Key Points
Brief lenalidomide consolidation after chemoimmunotherapy is acceptably tolerated and extends PFS and OS in CLL.
FCR has superior efficacy compared with FR chemoimmunotherapy for CLL.
Abstract
Prior to novel targeted agents for chronic lymphocytic leukemia (CLL), the best chemoimmunotherapy regimen in patients with non-del(11q) disease was unclear. The role of lenalidomide was also not defined. This phase 2 study randomized 342 untreated patients with non-del(11q) CLL requiring therapy to fludarabine plus rituximab (FR; n = 123), FR plus lenalidomide consolidation (FR+L; n = 109), or FR plus cyclophosphamide (FCR; n = 110) and compared 2-year progression-free survival (PFS) rates of each to the historical control rate with FC (60%). Patients with del(11q) in at least 20% of pretreatment cells continued with FCR (n = 27) or were reassigned to FCR+L (n = 31) and excluded from the primary analysis. Among non-del(11q) patients, 2-year PFS rates were 64% (90% confidence interval [CI], 57-71; FR), 72% (90% CI, 65-79; FR+L), and 74% (90% CI, 66-80; FCR); FR+L and FCR had rates significantly greater than historical control. Median PFS was significantly shorter with FR compared with FR+L (P = .04) and FCR (P < .001): 43 (95% CI, 33-50), 61 (95% CI, 45-71), and 97 (95% CI, 61 to not reached) months, respectively. Median follow-up was 73 months and median overall survival (OS) was only reached with FCR (101 months; 95% CI, 96 to not reached). With FR+L, the risk of death decreased over time and was lower than with FR at later time points (P = .01), but not significantly different from FCR (P = .21). Future studies incorporating short courses of lenalidomide into other novel treatment regimens are justified.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia. A plethora of prognostic factors have been described that identify patients more likely to progress with symptoms. In many cases, prognostic factors such as immunoglobulin heavy chain variable (IGHV) mutational status,1,2 ZAP-70 protein expression,3,4 recurrent somatic mutations,5 and cytogenetics6 tie to the biology of CLL. CLL is treated upon development of symptoms or cytopenias, as there is no advantage with early intervention.7 The evolution of CLL therapy has been dramatic with acceptance of fludarabine-based8-10 or fludarabine plus cyclophosphamide (FC)–based11 therapy given with rituximab (FCR).12 Phase 2 studies suggested that survival was prolonged with fludarabine plus rituximab (FR) or FCR as compared with historical controls and indeed that a subset of patients receiving each were disease-free at 10 years.13,14 Many years ago, this prompted phase 3 studies in both relapsed and symptomatic, untreated CLL with FCR compared with FC, which demonstrated significant improvement with FCR in complete response (CR), progression-free survival (PFS), and overall survival (OS).15 These mature chemoimmunotherapy studies indicated that patients with del(11q) may benefit from cyclophosphamide as part of their chemoimmunotherapy.16 Further, retrospective studies demonstrated the adverse risk previously identified with del(11q) patients receiving FR may be abrogated with the addition of cyclophosphamide.17 However, FCR is also associated with short-term toxicity, including a 2% treatment-related mortality often from infection, and also late onset therapy-related myeloid neoplasia.13,17 Thus, interest in the field at the time of this unreported, but very mature study existed to (1) determine the feasibility of risk stratification based upon interphase cytogenetic results in a multicenter study; (2) determine whether traditional and novel combination chemoimmunotherapy regimens might improve the 2-year PFS rate above the 60% reported in patients receiving FC18 and thereby justify subsequent studies of 1 or more of these; and (3) determine whether consolidation after chemoimmunotherapy was feasible and improved 2-year PFS rates. The value of the primary end point of the study is now of minimal value to the field, but of interest is the potential of short-term immune-modulation therapy after receipt of chemoimmunotherapy. Using lenalidomide as this immune modulator and only administering it for 6 months using a 21 days on/7 days off schedule, this trial differs from all other maintenance studies performed with lenalidomide. Despite the very modest value of the chemoimmunotherapy question addressed in this trial given advances in CLL therapy, the application of lenalidomide might be informative to the field.
Lenalidomide is one promising therapeutic agent active in CLL and is approved for treatment of multiple myeloma, transfusion-dependent anemia due to low- or intermediate-1-risk del 5q myelodysplastic syndromes, and relapsed or refractory mantle cell lymphoma. Lenalidomide promotes selective protein degradation through enhancing the activation of cereblon,19-21 resulting in depletion of IKZF1, IKZF3, and other target proteins that are important in these diseases. Despite promising early studies of lenalidomide in CLL that demonstrated durable responses,22-24 its development has been challenging due to toxicity and uncertainty about dose and schedule.24 One particular toxicity is tumor flare,25 which may be diminished with cytoreduction prior to administering lenalidomide.26,27 In 1 study examining low-dose lenalidomide as consolidation therapy following pentostatin, cyclophosphamide, and rituximab (PCR), lenalidomide improved response in ∼25% of patients and time to next treatment appeared to be improved when compared with historical control with PCR alone.27
This Cancer and Leukemia Group B (CALGB) 10404 study is a randomized phase 2 trial in untreated, symptomatic CLL patients that addressed both the feasibility of genomic classification by interphase cytogenetics and also the value of different chemoimmunotherapies (FR, FCR) and addition of brief lenalidomide consolidation. CALGB is now a part of the Alliance for Clinical Trials in Oncology.
Patients and methods
Patients
This National Cancer Institute (NCI)–sponsored, North American intergroup study (NCT00602459) led by Alliance/CALGB was approved by the local site institutional review board (locations in the supplemental Appendix). All patients provided written informed consent. Patients enrolled on this study were 18 years of age and older, had CLL with an absolute lymphocytosis of >5 × 109/L, immunophenotype typical of CLL, and features defined by the International Workshop on CLL (IWCLL) 2008 guidelines.28 All patients had symptomatic intermediate (Rai29 1 or 2) or high risk (Rai 3 or 4) stage CLL and a performance status of 2 or better. Patients had no prior therapy for CLL and a creatinine ≤1.5× the upper limit of normal. Patients were excluded if they required chronic administration of corticosteroids for other medical conditions. Due to the unknown teratogenic potential of lenalidomide, pregnant or nursing patients were not enrolled.
Treatment
Following enrollment, patients were assessed centrally for the presence or absence of del(11q) by interphase cytogenetics. Within strata defined by Rai stage at diagnosis, patients were then randomized to 1 of 3 treatment regimens: arm A, FR; arm B, FR with consolidative lenalidomide (FR+L); and arm C, FCR (Table 1). Interphase cytogenetics available prior to cycle 2 of therapy were then used to risk stratify patients. Patients without del(11q) in ≥20% of cells remained in their randomized treatment arms, whereas in view of better outcomes to FCR, patients with del(11q) in ≥20% of cells initially randomized to arms A and B were transitioned to arm D, where FCR was administered followed by consolidative lenalidomide (FCR+L). Patients initially randomized to arm C with FCR remained in that arm regardless of the del(11q) result. Allopurinol 300 mg orally once daily was given for days 1 to 14 of treatment. TMP-sulfa and acyclovir (or equivalent) prophylaxis was strongly recommended. Granulocyte colony-stimulating factors were allowed per American Society of Clinical Oncology guidelines.30 Six cycles of induction therapy were planned. Patients in all arms underwent response assessment ∼10 months after beginning induction therapy and patients in arms B and D who had stable disease or better with an absolute neutrophil count of ≥1 × 109/L, platelets ≥100 × 109/L, and estimated glomerular filtration rate ≥30 mL per minute proceeded to receive up to 6 lenalidomide consolidation cycles (21 days every 28 days). Details regarding dose modifications are outlined in the supplemental Methods.
Treatment in CALGB 10404
| Time* . | Arm A: FR . | Arm B: FR+L . | Arm C: FCR . | Arm D: FCR+L . |
|---|---|---|---|---|
| Month 1 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-3† | NA |
| Rituximab 50 mg/m2 IVPB day 1 | Rituximab 50 mg/m2 IVPB day 1 | Cyclophosphamide 250 mg/m2 days 1-3† | ||
| Rituximab 325 mg/m2 IVPB day 3 | Rituximab 325 mg/m2 IVPB day 3 | Rituximab 50 mg/m2 IVPB day 1 | ||
| Rituximab 375 mg/m2 IVPB day 5 | Rituximab 375 mg/m2 IVPB day 5 | Rituximab 325 mg/m2 IVPB day 2 | ||
| Months 2-6 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-3† | Fludarabine 25 mg/m2 IV days 1-3† |
| Rituximab 375 mg/m2 IVPB day 1 | Rituximab 375 mg/m2 IVPB day 1 | Cyclophosphamide 250 mg/m2 days 1-3† | Cyclophosphamide 250 mg/m2 days 1-3† | |
| Rituximab 500 mg/m2 IVPB day 1 | Rituximab 500 mg/m2 IVPB day 1 | |||
| End of 9 mo | Full staging | Full staging | Full staging | Full staging |
| Month 10 | NA | Lenalidomide 5 mg by mouth days 1-21 | NA | Lenalidomide 5 mg by mouth days 1-21 |
| Months 11-15 | NA | Lenalidomide 10 mg by mouth days 1-21 | NA | Lenalidomide 10 mg by mouth days 1-21 |
| End of 18 mo | NA | Full staging | NA | Full staging |
| End of 24 mo | Full staging | Full staging | Full staging | Full staging |
| Time* . | Arm A: FR . | Arm B: FR+L . | Arm C: FCR . | Arm D: FCR+L . |
|---|---|---|---|---|
| Month 1 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-3† | NA |
| Rituximab 50 mg/m2 IVPB day 1 | Rituximab 50 mg/m2 IVPB day 1 | Cyclophosphamide 250 mg/m2 days 1-3† | ||
| Rituximab 325 mg/m2 IVPB day 3 | Rituximab 325 mg/m2 IVPB day 3 | Rituximab 50 mg/m2 IVPB day 1 | ||
| Rituximab 375 mg/m2 IVPB day 5 | Rituximab 375 mg/m2 IVPB day 5 | Rituximab 325 mg/m2 IVPB day 2 | ||
| Months 2-6 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-5 | Fludarabine 25 mg/m2 IV days 1-3† | Fludarabine 25 mg/m2 IV days 1-3† |
| Rituximab 375 mg/m2 IVPB day 1 | Rituximab 375 mg/m2 IVPB day 1 | Cyclophosphamide 250 mg/m2 days 1-3† | Cyclophosphamide 250 mg/m2 days 1-3† | |
| Rituximab 500 mg/m2 IVPB day 1 | Rituximab 500 mg/m2 IVPB day 1 | |||
| End of 9 mo | Full staging | Full staging | Full staging | Full staging |
| Month 10 | NA | Lenalidomide 5 mg by mouth days 1-21 | NA | Lenalidomide 5 mg by mouth days 1-21 |
| Months 11-15 | NA | Lenalidomide 10 mg by mouth days 1-21 | NA | Lenalidomide 10 mg by mouth days 1-21 |
| End of 18 mo | NA | Full staging | NA | Full staging |
| End of 24 mo | Full staging | Full staging | Full staging | Full staging |
Patients with del(11q) disease initially randomized to arms A or B were reassigned to arm D after the first month.
IVPB, IV piggyback; NA, not applicable.
Each cycle of therapy was 28 days.
Patients 70 years of age and older received fludarabine 20 mg/m2 and cyclophosphamide 150 mg/m2.
Interphase cytogenetics
Stimulated fluorescent in situ hybridization (FISH) analyses were performed on peripheral blood or bone marrow samples, as previously described.31 FISH analyses probed for the chromosome 12 centromere, ATM (11q22.3), D13S319 (13q14.3), and TP53 (17p13.1) (Abbott Molecular Inc, Des Plaines, IL). For risk stratification based upon del(11)(q22.3) positivity, patients were required to have ≥20% of cells with loss of 1 copy.
Adverse event and response assessment
Adverse events were assessed by the NCI Common Terminology Criteria for Adverse Events version 3.0 and was changed to 4.0 in October 2010. As the regimens used in this trial have been widely studied and published, only grade 3-5 adverse events are reported, regardless of attribution. For response assessment, this study used the IWCLL 2008 response criteria which includes clinical, hematologic, and bone marrow features along with requirement of computed tomography scan examination.28 Evaluation of resolution of splenomegaly was by physical examination. Responses were assessed according to the schedule detailed in the supplemental Methods, and results were reviewed and confirmed by the principal investigator of this study (J.C.B.) in all cases.
Statistics
The primary objective of this randomized phase 2 study was to compare the 2-year PFS rates for FR, FR+L, and FCR with historical control data for FC18 in non-del(11q) patients. With a targeted 103 patients per arm with non-del(11q), there was at least 84% power to detect an improvement in 2-year PFS rate from 60% to 73%, using a critical value of 69% and constraining the one-sided type I error rate to 4%. Two-year PFS rate was defined as the percentage of evaluable patients who were alive and progression-free at 2 years. Patients who were lost to follow-up, received a nonprotocol CLL therapy, or had a documented progression or death prior to 2 years were considered treatment failures. Transplant prior to progression was only documented in 4 patients, and thus was not accounted for when calculating the PFS rate. Two-year PFS rates and their exact 90% confidence intervals (CIs) are reported within each arm separately as the primary analysis.
Secondary end points included time-to-event PFS and OS, overall response rate (ORR), and CR rate. PFS was defined as time from randomization until the first date of progression or death from any cause, censoring patients alive and progression-free at the date of last clinical assessment. Patients who received nonprotocol CLL therapy (other than transplant) prior to a documented progression were censored at the start date of the subsequent therapy. OS was defined as time from randomization until the date of death from any cause, censoring those alive at the date of last contact. Overall response was defined as complete or partial remission. Logistic regression and proportional hazard models were used to assess the impact of baseline characteristics of patients with non-del(11q) on response and PFS/OS, respectively.32,33
Detailed analysis methods for secondary comparisons are located in the supplemental Methods. All reported P values are 2-sided. All data were collected by the Alliance Statistics and Data Center (SDC) and analyses were performed by the Alliance SDC using SAS version 9.4. Data were locked for this analysis as of 23 August 2017.
Results
Enrollment, feasibility of treatment assignment, and patient characteristics
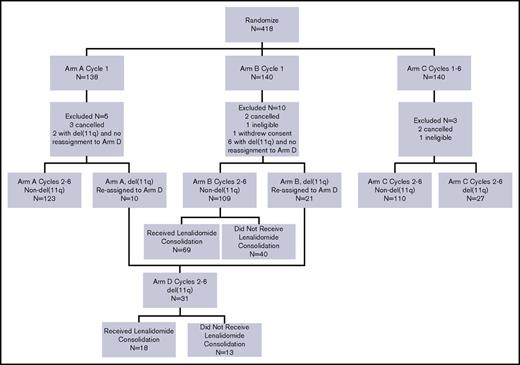
A total of 418 patients enrolled on this study between March 2008 and August 2012. As depicted in the CONSORT diagram (Figure 1), 400 patients are included in this analysis. Of the 18 patients not included, 7 never started treatment, 2 were ineligible, 1 withdrew consent for use of all data, and 8 with del(11q) had no reassignment to arm D (they were assigned to arm A or B prior to an amendment adding arm D). In addition, 15 patients did not have interphase cytogenetics results; a sample was not received by the central laboratory in 12, and a FISH result was not obtained in 3. These patients were included in the analysis within the arms to which they were initially randomized. Thus, central assignment of treatment by interphase cytogenetics by the second cycle of therapy was feasible in 385 patients (96%).
CONSORT diagram. Arm A = FR. Arm B = FR+L. Arm C = FCR. Arm D = FCR+L.
Baseline characteristics of the 400 patients are presented by treatment arm and by del(11q) status. Characteristics for 342 non-del(11q) patients were similar across treatment arms except for a higher frequency of hepatomegaly among the FCR treated patients (Table 2). Characteristics for 58 del(11q) patients were also similar between treatment arms (Table 3). Patients with del(11q) have similar characteristics as those without this abnormality, with exception of bulky (>5 cm) lymph nodes (supplemental Table 1).
Baseline characteristics of patients evaluated on the non-del(11q) treatment arms
| Variable . | FR, N = 123 . | FR+L, N = 109 . | FCR, N = 110 . | P* . |
|---|---|---|---|---|
| Median age (range), y | 61 (28-81) | 62 (36-79) | 60 (32-80) | .97 |
| Sex, n (%) | .76 | |||
| Male | 83 (67) | 69 (63) | 70 (64) | |
| Female | 40 (33) | 40 (37) | 40 (36) | |
| Rai stage, no. (%) | .98 | |||
| I | 26 (21) | 23 (21) | 23 (21) | |
| II | 40 (33) | 31 (28) | 37 (34) | |
| III | 25 (20) | 22 (20) | 21 (19) | |
| IV | 32 (26) | 33 (30) | 29 (26) | |
| ECOG PS, no. (%) | .81 | |||
| 0 | 61 (50) | 60 (55) | 60 (55) | |
| 1 | 58 (47) | 44 (40) | 47 (43) | |
| 2 | 4 (3) | 5 (5) | 3 (3) | |
| Median hemoglobin (range), g/L | 128 (59-161) | 120 (59-162) | 119 (69-173) | .18 |
| Median platelets (range), ×109/L | 151 (48-486) | 133 (24-410) | 133 (12-303) | .46 |
| Median WBC (range), ×109/L | 72.9 (2.2-424.9) | 92.6 (6.2-476.9) | 80.8 (1.2-899.8) | .52 |
| B2M above normal, no. (%) | .79 | |||
| No | 22 (18) | 16 (15) | 17 (16) | |
| Yes | 97 (82) | 89 (85) | 87 (84) | |
| LDH above normal, no. (%) | .07 | |||
| No | 57 (46) | 64 (59) | 48 (44) | |
| Yes | 66 (54) | 45 (41) | 60 (56) | |
| Serum creatinine above normal, no. (%) | .44 | |||
| No | 109 (90) | 100 (92) | 95 (86) | |
| Yes | 12 (10) | 9 (8) | 15 (14) | |
| Palpable splenomegaly, no. (%) | .37 | |||
| No | 71 (60) | 56 (52) | 55 (51) | |
| Yes | 48 (40) | 52 (48) | 52 (49) | |
| Palpable hepatomegaly, no. (%) | .005 | |||
| No | 116 (98) | 98 (94) | 93 (88) | |
| Yes | 2 (2) | 6 (6) | 13 (12) | |
| Adenopathy, no. (%) | .50 | |||
| No adenopathy | 4 (3) | 2 (2) | 3 (3) | |
| Yes, lymph node <5cm | 80 (70) | 74 (72) | 80 (74) | |
| Yes, lymph node 5-10cm | 24 (21) | 23 (22) | 15 (14) | |
| Yes, lymph node >10cm | 7 (6) | 4 (4) | 10 (9) | |
| del(17p), no. (%) | .90 | |||
| Absent | 106 (89) | 92 (91) | 96 (90) | |
| Present | 13 (11) | 9 (9) | 11 (10) | |
| del(11q),†no. (%) | .92 | |||
| Absent | 111 (93) | 94 (93) | 101 (94) | |
| Present | 8 (7) | 7 (7) | 6 (6) | |
| Trisomy 12, no. (%) | .30 | |||
| Absent | 90 (76) | 79 (78) | 74 (69) | |
| Present | 29 (24) | 22 (22) | 33 (31) | |
| del(13q), no. (%) | .14 | |||
| Absent | 60 (50) | 39 (39) | 54 (50) | |
| Present | 59 (50) | 62 (61) | 53 (50) | |
| Hierarchical cytogenetic classification, no. (%) | .56 | |||
| del(17p) | 13 (11) | 9 (9) | 11 (10) | |
| del(11q) | 7 (6) | 6 (6) | 4 (4) | |
| Trisomy 12 | 25 (21) | 20 (20) | 31 (29) | |
| None | 23 (19) | 19 (19) | 26 (24) | |
| del(13q) | 51 (43) | 47 (47) | 35 (33) |
| Variable . | FR, N = 123 . | FR+L, N = 109 . | FCR, N = 110 . | P* . |
|---|---|---|---|---|
| Median age (range), y | 61 (28-81) | 62 (36-79) | 60 (32-80) | .97 |
| Sex, n (%) | .76 | |||
| Male | 83 (67) | 69 (63) | 70 (64) | |
| Female | 40 (33) | 40 (37) | 40 (36) | |
| Rai stage, no. (%) | .98 | |||
| I | 26 (21) | 23 (21) | 23 (21) | |
| II | 40 (33) | 31 (28) | 37 (34) | |
| III | 25 (20) | 22 (20) | 21 (19) | |
| IV | 32 (26) | 33 (30) | 29 (26) | |
| ECOG PS, no. (%) | .81 | |||
| 0 | 61 (50) | 60 (55) | 60 (55) | |
| 1 | 58 (47) | 44 (40) | 47 (43) | |
| 2 | 4 (3) | 5 (5) | 3 (3) | |
| Median hemoglobin (range), g/L | 128 (59-161) | 120 (59-162) | 119 (69-173) | .18 |
| Median platelets (range), ×109/L | 151 (48-486) | 133 (24-410) | 133 (12-303) | .46 |
| Median WBC (range), ×109/L | 72.9 (2.2-424.9) | 92.6 (6.2-476.9) | 80.8 (1.2-899.8) | .52 |
| B2M above normal, no. (%) | .79 | |||
| No | 22 (18) | 16 (15) | 17 (16) | |
| Yes | 97 (82) | 89 (85) | 87 (84) | |
| LDH above normal, no. (%) | .07 | |||
| No | 57 (46) | 64 (59) | 48 (44) | |
| Yes | 66 (54) | 45 (41) | 60 (56) | |
| Serum creatinine above normal, no. (%) | .44 | |||
| No | 109 (90) | 100 (92) | 95 (86) | |
| Yes | 12 (10) | 9 (8) | 15 (14) | |
| Palpable splenomegaly, no. (%) | .37 | |||
| No | 71 (60) | 56 (52) | 55 (51) | |
| Yes | 48 (40) | 52 (48) | 52 (49) | |
| Palpable hepatomegaly, no. (%) | .005 | |||
| No | 116 (98) | 98 (94) | 93 (88) | |
| Yes | 2 (2) | 6 (6) | 13 (12) | |
| Adenopathy, no. (%) | .50 | |||
| No adenopathy | 4 (3) | 2 (2) | 3 (3) | |
| Yes, lymph node <5cm | 80 (70) | 74 (72) | 80 (74) | |
| Yes, lymph node 5-10cm | 24 (21) | 23 (22) | 15 (14) | |
| Yes, lymph node >10cm | 7 (6) | 4 (4) | 10 (9) | |
| del(17p), no. (%) | .90 | |||
| Absent | 106 (89) | 92 (91) | 96 (90) | |
| Present | 13 (11) | 9 (9) | 11 (10) | |
| del(11q),†no. (%) | .92 | |||
| Absent | 111 (93) | 94 (93) | 101 (94) | |
| Present | 8 (7) | 7 (7) | 6 (6) | |
| Trisomy 12, no. (%) | .30 | |||
| Absent | 90 (76) | 79 (78) | 74 (69) | |
| Present | 29 (24) | 22 (22) | 33 (31) | |
| del(13q), no. (%) | .14 | |||
| Absent | 60 (50) | 39 (39) | 54 (50) | |
| Present | 59 (50) | 62 (61) | 53 (50) | |
| Hierarchical cytogenetic classification, no. (%) | .56 | |||
| del(17p) | 13 (11) | 9 (9) | 11 (10) | |
| del(11q) | 7 (6) | 6 (6) | 4 (4) | |
| Trisomy 12 | 25 (21) | 20 (20) | 31 (29) | |
| None | 23 (19) | 19 (19) | 26 (24) | |
| del(13q) | 51 (43) | 47 (47) | 35 (33) |
Percent values are calculated out of the number of patients with nonmissing data in each category.
B2M, β-2 microglobulin; ECOG PS indicates Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase; WBC, white blood cell count.
χ2 or Fisher’s exact and Kruskal-Wallis P values are presented for categoric and continuous variables, respectively.
The del(11q) category includes patients with del(11q) detected in >2.3% of cells (above background) and in <20% of cells.
Baseline characteristics of patients with del(11q) disease
| Variable . | FCR, N = 27 . | FCR+L, N = 31 . | P* . |
|---|---|---|---|
| Median age (range), y | 60 (41-78) | 59 (30-75) | .50 |
| Sex, no. (%) | .27 | ||
| Male | 16 (59) | 23 (74) | |
| Female | 11 (41) | 8 (26) | |
| Rai stage, no. (%) | .25 | ||
| I | 4 (15) | 8 (26) | |
| II | 8 (30) | 14 (45) | |
| III | 6 (22) | 4 (13) | |
| IV | 9 (33) | 5 (16) | |
| ECOG PS, no. (%) | .89 | ||
| 0 | 16 (59) | 17 (55) | |
| 1 | 10 (37) | 13 (42) | |
| 2 | 1 (4) | 1 (3) | |
| Median hemoglobin (range), g/L | 121 (70-154) | 120 (35-151) | .83 |
| Median platelets (range), ×109/L | 125 (37-275) | 159 (44-375) | .34 |
| Median WBC (range), ×109/L | 69.1 (10.6-289.0) | 77.1 (6.3-244.9) | .86 |
| B2M above normal, no. (%) | .51 | ||
| No | 7 (29) | 5 (18) | |
| Yes | 17 (71) | 23 (82) | |
| LDH above normal, no. (%) | .59 | ||
| No | 15 (58) | 14 (48) | |
| Yes | 11 (42) | 15 (52) | |
| Serum creatinine above normal, no. (%) | 1.00 | ||
| No | 25 (96) | 28 (93) | |
| Yes | 1 (4) | 2 (7) | |
| Palpable splenomegaly, no. (%) | .79 | ||
| No | 14 (52) | 18 (58) | |
| Yes | 13 (48) | 13 (42) | |
| Palpable hepatomegaly, no. (%) | .36 | ||
| No | 26 (96) | 26 (87) | |
| Yes | 1 (4) | 4 (13) | |
| Adenopathy, no. (%) | 1.00 | ||
| No adenopathy | 0 (0) | 0 (0) | |
| Yes, lymph node <5 cm | 12 (50) | 15 (52) | |
| Yes, lymph node 5-10 cm | 10 (42) | 12 (41) | |
| Yes, lymph node >10 cm | 2 (8) | 2 (7) | |
| del(17p), no. (%) | 1.00 | ||
| Absent | 26 (96) | 29 (94) | |
| Present | 1 (4) | 2 (6) | |
| Trisomy 12, no. (%) | .74 | ||
| Absent | 23 (85) | 25 (81) | |
| Present | 4 (15) | 6 (19) | |
| del(13q), no. (%) | .18 | ||
| Absent | 13 (48) | 9 (29) | |
| Present | 14 (52) | 22 (71) |
| Variable . | FCR, N = 27 . | FCR+L, N = 31 . | P* . |
|---|---|---|---|
| Median age (range), y | 60 (41-78) | 59 (30-75) | .50 |
| Sex, no. (%) | .27 | ||
| Male | 16 (59) | 23 (74) | |
| Female | 11 (41) | 8 (26) | |
| Rai stage, no. (%) | .25 | ||
| I | 4 (15) | 8 (26) | |
| II | 8 (30) | 14 (45) | |
| III | 6 (22) | 4 (13) | |
| IV | 9 (33) | 5 (16) | |
| ECOG PS, no. (%) | .89 | ||
| 0 | 16 (59) | 17 (55) | |
| 1 | 10 (37) | 13 (42) | |
| 2 | 1 (4) | 1 (3) | |
| Median hemoglobin (range), g/L | 121 (70-154) | 120 (35-151) | .83 |
| Median platelets (range), ×109/L | 125 (37-275) | 159 (44-375) | .34 |
| Median WBC (range), ×109/L | 69.1 (10.6-289.0) | 77.1 (6.3-244.9) | .86 |
| B2M above normal, no. (%) | .51 | ||
| No | 7 (29) | 5 (18) | |
| Yes | 17 (71) | 23 (82) | |
| LDH above normal, no. (%) | .59 | ||
| No | 15 (58) | 14 (48) | |
| Yes | 11 (42) | 15 (52) | |
| Serum creatinine above normal, no. (%) | 1.00 | ||
| No | 25 (96) | 28 (93) | |
| Yes | 1 (4) | 2 (7) | |
| Palpable splenomegaly, no. (%) | .79 | ||
| No | 14 (52) | 18 (58) | |
| Yes | 13 (48) | 13 (42) | |
| Palpable hepatomegaly, no. (%) | .36 | ||
| No | 26 (96) | 26 (87) | |
| Yes | 1 (4) | 4 (13) | |
| Adenopathy, no. (%) | 1.00 | ||
| No adenopathy | 0 (0) | 0 (0) | |
| Yes, lymph node <5 cm | 12 (50) | 15 (52) | |
| Yes, lymph node 5-10 cm | 10 (42) | 12 (41) | |
| Yes, lymph node >10 cm | 2 (8) | 2 (7) | |
| del(17p), no. (%) | 1.00 | ||
| Absent | 26 (96) | 29 (94) | |
| Present | 1 (4) | 2 (6) | |
| Trisomy 12, no. (%) | .74 | ||
| Absent | 23 (85) | 25 (81) | |
| Present | 4 (15) | 6 (19) | |
| del(13q), no. (%) | .18 | ||
| Absent | 13 (48) | 9 (29) | |
| Present | 14 (52) | 22 (71) |
Percent values are calculated out of the number of patients with nonmissing data in each category.
Abbreviations are explained in Table 2.
Fisher’s exact and Kruskal-Wallis P values are presented for categoric and continuous variables, respectively.
Tolerability and adverse events
The majority of patients received all 6 cycles of induction therapy (non-del(11q): FR, 71%; FR+L, 66%; FCR, 61%; del(11q): FCR, 30%, FCR+L, 65%), although fewer patients received all assigned induction therapy in the 2 FCR-treated groups. Among patients who received lenalidomide consolidation, most patients received all 6 planned cycles (non-del(11q): FR+L, 78%; del(11q): FCR+L, 61%). Among those who did not receive all 6 cycles, most have off-treatment reason as adverse event (60% for FR+L and 57% for FCR+L). Specific details related to treatment administration are in supplemental Table 2. Failure to complete therapy in each of the groups was most commonly due to failure to recover blood counts, toxicity of therapy, or progression on therapy.
Six fatal events occurred during induction therapy: 3 with FR and 3 with FCR. These include a case of John Cunningham (JC) virus encephalopathy (non-del(11q) with FR), autoimmune hemolytic anemia following cycle 2 of FR (non-del(11q) with FR+L), death related to disease progression (non-del(11q) with FR+L), pneumonitis (non-del(11q) with FCR), sepsis (non-del(11q) with FCR), and sepsis/diarrhea (del(11q) with FCR). There were no grade 5 events with lenalidomide consolidation.
Grade 3 or greater adverse events occurring in at least 5% of patients are summarized by treatment arm and by del(11q) status in Table 4. Summaries of all reported grade 3 or greater adverse events and new primary or new secondary malignancies are provided in supplemental Tables 4 and 5. In general, adverse events were manageable and typical of those reported with these different chemoimmunotherapy regimens or monotherapy with lenalidomide.
Grade 3-5 adverse events occurring in at least 5% of patients
| Adverse event type . | Arm/group . | Grade 3, no. (%) . | Grade 4, no. (%) . | Grade 5, no. (%) . |
|---|---|---|---|---|
| Hematologic | ||||
| Hemoglobin | A: FR, non-del(11q) | 15 (13) | 3 (3) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 4 (4) | 0 (0) | |
| C: FCR, non-del(11q) | 22 (21) | 4 (4) | 0 (0) | |
| C: FCR, del(11q) | 4 (15) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 2 (6) | 0 (0) | |
| Hemolysis | A: FR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 3 (3) | 0 (0) | 1 (1) | |
| C: FCR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 1 (3) | 0 (0) | |
| Leukocytes (total WBC) | A: FR, non-del(11q) | 29 (25) | 7 (6) | 0 (0) |
| B: FR+L, non-del(11q) | 22 (20) | 12 (11) | 0 (0) | |
| C: FCR, non-del(11q) | 22 (21) | 11 (10) | 0 (0) | |
| C: FCR, del(11q) | 6 (23) | 3 (12) | 0 (0) | |
| D: FCR+L, del(11q) | 6 (19) | 3 (10) | 0 (0) | |
| Lymphopenia | A: FR, non-del(11q) | 25 (21) | 11 (9) | 0 (0) |
| B: FR+L, non-del(11q) | 19 (18) | 13 (12) | 0 (0) | |
| C: FCR, non-del(11q) | 23 (22) | 18 (17) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 6 (23) | 0 (0) | |
| D: FCR+L, del(11q) | 5 (16) | 4 (13) | 0 (0) | |
| Neutrophils/granulocytes (ANC/AGC) | A: FR, non-del(11q) | 43 (37) | 33 (28) | 0 (0) |
| B: FR+L, non-del(11q) | 35 (32) | 42 (39) | 0 (0) | |
| C: FCR, non-del(11q) | 30 (28) | 44 (42) | 0 (0) | |
| C: FCR, del(11q) | 9 (35) | 13 (50) | 0 (0) | |
| D: FCR+L, del(11q) | 8 (26) | 14 (45) | 0 (0) | |
| Platelets | A: FR, non-del(11q) | 9 (8) | 1 (1) | 0 (0) |
| B: FR+L, non-del(11q) | 9 (8) | 4 (4) | 0 (0) | |
| C: FCR, non-del(11q) | 15 (14) | 7 (7) | 0 (0) | |
| C: FCR, del(11q) | 3 (12) | 1 (4) | 0 (0) | |
| D: FCR+L, del(11q) | 3 (10) | 1 (3) | 0 (0) | |
| Nonhematologic | ||||
| Hypertension | A: FR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 6 (6) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Fatigue | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 15 (14) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 11 (10) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Rash (desquamation) | A: FR, non-del(11q) | 6 (5) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 7 (7) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
| Diarrhea | A: FR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 5 (5) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Febrile neutropenia | A: FR, non-del(11q) | 14 (12) | 1 (1) | 0 (0) |
| B: FR+L, non-del(11q) | 16 (15) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 17 (16) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 3 (12) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 5 (16) | 0 (0) | 0 (0) | |
| Infection with ANC grade <3 | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 5 (5) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 4 (4) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Infection with ANC grade ≥3 | A: FR, non-del(11q) | 5 (4) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 7 (7) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| ALT, SGPT | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 6 (6) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 1 (3) | 0 (0) | |
| Glucose, serum-high (hyperglycemia) | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 8 (7) | 2 (2) | 0 (0) | |
| C: FCR, non-del(11q) | 11 (10) | 3 (3) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 1 (3) | 0 (0) | 0 (0) | |
| Phosphate, serum-low (hypophosphatemia) | A: FR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 6 (6) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 1 (4) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 1 (3) | 0 (0) | 0 (0) | |
| Potassium, serum-low (hypokalemia) | A: FR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
| Sodium, serum-low (hyponatremia) | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 5 (5) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Treatment-related secondary malignancy | A: FR, non-del(11q) | 0 (0) | 2 (2) | 0 (0) |
| B: FR+L, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 1 (3) | 0 (0) | |
| Pain | A: FR, non-del(11q) | 8 (7) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 13 (12) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Dsypnea | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 2 (2) | 0 (0) | |
| C: FCR, non-del(11q) | 3 (3) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Hypoxia | A: FR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 0 (0) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
| Adverse event type . | Arm/group . | Grade 3, no. (%) . | Grade 4, no. (%) . | Grade 5, no. (%) . |
|---|---|---|---|---|
| Hematologic | ||||
| Hemoglobin | A: FR, non-del(11q) | 15 (13) | 3 (3) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 4 (4) | 0 (0) | |
| C: FCR, non-del(11q) | 22 (21) | 4 (4) | 0 (0) | |
| C: FCR, del(11q) | 4 (15) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 2 (6) | 0 (0) | |
| Hemolysis | A: FR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 3 (3) | 0 (0) | 1 (1) | |
| C: FCR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 1 (3) | 0 (0) | |
| Leukocytes (total WBC) | A: FR, non-del(11q) | 29 (25) | 7 (6) | 0 (0) |
| B: FR+L, non-del(11q) | 22 (20) | 12 (11) | 0 (0) | |
| C: FCR, non-del(11q) | 22 (21) | 11 (10) | 0 (0) | |
| C: FCR, del(11q) | 6 (23) | 3 (12) | 0 (0) | |
| D: FCR+L, del(11q) | 6 (19) | 3 (10) | 0 (0) | |
| Lymphopenia | A: FR, non-del(11q) | 25 (21) | 11 (9) | 0 (0) |
| B: FR+L, non-del(11q) | 19 (18) | 13 (12) | 0 (0) | |
| C: FCR, non-del(11q) | 23 (22) | 18 (17) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 6 (23) | 0 (0) | |
| D: FCR+L, del(11q) | 5 (16) | 4 (13) | 0 (0) | |
| Neutrophils/granulocytes (ANC/AGC) | A: FR, non-del(11q) | 43 (37) | 33 (28) | 0 (0) |
| B: FR+L, non-del(11q) | 35 (32) | 42 (39) | 0 (0) | |
| C: FCR, non-del(11q) | 30 (28) | 44 (42) | 0 (0) | |
| C: FCR, del(11q) | 9 (35) | 13 (50) | 0 (0) | |
| D: FCR+L, del(11q) | 8 (26) | 14 (45) | 0 (0) | |
| Platelets | A: FR, non-del(11q) | 9 (8) | 1 (1) | 0 (0) |
| B: FR+L, non-del(11q) | 9 (8) | 4 (4) | 0 (0) | |
| C: FCR, non-del(11q) | 15 (14) | 7 (7) | 0 (0) | |
| C: FCR, del(11q) | 3 (12) | 1 (4) | 0 (0) | |
| D: FCR+L, del(11q) | 3 (10) | 1 (3) | 0 (0) | |
| Nonhematologic | ||||
| Hypertension | A: FR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 6 (6) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Fatigue | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 15 (14) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 11 (10) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Rash (desquamation) | A: FR, non-del(11q) | 6 (5) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 7 (7) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
| Diarrhea | A: FR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 5 (5) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Febrile neutropenia | A: FR, non-del(11q) | 14 (12) | 1 (1) | 0 (0) |
| B: FR+L, non-del(11q) | 16 (15) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 17 (16) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 3 (12) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 5 (16) | 0 (0) | 0 (0) | |
| Infection with ANC grade <3 | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 5 (5) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 4 (4) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Infection with ANC grade ≥3 | A: FR, non-del(11q) | 5 (4) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 7 (7) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| ALT, SGPT | A: FR, non-del(11q) | 4 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 6 (6) | 1 (1) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 1 (3) | 0 (0) | |
| Glucose, serum-high (hyperglycemia) | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 8 (7) | 2 (2) | 0 (0) | |
| C: FCR, non-del(11q) | 11 (10) | 3 (3) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 1 (3) | 0 (0) | 0 (0) | |
| Phosphate, serum-low (hypophosphatemia) | A: FR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 6 (6) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 1 (4) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 1 (3) | 0 (0) | 0 (0) | |
| Potassium, serum-low (hypokalemia) | A: FR, non-del(11q) | 2 (2) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
| Sodium, serum-low (hyponatremia) | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 5 (5) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Treatment-related secondary malignancy | A: FR, non-del(11q) | 0 (0) | 2 (2) | 0 (0) |
| B: FR+L, non-del(11q) | 1 (1) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 2 (2) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 1 (3) | 0 (0) | |
| Pain | A: FR, non-del(11q) | 8 (7) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 12 (11) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 13 (12) | 0 (0) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Dsypnea | A: FR, non-del(11q) | 3 (3) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 4 (4) | 2 (2) | 0 (0) | |
| C: FCR, non-del(11q) | 3 (3) | 2 (2) | 0 (0) | |
| C: FCR, del(11q) | 2 (8) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| Hypoxia | A: FR, non-del(11q) | 0 (0) | 0 (0) | 0 (0) |
| B: FR+L, non-del(11q) | 2 (2) | 0 (0) | 0 (0) | |
| C: FCR, non-del(11q) | 0 (0) | 1 (1) | 0 (0) | |
| C: FCR, del(11q) | 0 (0) | 0 (0) | 0 (0) | |
| D: FCR+L, del(11q) | 2 (6) | 0 (0) | 0 (0) | |
Percent values are calculated out of the number of patients who started treatment and submitted at least 1 adverse event form for each group: FR in non-del(11q) (n = 117), FR + L in non-del(11q) (n = 109), FCR in non-del(11q) (n = 106), FCR in del(11q) (n = 26), FCR + L in del(11q) (n = 31).
Outcome of therapy
The primary objective of this study was to determine whether PFS rate at 2 years for non-del(11q) patients treated in each of 3 experimental arms (arm A, FR; arm B, FR+L; and arm C, FCR) was sufficiently improved relative to historical control with FC to warrant further study. Non-del(11q) patients treated with FR had a 2-year PFS rate of 64% (90% CI, 57-71), whereas those in the FR+L and FCR groups had higher 2-year PFS rates of 72% (90% CI, 65-79) and 74% (90% CI, 66-80), respectively. Only the FR+L and FCR groups had 2-year PFS rates significantly higher than the 60% previously reported with FC18 to be considered for a future phase 3 trial (P = .008 and P = .004, respectively). Table 5 summarizes 2-year PFS rates with exact 90% CIs by treatment arm and del(11q) status.
PFS rates at 2 y
| Arm/group . | N . | No. of successes* . | % . | Exact 90% CI, % . |
|---|---|---|---|---|
| A: FR, non-del(11q) | 123 | 79 | 64 | 57-71 |
| B: FR + L, non-del(11q) | 109 | 79 | 72 | 65-79 |
| C: FCR, non-del(11q) | 110 | 81 | 74 | 66-80 |
| C: FCR, del(11q) | 27 | 15 | 56 | 38-72 |
| D: FCR + L, del(11q) | 31 | 20 | 65 | 48-79 |
| Arm/group . | N . | No. of successes* . | % . | Exact 90% CI, % . |
|---|---|---|---|---|
| A: FR, non-del(11q) | 123 | 79 | 64 | 57-71 |
| B: FR + L, non-del(11q) | 109 | 79 | 72 | 65-79 |
| C: FCR, non-del(11q) | 110 | 81 | 74 | 66-80 |
| C: FCR, del(11q) | 27 | 15 | 56 | 38-72 |
| D: FCR + L, del(11q) | 31 | 20 | 65 | 48-79 |
Patients lost to follow-up, starting nonprotocol therapy, or with progressions or deaths prior to 2 years were treated as failures.
Secondary end points of the study included best ORR, CR rate, and time-to-event PFS and OS. The best ORR occurring with therapy did not differ significantly across the groups (non-del(11q): FR, 75%; FR+L, 69%; FCR, 71%; del(11q): FCR, 59%, FCR+L, 74%), although CR rates did (non-del(11q): FR, 32%; FR+L, 32%; FCR, 51%; del(11q): FCR, 19%, FCR+L, 35%). For non-del(11q) patients, those with FCR treatment had a significantly higher CR rate than those with FR and FR+L treatment (P < .01 for each). A summary of response rates with 90% CIs is shown by treatment arm and del(11q) status in supplemental Table 5.
Impact of lenalidomide
Among the 69 non-del(11q) patients who received lenalidomide consolidation as part of the FR+L group, 3 of 31 patients who had a partial response (PR) with induction therapy converted to CR with time and continued therapy. Among the 18 del(11q) patients who received lenalidomide consolidation as part of the FCR+L group, 2 of 10 patients who had a PR with induction therapy converted to CR with time and continued therapy.
Despite the absence of marked response improvement with lenalidomide, the 2-year PFS rates nonetheless suggest clinical benefit with lenalidomide consolidation following FR in non-del(11q) patients. Furthermore, time-to-event PFS was significantly improved in the non-del(11q) patients in the FR+L group vs the non-del(11q) patients who received FR alone (P = .04; Figure 2A). The median PFS was 60.7 months (95% CI, 44.8-71.3) with FR+L vs 43.5 months (95% CI, 32.8-50.2) with FR. In non-del(11q) patients receiving FCR, the median PFS was 97.2 months (95% CI, 61.4 to not reached) and significantly longer from those treated with FR alone (P < .001; Figure 2A). Even though the median PFS was longer with FCR than with FR+L, the difference in PFS did not reach statistical significance (P = .07).
PFS and OS. (A) PFS for patients with non-del(11q) disease and assigned to FR, FR+L, or FCR. (B) PFS for patients with del(11q) disease and assigned to FCR or reassigned to FCR+L. (C) OS for patients with non-del(11q) disease and assigned FR, FR+L, or FCR. (D) OS for patients with del(11q) disease and assigned to FCR or reassigned to FCR+L.
PFS and OS. (A) PFS for patients with non-del(11q) disease and assigned to FR, FR+L, or FCR. (B) PFS for patients with del(11q) disease and assigned to FCR or reassigned to FCR+L. (C) OS for patients with non-del(11q) disease and assigned FR, FR+L, or FCR. (D) OS for patients with del(11q) disease and assigned to FCR or reassigned to FCR+L.
For del(11q) patients treated with FCR, median PFS was 35.5 months (95% CI, 21.8-65.5), significantly shorter than that observed in non-del(11q) patients receiving FCR (P = .006). Thus, the data fail to support the hypothesis that the inclusion of cyclophosphamide abrogates the poor outcome associated with del(11q). In del(11q) patients treated with FCR+L, the median PFS was 41.2 months (95% CI, 25.7-50.7). Even though this was longer than the median PFS with FCR alone in del(11q) patients, the curves for the 2 groups overlapped (P = .98; Figure 2B).
Overall survival
OS was estimated according to treatment arm and del(11q) status (Figure 2C-D). With a median follow-up of 73 months (range, 2-112 months), the median OS has only been reached in the group of non-del(11q) patients receiving FCR (median = 101.1 months; 95% CI, 95.6 to not reached). Among the non-del(11q) patients, OS was not significantly different between the FR+L and FR/FCR treatment groups (P = .20 and P = .26, respectively; Figure 2C). However, in the FR+L treatment group, there have been only 5 deaths beyond 48 months in 89 at-risk patients compared with 21 and 12 deaths beyond 48 months among 108 and 90 at-risk patients in the FR and FCR groups, respectively.
Univariable and multivariable analyses of outcome
Secondary objectives of this study included evaluating baseline features associated with end points of clinical benefit, such as ORR, PFS, and OS among the non-del(11q) patients.
For ORR, interphase cytogenetics was significantly associated with outcome when adjusting for other variables (supplemental Table 6). Using the Dohner hierarchical classification for cytogenetics,6 patients with del(17p) had lower odds of responding compared with patients with the low-risk classification of del(13q); only 11 of 30 patients (37%) with del(17p) responded to therapy, all with partial responses, as opposed to 79 of 112 patients (71%) with del(13q). There were no significant differences in the odds of response according to treatment group (P > .20 for all pairwise comparisons).
For PFS, higher WBC count, bulky nodes, interphase cytogenetics and treatment group were significant in the multivariable analysis (supplemental Table 7). Both interphase cytogenetics and treatment group violated the assumption of proportional hazards (P < .01), and therefore the hazard ratio (HR) was allowed to change over time in the model. The risk of an event was significantly higher for patients classified with del(17p) and those classified with del(11q) in <20% of cells compared with those classified with del(13q). The risk of an event for patients with del(17p) was highest at early time points in the study, with an HR of 2.39 (95% CI, 1.24-4.61) at year 2 and an HR of 1.29 (95% CI, 0.48-3.50) for those still at risk at year 5. Conversely, the risk of an event for patients with low levels of del(11q) increased over time, with an HR of 2.85 (95% CI, 1.39-5.82) at year 2 and an HR of 8.26 (95% CI, 2.60, 26.27) for those still at risk at year 5. For patients who were in treatment groups FR+L and FCR, the risk of an event was increasingly lower than for patients who received FR; at year 5, the HR was 0.43 (95% CI, 0.24-0.79) and 0.21 (95% CI, 0.11-0.41), respectively, relative to the FR treatment group. There was no significant difference in PFS between the FR+L and FCR treatment groups (P = .12).
For OS, age ≥65 years, interphase cytogenetics and treatment group were significant in the multivariable analysis (supplemental Table 8). Specifically, the risk of death was significantly higher for patients classified with del(17p) compared with those classified with del(13q) (P = .0002). With respect to treatment group, the proportional hazards assumption was violated (P = .04) and the HR was allowed to change over time in the model. Accounting for nonproportional hazards, the risk of death for patients in treatment group FR+L decreased over time and was significantly lower than the risk of death for patients in treatment group FR at later time points (P = .01). The risk of death for those in the FCR treatment group was not significantly different from those in the FR or FR+L groups (P = .12 and P = .21, respectively).
A post hoc exploratory data analysis was conducted in the subgroup of patients aged 65 years or older because these patients have historically been underrepresented in clinical trials. Among the 400 patients analyzed, 144 (36%) were at least 65 years old. In this subgroup of patients, the CR rate was 38% (95% CI, 30-46) and the ORR was 66% (95% CI, 58-74). The median PFS was 55 months (95% CI, 45-66) and the median OS was 96 months (95% CI, 76 to not reached). Large differences in response rates, PFS, and OS were not observed according to treatment arm in this subgroup of patients (supplemental Table 9).
Discussion
This large randomized phase 2 study has identified 2 treatment strategies that could be pursued in future phase 3 trials for previously untreated non-del(11q) CLL patients requiring therapy, including chemoimmunotherapy with either FR+L or FCR. Both treatment strategies exceeded the 2-year PFS rate observed with FC in earlier studies.19 FR was not as effective as measured by PFS, being more similar to reported outcomes for FC.20,34 Adverse events with each induction therapy were manageable and the majority of non-del(11q) patients received all 6 cycles. Additionally, this trial demonstrated in a randomized phase 2 trial the benefit of short-term lenalidomide therapy as consolidation treatment. Collectively, these findings provide further justification together with other reported phase 3 trials performed with consolidative lenalidomide35,36 to justify its use in future clinical trials.
This trial successfully used for the first time centrally assessed interphase cytogenetics to risk stratify patients in a multicenter study based upon the presence of del(11q). Despite earlier data suggesting that FCR might abrogate the poor outcome associated with this aberration,17 in our cohort, patients with del(11q) had a considerably shorter PFS compared with non-del(11q) patients who received FCR. In addition, the CR rate with FCR in patients with del(11q) was lower than that observed in non-del(11q) patients. Because this trial required ≥20% of cells with loss of 1 copy to categorize as del(11q), which is a higher threshold than what is typically used in practice, the discrepant results could be due to enrichment of more aggressive cases.
A novel observation of our study is that lenalidomide consolidation for 6 cycles after FR, despite producing no measurable improvement in response rate, significantly impacted both PFS and OS when compared with chemoimmunotherapy with FR alone. In a multivariable analysis for OS, the risk of death for those in the FR+L group decreased over time and was lower relative to those receiving FR at later time points (P = .01) Collectively, this provides increased interest in investigating lenalidomide or similar therapeutic agents as consolidation therapy for CLL.
The mechanism accounting for clinical benefit of lenalidomide consolidation therapy in our study is unknown. However, the findings are consistent with positive data from lenalidomide maintenance trials in CLL35,36 and lymphoma37 despite lack of deepening response. An earlier study examining lenalidomide consolidation after completion of pentostatin, cyclophosphamide, and rituximab (PCR) demonstrated improved time to next treatment over previous PCR outcomes.38 In this PCR+L trial, the authors demonstrated improved T-cell function in vivo, similar to previous reports with this agent.38,39 Thus, an immunologic anti-tumor response to lenalidomide could explain our observation. In addition, lenalidomide mediates degradation of IKZF1 and IKZF3, both transcription factors expressed in T cells as well as in CLL cells.40,41 IKZF3 has recently been shown to be mutated in a small subset of CLL patients, and is over-expressed in the majority of CLL patients, suggesting a role in CLL pathogenesis.5 IKZF3 disrupts apoptosis, possibly through promoting upregulation of bcl-2 family proteins.42 Further investigation of lenalidomide and other cereblon-modulating agents is warranted given these novel findings.
The relevance of this study’s findings in the context of a rapidly moving field of CLL therapeutics must be considered and outside of the lenalidomide consolidation arm are of little impact. High-sensitivity flow cytometry and IGHV mutational analysis was not an integral biomarker and was not performed as part of the trial nor was minimal residual disease assessment. Recent observations have suggested that for the small subset of young CLL patients with IGHV-mutated CLL, treatment with FCR may promote prolonged durable remissions and cure.13 Bruton tyrosine kinase (BTK) inhibitors such as ibrutinib43,44 are being increasingly used as initial therapy for CLL treatment. In addition to inhibiting BTK, ibrutinib also inhibits interleukin-2–inducible kinase, which favorably modulates the immune system.45,46 Thus, combining ibrutinib with lenalidomide might provide dual modulation of the immune system through different mechanisms. Several studies are ongoing with this combination treatment. If effective, such approaches might offer potential benefits such as allowing discontinuation of ibrutinib or alternatively preventing relapse of high-risk CLL patients such as those with complex karyotype.47,48 The data from our study justify continued study of lenalidomide or related compounds in the treatment of CLL.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the patients and families for their participation in this trial. Additionally, they acknowledge Abbott Molecular Inc for providing the FISH reagents used in this study, Molly Boyd for providing quality assurance of the data, and the many site co-investigators, study coordinators, health care providers, and pharmacists within the 4 North American cooperative groups who aided in completion of this study.
This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180833, U10CA180836, U10CA180850, U10CA180867; Canadian Cancer Trials Group: National Clinical Trials Network U10CA180863 and Canadian Cancer Society Research Institute 704970; Eastern Cooperative Oncology Group/American College of Radiology Imaging Network: U10CA180791, U10CA180799, and U10CA180802; Southwest Oncology Group U10CA180828 and U10CA180888. This work was also supported in part by funding from the Four Winds Foundation, the Leukemia & Lymphoma Society, Vysis Inc (part of Abbott Molecular), and the National Cancer Institute (P01 CA095426 and R35 CA197734) (J.C.B.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.C.B. and A.S.R. planned the study, assessed response and toxicity, analyzed the data, and wrote the manuscript; N.A.H. performed interphase cytogenetics, reviewed the draft of the manuscript, and approved the final version; A.E.H. compiled data, analyzed data, reviewed drafts of the manuscript, and approved the final version; E.H. provided quality assurance of the data and approved the final version of the manuscript; M.R.S., J.E.G., S.C., T.A.F., M.J.T., M.S.T., F.R.A., R.M.S., S.R., J.E.C., and R.A.L. contributed to the accrual of patients, reviewed drafts of the manuscript, and approved the final version; S.J.M. is the faculty statistician who worked with A.S.R. on the statistical analysis plan of the study, edited and reviewed the manuscript, and approved the final version; and R.A.L. was involved in the study planning, manuscript review, and final approval of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John C. Byrd, The Ohio State University, 455B OSUCCC, 410 West 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.
References
Author notes
J.C.B. and A.S.R. equally contributed to this work.