzed for Venus intensity. In accordance with the results from inhibitor-based assays, knockdown of only AhR seemed to enhance the Venus intensity (Figure 5A). Upon maturation in the presence or absence of siRNAs that target AhR or the AhR downstream target CYP1B1,31 siRNAs that targeted AhR-enhanced, but not CYP1B1-enhanced, Venus intensity were consistent with SR1 treatment (Figure 5B). In accordance with the role of CYP1B1 as a target of AhR, AhR knockdown resulted in downregulation of the mRNA expression of AHR and CYP1B1, whereas CYP1B1 knockdown did not affect AHR levels (Figure 5C-D; supplemental Figure 9). TUBB1 mRNA level and PLP yield were increased by siRNA for AhR knockdown, whereas CYP1B1 knockdown lowered TUBB1 levels but had no effect on PLP yield (Figure 5E-F). These results using siRNA are in accordance with the regulatory role of AhR in thrombopoiesis32 and CYP1B1 as a gene induced by AhR,25 as well as suggest that this reporter system should also be applicable to siRNA-based screening.
Application of β1-tubulin–Venus reporter imMKCLs to siRNA-based assays. (A) Relative Venus intensity of β1-tubulin–Venus imMKCLs that were transfected with siRNAs specific for the indicated genes or nontarget. Cells were reversed transfected using the Stemfect RNA Transfection Kit and further matured for 7 days. Venus fluorescence intensities were detected using the ArrayScan and normalized to values of cells treated with mock siRNA in the presence of SR1 or DMSO. (B) Relative Venus intensity of β1-tubulin–Venus imMKCLs that were transfected with siRNAs specific for AhR, CYP1B1, or nontarget (Mock). Samples were also assessed for mRNA expression of AhR (C), CYP1B1 (D), or TUBB1 (E), as well as PLP yield (F). The mRNA expression levels were measured using quantitative real-time reverse transcription polymerase chain reaction and normalized to GAPDH. For PLP production, the cells were incubated for 7 days. CD41+CD42b+ PLPs were detected and counted using flow cytometry. Data are expressed as the mean ± SD from 3 independent experiments. *P < .05, **P < .01 vs Mock (without SR1), 1-way ANOVA. n.s., not significant (P > .5).
Application of β1-tubulin–Venus reporter imMKCLs to siRNA-based assays. (A) Relative Venus intensity of β1-tubulin–Venus imMKCLs that were transfected with siRNAs specific for the indicated genes or nontarget. Cells were reversed transfected using the Stemfect RNA Transfection Kit and further matured for 7 days. Venus fluorescence intensities were detected using the ArrayScan and normalized to values of cells treated with mock siRNA in the presence of SR1 or DMSO. (B) Relative Venus intensity of β1-tubulin–Venus imMKCLs that were transfected with siRNAs specific for AhR, CYP1B1, or nontarget (Mock). Samples were also assessed for mRNA expression of AhR (C), CYP1B1 (D), or TUBB1 (E), as well as PLP yield (F). The mRNA expression levels were measured using quantitative real-time reverse transcription polymerase chain reaction and normalized to GAPDH. For PLP production, the cells were incubated for 7 days. CD41+CD42b+ PLPs were detected and counted using flow cytometry. Data are expressed as the mean ± SD from 3 independent experiments. *P < .05, **P < .01 vs Mock (without SR1), 1-way ANOVA. n.s., not significant (P > .5).
Discussion
The establishment of imMKCLs represents an important advance toward generating large numbers of iPSC-derived platelets ex vivo, but the low PLP yield and requirement of mouse somatic feeder cells are major hurdles that must be overcome before clinical realization.33 In the current study, we present experimental and practical details of a β1-tubulin–Venus reporter line that promises to accelerate our search for optimal conditions for imMKCL maturation under feeder-free conditions. Key features of this reporter line are that the degree and behavior of MK maturation, including the size of MKs and the number of proplatelet-bearing MKs, can be monitored in real time by fluorescent signals quantitatively without much effort. This feature enables β1-tubulin–Venus reporter imMKCL to be an ideal reporter line for HTS.
By screening >5000 compounds, a series of candidates were identified from distinct categories that were not reported to promote MK maturation and PLP production (Figure 2). Wnt-C59 and TCS 359 are known inhibitors of WNT signaling and FLT3, respectively; however, other compounds exhibiting similar inhibitor effects on WNT and FLT3 signaling did not show similar effects in the reporter cell line (Figure 4A). This unique thrombopoietic property of Wnt-C59 and TCS 359 (Figure 3) may be advantageous in clinical settings, given that WNT and FLT3 inhibitors are used to treat leukemia and cancers34,35 that often manifest thrombocytopenia due to the disease itself or as a result of chemotherapy.
MK maturation is difficult to evaluate and quantify. During development, MKs increase in size, synthesize granules, and develop the DMS.8 Previous studies of MK maturation focused mainly on the expression of cell surface markers and MK-related genes36 or cellular structure.37 However, these approaches lack specificity, efficiency, or quantifiable outcomes. β1-tubulin is thought to be an effector protein, the level of which increases during MK development,16,38 and a key component in platelet release.39 The generated β1-tubulin–Venus reporter cell line allows the real-time monitoring of living MK development using fluorescence intensity without staining procedures, thus enabling the visualization and quantitative evaluation of maturation behaviors (Figure 2C-D; supplemental Figure 4). Development of the novel assay of proplatelet-bearing MKs would contribute to a detailed understanding of improved MK maturation induced by candidate compounds (supplemental Figure 4). The measured levels of Venus fluorescence were consistent with CD42b, a well-known marker of MK maturation (Figure 1F), which supports the reliability of the reporter cell line in maturation assays. Moreover, the high-throughput nature of the established platform allows an extensive and reproducible screening that cannot be achieved by conventional methods to assess MK maturation.
Interestingly, many of the identified candidates showed AhR antagonistic properties, whereas others were found to act through an AhR-independent mechanism at physiological doses (Figure 4). The AhR antagonistic properties of candidate compounds observed in this study are consistent with a positive effect of AhR activation during the progenitor stage, as well as a positive effect of AhR repression during the MK-differentiation stage.24 In addition, Strassel et al25 showed that AhR blockade, which is inversely correlated with CYP1B1 expression, improves the yield of proplatelet-producing MKs presumably by supplementing the roles of mesenchymal stromal cells in bone marrow. In the present study, the antagonistic action of SR1 or Wnt-C59 against AhR signaling markedly upregulated the expression of β1-tubulin but not that of NF-E2, a putative upstream regulator of β1-tubulin transcription. Although the exact mechanism is unclear, this effect suggests that AhR might contribute to upregulated β1-tubulin expression levels directly and independently of NF-E2. Notably, another promising application of this reporter cell line was the combination with siRNA technology (Figure 5). Although further studies are required to elucidate the mechanism, we are excited by the possibility of this reporter line uncovering the mechanisms involved in megakaryopoiesis and thrombopoiesis.
In summary, we have established a visible and quantifiable screening system for MK maturation based on β1-tubulin expression. The reporter line–based platforms should allow for more comprehensive investigations of the mechanisms underlying thrombopoiesis and fuel the development of novel compounds for manufacturing platelets ex vivo.
Acknowledgments
The authors thank Peter Karagiannis for critical reading of the manuscript and Natsumi Higashi for assisting with the animal experiments.
This work was supported in part by the Highway Program for Realization of Regenerative Medicine (JP17bm0504008) (K.E.), Practical Applications of Regenerative Medicine (JP17bk0104039) (K.E.), the Core Center for iPS Cell Research (JP17bm0104001) (N.S. and K.E.), the Japan Agency for Medical Research and Development, a Grant-in-Aid for Scientific Research (15H03005) (K.E.)/Japan Society for the Promotion of Science, and the iPS Cell Research Fund (S.J.C.). S.J.C. acknowledges financial support from the Japan Society for the Promotion of Science Postdoctoral Fellowship Program (P17122).
Koji Eto, Department of Clinical Application, Center for iPS Cell Research and Application, Kyoto University, 53 Kawaharacho, Shogoin, Sakyoku, Kyoto 606-8507, Japan email: kojieto@cira.kyoto-u.ac.jp.
Authorship
Contribution: H.S. and S.J.C. designed and performed the experiments, evaluated the data, and prepared the manuscript; K.H. assisted with the experiments; H.E. designed the reprogramming of imMKCL to MK iPSCs; Y.N. and A.O. provided guidance on ArrayScan technology; T.Y. provided guidance on data analysis of the microarray studies; A.H. provided guidance on the CRISPR-Cas9 experiments; A.S. performed the morphological analysis; H.H. and N.K. performed the luciferase AhR reporter gene assay; G.J.M. and K.F. provided guidance and intellectual contributions to the manuscript; N.S. provided guidance on the data interpretation and manuscript preparation; and K.E. managed the overall project, contributed to the data interpretation, and edited the manuscript.
Conflict-of-interest disclosure: H.S., S.J.C., K.H., Y.N., A.O., and K.E. have applied for patents related to this study. K.E. is a founder of Megakaryon Co. Ltd. The interests of K.E. were reviewed and are managed by Kyoto University in accordance with its conflict-of-interest policies. The remaining authors declare no competing financial interests.
References
Author notes
The full-text version of this article contains a data supplement.
H.S. and S.J.C. contributed equally to this work.

![Candidate compounds promote MK maturation and subsequent PLP production in vitro and in vivo. (A) Enlargement of imMKCL surface area upon exposure to candidate compounds. imMKCLs were incubated with SR1 (0.75 μM), TCS 359 (3.3 μM), Wnt-C59 (1.1 μM), or vehicle. (B) Representative electronic micrographs of imMKCLs showing extensive DMS development in the presence of the candidate compounds. imMKCLs were incubated for 6 days with each candidate compound or vehicle. Scale bars, 2μm. (C) PAC-1 binding to human donor platelets and imMKCL-derived PLPs was quantified in the absence or presence of 100 μM ADP and 40 μM TRAP-6 using flow cytometry. The change in mean fluorescence intensity (ΔMFI) was calculated as agonist (+) − agonist (−). (D) Candidate compounds promote PLP production from CB MNC-derived MKs. MNCs were isolated from human CB and differentiated into MK lineage. The CB MNC–derived MKs were incubated for 7 days with each candidate compound or vehicle. PLP yield per initial CD41a+ MK in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (E) Candidate compounds promote PLP production from MKs differentiated directly from iPSCs (direct iPSC-derived MKs). iPSCs (TkDN 3-4 clone) were first differentiated into hematopoietic progenitor cells in the presence of feeder cells, followed by MK lineage differentiation without intervention. Direct iPSC-derived MKs were incubated for 10 days with each candidate compound or vehicle. PLP yield per initial cell in the presence of vehicle (DMSO alone) was assigned a value of 1.0. (F) Schema of mice experiments to evaluate the in vivo thrombopoietic effect of the candidate compounds. Platelets were depleted by the intravenous (i.v.) administration of anti-GPIbα (200 ng/g body weight) to male C57BL/6JRj mice. The mice were then divided into 4 groups and administered 100 μg/kg CH-223191, Wnt-C59, or TCS 359, or PBS (intraperitoneally [i.p.]) on the days indicated. Blood samples were collected before and after anti-GPIbα injection. (G) Promotion of in vivo platelet recovery in an anti-GPIbα–induced thrombocytopenic mouse model by the candidate compounds. Data are expressed as the mean ± S.D. from 3 to 5 independent experiments. *P < .05, **P < .01, ***P < .001 vs vehicle or PBS, 2-way ANOVA, followed by multiple comparisons (G) or 1-way analysis (C-E). N.S., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/17/10.1182_bloodadvances.2018019547/1/m_blooda_adv-2024-716-gr3.jpeg?Expires=1763474084&Signature=MND3jhud92fbPrxyLAR0F3bykUedI5WTpIL-yw9ltj7ZZ-QKWu1OwwXgYTTj99S-36~t-L4DpPORryvspSh-L-wwtHTU3oBZEzO8v2KA6XXt8p6TwlEqoFayDbWrKbS8EnDqFfkpnqFFvwEeAxoyW7ODE5vhzGcQfhwCbwgr3rac6JAT-D52oXFzIk0jqLcJMlf4wzh~k1SxVfo5DBJJRrSCuTg1lqiAv4IoVyk2UELdH5Mxjn7L11PHde-m1K~hmnB6QsgsvYG-lSTn~WwisqJj43Z9~5scjtg2hJUalsdgZ3xduGU-0SVTtv3keOzByD7e-N82SiiTCOQu2TqQZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)