Key Points
Early platelet administration is associated with improved hemostasis and reduced mortality in severely injured, bleeding trauma patients.
Abstract
Transfusing platelets during massive hemorrhage is debated because of a lack of high-quality evidence concerning outcomes in trauma patients. The objective of this study was to examine the effect of platelet transfusions on mortality in severely injured trauma patients. This work analyzed PROPPR (Pragmatic, Randomized Optimal Platelet and Plasma Ratios) trial patients who received only the first cooler of blood products, which either did or did not contain platelets. Primary outcomes were all-cause mortality at 24 hours and 30 days and hemostasis. Secondary outcomes included cause of death, complications, and hospital-, intensive care unit (ICU)–, and ventilator-free days. Continuous variables were compared using Wilcoxon rank sum tests. Categorical variables were compared using Fisher’s exact tests. There were 261 PROPPR patients who achieved hemostasis or died before receiving a second cooler of blood products (137 received platelets and 124 did not). Patients who received platelets also received more total plasma (median, 3 vs 2 U; P < .05) by PROPPR intervention design. There were no differences in total red blood cell transfusions between groups. After controlling for plasma volume, patients who received platelets had significantly decreased 24-hour (5.8% vs 16.9%; P < .05) and 30-day mortality (9.5% vs 20.2%; P < .05). More patients in the platelet group achieved hemostasis (94.9% vs 73.4%; P < .01), and fewer died as a result of exsanguination (1.5% vs 12.9%; P < .01). Patients who received platelets had a shorter time on mechanical ventilation (P < .05); however, no differences in hospital- or ICU-free days were observed. In conclusion, early platelet administration is associated with improved hemostasis and reduced mortality in severely injured, bleeding patients. This trial was registered at www.clinicaltrials.gov as # NCT01545232.
Introduction
Achieving hemostasis after injury is critical for survival. Hemorrhage accounts for 30% to 40% of trauma-related fatalities, with one quarter of all trauma patients experiencing some degree of prolonged hemorrhage associated with coagulopathy.1-5 Hemorrhage-related trauma fatalities usually occur within the first 2 hours of admission, making this immediate postadmission period critical for rapidly delivering lifesaving interventions.6 Previous studies have examined the utility of early red blood cells (RBCs) and plasma, but few data exist on early platelet transfusion.7-9 Platelets are critical regulators of hemostasis, serving as the matrix for initial vascular plug formation, providing a lipid-rich surface to support coagulation factor assembly, creating a scaffold for the generation of fibrin clots, and releasing pro–wound-healing cytokines and procoagulant microparticles.10,11 Given the importance of endogenous platelets to hemostasis and their observed inability to function appropriately in severely injured patients, early platelet transfusions would seem intuitive.12-14 Several retrospective studies and metaanalyses have demonstrated a benefit of platelet transfusions on outcomes in trauma patients.15-19 These data are supported by 2 multicenter, prospective observational studies. In 2013, Holcomb et al20 published findings of the PROMMTT (Prospective, Observational, Multicenter, Major Trauma Transfusion) study showing that although only 72% of patients received platelets within 3 hours, the early delivery of higher platelet and plasma ratios was associated with decreased 24-hour mortality. Similar conclusions were drawn from the ACIT (Activation of Coagulation and Inflammation in Trauma) study, where Balvers et al21 reported higher ratios of platelets or plasma/RBCs, in combination with tranexamic acid, reduced both the rate of massive transfusion and the incidence of mortality in a comparable patient population. Furthermore, in the PROPPR (Pragmatic, Randomized Optimal Platelet and Plasma Ratios) trial, a significant increase in the incidence of hemostasis and a reduction in exsanguinating deaths were found in patients receiving a higher ratio of platelets and plasma/RBCs.22 These findings were corroborated in a recent metaanalysis of 15 studies demonstrating a significant survival benefit when high platelet and plasma transfusion ratios were administered during damage control resuscitation.23 Conversely, a separate systematic review concluded that there was insufficient evidence to administer a high ratio of platelets and plasma to bleeding trauma patients, given the limited observed clinical benefits.24 In summary, there are conflicting conclusions, reflecting a lack of high-quality, randomized trial data on the isolated effect of platelet transfusions on outcomes in bleeding trauma patients.
In addition to a lack of robust clinical findings establishing an international consensus on platelet transfusions in trauma patients, recent data suggest that platelet transfusions do not affect circulating platelet count or function and may further debilitate them.25,26 These observations likely reflect both the well-described storage lesions that affect transfused platelet number and function and the differential removal from the circulation by adhesion to the site of injury of better-functioning platelets in injured patients.27,28
The recently completed PROPPR trial provided an opportunity to examine a subset of severely injured patients who were prospectively randomized to receive early platelets or not. The aim of this study was to determine the effects of early platelet transfusions on outcomes in Level 1 trauma patients predicted to need a massive transfusion. We hypothesized that early platelet transfusion would be associated with improvements in early and late survival.
Materials and methods
Study design
The PROPPR study was a multicenter, pragmatic, randomized trial conducted at 12 Level 1 trauma centers designed to evaluate the effectiveness of a 1:1:1 transfusion ratio of blood products compared with a 1:1:2 ratio in critically ill patients predicted to receive a massive transfusion.29 This study was conducted with approval from the US Food and Drug Administration and the Department of Defense and in accordance with all local committees for the protection of human subjects under exception from informed consent.30 All authors had access to and approval to access the primary clinical trial data.
The PROPPR trial design has been previously described in detail.22,30 Briefly, patients were eligible for enrollment if they were an institutional highest-level trauma activation, were ≥15 years of age, were received directly from the injury scene, had been transfused with at least 1 unit of blood product within the first hour of arrival or prehospital, and were predicted to receive a massive transfusion. Patients were excluded if they were transferred from another hospital, had a lethal traumatic brain injury, were prisoners, were pregnant, were <15 years of age, had received >5 minutes of cardiopulmonary resuscitation, had a >20% total body-surface area burn, had an inhalation injury, or had >3 units of RBCs transfused.
The transfusion regimens were as follows: individuals randomized to the 1:1:1 group received coolers containing 1 dose of platelets (an apheresis unit containing 300+ billion platelets in 300 mL of plasma equivalent to 6 whole blood–derived units), 6 units of plasma, and 6 units of RBCs. Platelets were transfused first, followed by alternating RBC and plasma units. For individuals randomized to the 1:1:2 group, the initial cooler and all subsequent odd-numbered coolers contained 3 units of plasma, 0 doses of platelets, and 6 units of RBCs. These units were transfused by alternating 2 units of RBCs and 1 unit of plasma. Even-numbered coolers contained 3 units of plasma, 1 dose of platelets, and 6 units of RBCs, with platelets transfused first, followed by alternating RBC and plasma units.
For this study, the term prerandomization refers to any event, treatment, or blood product administered before being randomized to a treatment group. The term randomized treatment phase refers to the time period between being randomized and the end of the initial resuscitation with treatment-phase blood products (1:1:1 or 1:1:2). The term post–randomized treatment refers to any event, treatment, or blood products administered after the randomized treatment phase ended. Anything occurring post–randomized treatment was not standardized and the clinical course was subject to the attending physician’s discretion.
Secondary analysis
For this study, we were interested in determining the effects of platelet transfusion on outcomes, comparing patients who received platelets with those who did not. Because of the differing contents of the randomized coolers in the 2 PROPPR groups, we limited the analysis to patients who received only the first cooler of blood products during the randomized treatment phase and received no additional platelet transfusions post–randomized treatment. However, patients could receive additional plasma and/or RBC units post–randomized treatment. Therefore, the selected patients in the 1:1:1 group received 1 dose of platelets, whereas the selected patients in the 1:1:2 group did not receive any platelet transfusions during the randomized treatment phase. In this analysis, no patients in either group received platelets in the post–randomized treatment phase.
Blood sample collection and testing
Whole-blood samples were collected into citrated vacutainer tubes at the time of admission and at 2, 4, 6, 12, 24, 48, and 72 hours after admission. Platelet count was determined using flow cytometric analysis (Beckman Coulter Gallios 3L 10C instrument; Beckman Coulter, Indianapolis, IN). Whole blood (5 µL) was incubated with APC-conjugated antibodies against CD42b (GPIbα receptor antibody; BD Biosciences, San Jose, CA). The total number of CD42b+ platelets was recorded and expressed as the number of platelets × 109/L (normal platelet counts are 250 × 109/L).
Statistical analysis
All continuous variables were summarized as medians and interquartile ranges (IQRs) and compared using the Wilcoxon rank sum test. Categorical variables were compared using Fisher’s exact test. The Kaplan-Meier method was used to estimate mortality. Because the 1:1:2 treatment group in PROPPR received half the amount of plasma by design, plasma transfusions differed significantly between groups and required adjustment. For adjustment purposes, total plasma received was categorized into 7 strata (0, 1, 2, 3, 4, 5, and ≥6 units). Stratification methods were used for comparing treatment groups, to adjust for different amounts of plasma in these 2 groups. Mortality was tested using the Cochran-Mantel-Haenszel test (24 hours) or stratified log-rank test for censored data (30 days), adjusting for plasma. The van Elteren test was used for continuous outcomes while adjusting for plasma. In these tests, outcome comparisons between treatment groups were evaluated in each stratum of the same (strata of 0, 1, 2, 3, 4, and 5 units) or similar (stratum of ≥6 units) amounts of plasma. The test statistics of all strata were subsequently combined to yield the overall test result for the effects of platelets. The generalized logit mixed model was used for disposition location. The plasma strata were included as random effects in this model to accommodate evaluation at the same or similar amount of plasma. The Wald test was used to compare all-cause and cause-specific differences in mortality. All P values were 2 sided, and P < .05 was considered significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
During the study period (August 2012 to December 2013), 680 patients were enrolled in the PROPPR trial. Of these patients, 261 received only products within the first cooler of blood products and no platelets during the post–randomized treatment phase, with 137 patients receiving platelets (in 1:1:1 group) and 124 patients receiving no platelets (in 1:1:2 group; Table 1). Patients in this subgroup were less severely injured than other patients enrolled in the PROPPR trial (median Injury Severity Score, 22 [IQR, 12-34) vs 30 [IQR, 21-41], respectively; P < .01), thus explaining why only 1 cooler of blood products was necessary during initial resuscitation. Between those patients who did and did not receive platelets, we did not observe any differences in demographics, injury severity or mechanism, hemodynamics, or coagulation test parameters. We did observe a longer median time to randomization in the platelet group compared with the no-platelet group (35 vs 28 minutes; P = .01); however, when added as a covariate in both mortality models, it did not affect the results (Table 1).
Patient characteristics by treatment group
| . | Platelets (n = 137) . | No platelets (n = 124) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 35 (25-50) | 35 (26-49.5) | .82 |
| Male sex, n (%) | 109 (79.6) | 103 (83.1) | .53 |
| Race, n (%) | .78 | ||
| White | 88 (64.2) | 77 (62.1) | |
| Black | 35 (25.6) | 36 (29.0) | |
| Other | 14 (10.2) | 11 (8.9) | |
| Glasgow Coma Scale score, median (IQR) | 14 (3-15) | 14 (3-15) | .28 |
| Systolic blood pressure, mm Hg | |||
| Median (IQR) | 102 (85-123) | 105 (80-126) | .56 |
| ≤90, n (%) | 51 (37.8) | 51 (42.9) | .44 |
| Diastolic blood pressure, median (IQR), mm Hg | 72 (57-89) | 65 (50-89) | .10 |
| Heart rate, beats per min | |||
| Median (IQR) | 110 (93-130) | 103 (87-125) | .07 |
| ≥120, n (%) | 52 (38.0) | 41 (33.1) | .44 |
| Respiratory rate, median (IQR), breaths per min | 20 (16-25) | 21 (18-26) | .25 |
| Blood consumption score ≥2, n (%) | 83 (60.6) | 78 (62.9) | .71 |
| Critical administration threshold, N (%) | 66 (48.2) | 65 (52.4) | .54 |
| Mechanism of injury, n (%) | |||
| Any blunt injury | 70 (51.1) | 57 (46.0) | .46 |
| Any penetrating injury | 70 (51.1) | 67 (54.0) | .71 |
| Time to randomization, median (IQR), min | 35 (20-54) | 27.5 (18-43) | .01 |
| Hemoglobin level, g/dL | |||
| Median (IQR) | 12.6 (11.1-13.7) | 12.7 (10.6-13.7) | .49 |
| ≤11, n (%) | 33 (24.1) | 34 (27.4) | .30 |
| >11, n (%) | 100 (73.0) | 82 (66.1) | |
| Unknown, n (%) | 4 (2.9) | 8 (6.5) | |
| INR | |||
| Median (IQR) | 1.20 (1.10-1.32) | 1.20 (1.14-1.40) | .18 |
| >1.5, n (%) | 12 (8.8) | 11 (8.9) | .66 |
| ≤1.5, n (%) | 78 (56.9) | 64 (51.6) | |
| Unknown, n (%) | 47 (34.3) | 49 (39.5) | |
| Thromboelastography R time, min | |||
| Median (IQR) | 3.6 (2.8-4.4) | 3.9 (2.8-4.7) | .31 |
| >8, n (%) | 6 (4.4) | 4 (3.2) | .94 |
| ≤8, n (%) | 101 (73.7) | 92 (74.2) | |
| Unknown, n (%) | 30 (21.9) | 28 (22.6) | |
| Platelet count, ×109/L | |||
| Median (IQR) | 244 (195-297) | 243 (184-285) | .60 |
| <150, n (%) | 10 (7.3) | 14 (11.3) | .17 |
| <100, n (%) | 3 (2.2) | 2 (1.6) | .33 |
| <50, n (%) | 0 (0) | 0 (0) | |
| Unknown, n (%) | 6 (4.4) | 11 (8.9) | |
| Base excess, median (IQR), mmol/L | −6.1 (−9.8 to −2.9) | −6.8 (−11.3 to −3.0) | .40 |
| Injury Severity Score, median (IQR) | 22 (14-34) | 21.5 (11-29.5) | .15 |
| AIS Head score ≥3, n (%) | 22 (16.1) | 18 (14.5) | .86 |
| Revised Trauma Score, median (IQR) | 7.11 (4.09-7.84) | 6.90 (4.09-7.84) | .28 |
| . | Platelets (n = 137) . | No platelets (n = 124) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 35 (25-50) | 35 (26-49.5) | .82 |
| Male sex, n (%) | 109 (79.6) | 103 (83.1) | .53 |
| Race, n (%) | .78 | ||
| White | 88 (64.2) | 77 (62.1) | |
| Black | 35 (25.6) | 36 (29.0) | |
| Other | 14 (10.2) | 11 (8.9) | |
| Glasgow Coma Scale score, median (IQR) | 14 (3-15) | 14 (3-15) | .28 |
| Systolic blood pressure, mm Hg | |||
| Median (IQR) | 102 (85-123) | 105 (80-126) | .56 |
| ≤90, n (%) | 51 (37.8) | 51 (42.9) | .44 |
| Diastolic blood pressure, median (IQR), mm Hg | 72 (57-89) | 65 (50-89) | .10 |
| Heart rate, beats per min | |||
| Median (IQR) | 110 (93-130) | 103 (87-125) | .07 |
| ≥120, n (%) | 52 (38.0) | 41 (33.1) | .44 |
| Respiratory rate, median (IQR), breaths per min | 20 (16-25) | 21 (18-26) | .25 |
| Blood consumption score ≥2, n (%) | 83 (60.6) | 78 (62.9) | .71 |
| Critical administration threshold, N (%) | 66 (48.2) | 65 (52.4) | .54 |
| Mechanism of injury, n (%) | |||
| Any blunt injury | 70 (51.1) | 57 (46.0) | .46 |
| Any penetrating injury | 70 (51.1) | 67 (54.0) | .71 |
| Time to randomization, median (IQR), min | 35 (20-54) | 27.5 (18-43) | .01 |
| Hemoglobin level, g/dL | |||
| Median (IQR) | 12.6 (11.1-13.7) | 12.7 (10.6-13.7) | .49 |
| ≤11, n (%) | 33 (24.1) | 34 (27.4) | .30 |
| >11, n (%) | 100 (73.0) | 82 (66.1) | |
| Unknown, n (%) | 4 (2.9) | 8 (6.5) | |
| INR | |||
| Median (IQR) | 1.20 (1.10-1.32) | 1.20 (1.14-1.40) | .18 |
| >1.5, n (%) | 12 (8.8) | 11 (8.9) | .66 |
| ≤1.5, n (%) | 78 (56.9) | 64 (51.6) | |
| Unknown, n (%) | 47 (34.3) | 49 (39.5) | |
| Thromboelastography R time, min | |||
| Median (IQR) | 3.6 (2.8-4.4) | 3.9 (2.8-4.7) | .31 |
| >8, n (%) | 6 (4.4) | 4 (3.2) | .94 |
| ≤8, n (%) | 101 (73.7) | 92 (74.2) | |
| Unknown, n (%) | 30 (21.9) | 28 (22.6) | |
| Platelet count, ×109/L | |||
| Median (IQR) | 244 (195-297) | 243 (184-285) | .60 |
| <150, n (%) | 10 (7.3) | 14 (11.3) | .17 |
| <100, n (%) | 3 (2.2) | 2 (1.6) | .33 |
| <50, n (%) | 0 (0) | 0 (0) | |
| Unknown, n (%) | 6 (4.4) | 11 (8.9) | |
| Base excess, median (IQR), mmol/L | −6.1 (−9.8 to −2.9) | −6.8 (−11.3 to −3.0) | .40 |
| Injury Severity Score, median (IQR) | 22 (14-34) | 21.5 (11-29.5) | .15 |
| AIS Head score ≥3, n (%) | 22 (16.1) | 18 (14.5) | .86 |
| Revised Trauma Score, median (IQR) | 7.11 (4.09-7.84) | 6.90 (4.09-7.84) | .28 |
Associations between characteristics and treatment were evaluated using the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. All laboratory values were drawn on admission.
AIS, Abbreviated Injury Scale; INR, international normalized ratio.
There were no differences in the volumes of prerandomization blood products or fluids administered to each group (Table 2). Both cohorts received a similar volume of RBCs during the prerandomization period (median, 2 units for both groups) and randomized treatment phase (median, 3 units for both groups). However, patients in the platelet group received more units of plasma than those in the no-platelet group (median, 2 [IQR, 1-3] vs 1 [IQR, 0-2] unit; P < .01) during the randomized treatment phase. Both cohorts received a median of 5 units of RBCs total in the first 24 hours. The total 24-hour plasma received was greater in the platelet group (median, 3 [IQR, 2-5] vs 2 [IQR, 1-3] units; P < .01). The total amount of platelets administered to the platelet group during the randomized treatment phase and over 24 hours was 6 units. Platelets were transfused at a median of 2 minutes (IQR, 1-5 minutes) after randomization. There was no difference in the amount of cryoprecipitate, colloids, or crystalloids between the 2 cohorts.
IV infusions by treatment group
| . | Median (IQR) . | P . | |
|---|---|---|---|
| Platelets (n = 137) . | No platelets (n = 124) . | ||
| Prerandomized products, units | |||
| RBCs | 2 (1-3) | 2 (1-2) | .26 |
| Plasma | 0 (0-1) | 0 (0-0) | .40 |
| Platelets | 0 (0-0) | 0 (0-0) | .35 |
| Cryoprecipitate | 0 (0-0) | 0 (0-0) | 1.00 |
| Colloids | 0 (0-0) | 0 (0-0) | .37 |
| Crystalloids, L | 1.75 (1.0-3.0) | 1.35 (0.40-2.73) | .07 |
| Randomized products, units | |||
| RBCs | 3 (2-4) | 3 (2-4) | .06 |
| Plasma | 2 (1-3) | 1 (0-2) | <.01 |
| Platelets | 6 (6-6) | 0 (0-0) | <.01 |
| Time from randomization to platelet infusion, min | 2 (1-5) | NA | |
| Total product from prehospital to first 24 h, units | |||
| RBCs | 5 (3-7) | 5 (4-7) | .64 |
| Plasma | 3 (2-5) | 2 (1-3) | <.01 |
| Platelets | 6 (6-6) | 0 (0-0) | <.01 |
| Cryoprecipitate | 0 (0-0) | 0 (0-0) | .44 |
| Colloids | 0 (0-0.25) | 0 (0-0) | .10 |
| Crystalloids, L | 6.30 (3.95-8.70) | 5.00 (2.45-8.88) | .13 |
| . | Median (IQR) . | P . | |
|---|---|---|---|
| Platelets (n = 137) . | No platelets (n = 124) . | ||
| Prerandomized products, units | |||
| RBCs | 2 (1-3) | 2 (1-2) | .26 |
| Plasma | 0 (0-1) | 0 (0-0) | .40 |
| Platelets | 0 (0-0) | 0 (0-0) | .35 |
| Cryoprecipitate | 0 (0-0) | 0 (0-0) | 1.00 |
| Colloids | 0 (0-0) | 0 (0-0) | .37 |
| Crystalloids, L | 1.75 (1.0-3.0) | 1.35 (0.40-2.73) | .07 |
| Randomized products, units | |||
| RBCs | 3 (2-4) | 3 (2-4) | .06 |
| Plasma | 2 (1-3) | 1 (0-2) | <.01 |
| Platelets | 6 (6-6) | 0 (0-0) | <.01 |
| Time from randomization to platelet infusion, min | 2 (1-5) | NA | |
| Total product from prehospital to first 24 h, units | |||
| RBCs | 5 (3-7) | 5 (4-7) | .64 |
| Plasma | 3 (2-5) | 2 (1-3) | <.01 |
| Platelets | 6 (6-6) | 0 (0-0) | <.01 |
| Cryoprecipitate | 0 (0-0) | 0 (0-0) | .44 |
| Colloids | 0 (0-0.25) | 0 (0-0) | .10 |
| Crystalloids, L | 6.30 (3.95-8.70) | 5.00 (2.45-8.88) | .13 |
Comparison of continuous variable between 2 groups was evaluated by the Wilcoxon rank sum test. Total blood products include all prehospital, prerandomized, randomized, and postrandomized products up to 24 hours after admission. No postrandomized platelets were administered in either group.
NA, not applicable.
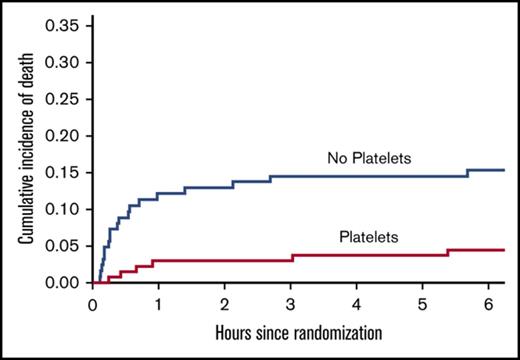
Patients who received platelets had significantly lower incidences of both 24-hour (5.8% vs 16.9%; P < .01) and 30-day mortality (9.5% vs 20.2%; P = .01) compared with those who did not receive platelets (Table 3). Among those patients who died, patients who received platelets had significantly longer times to death compared with those who did not (13.8 vs 0.6 hours, respectively; P = .02), suggesting that hemorrhage was more readily managed after transfusion of platelets. Kaplan-Meier curve analysis demonstrated a significant reduction in the cumulative incidence of early (Figure 1A) and late (Figure 1B) death in patients who received platelets (P < .01). It is clear from these curves that this effect mainly resulted from the reduction in early mortality in the patients who received platelets in the first few minutes of arrival. Similarly, a significantly larger percentage of patients in the platelet group achieved hemostasis compared with those in the no-platelet group (94.9% vs 73.4%, respectively; P < .01); however, there was no significant difference in the median time to hemostasis (81.5 [IQR, 46-135] vs 59 [IQR, 36-109] minutes; P = .14). Although patients in the platelet group had more ventilator-free days (P = .03), no significant differences were observed in hospital- or ICU-free days or in disposition location (Table 3).
Outcomes by treatment group
| Outcome . | Platelets (n = 137) . | No platelets (n = 124) . | P . |
|---|---|---|---|
| 24-h mortality, n (%) | 8 (5.8) | 21 (16.9) | <.01* |
| 30-d mortality, n (%) | 13 (9.5) | 25 (20.2) | <.01† |
| Time to death, median (IQR), h | 13.8 (0.9-69.5) | 0.6 (0.3-5.7) | .02‡ |
| Achieved hemostasis, n (%) | 130 (94.9) | 91 (73.4) | <.01* |
| Anatomic, median (IQR), min | 81.5 (46-135) | 59 (36-109) | .14§ |
| Hospital-free days, median (IQR) | 13 (0-22) | 15 (0-22) | .77§ |
| Ventilator-free days, median (IQR) | 28 (23-29) | 28 (9-29) | .03§ |
| ICU-free days, median (IQR) | 25 (15-27) | 25 (7-27) | .09§ |
| Disposition location, n (%) | .07‖ | ||
| Home | 65 (47.5) | 51 (41.1) | |
| Other¶ | 33 (24.1) | 31 (25.0) | |
| Remained hospitalized | 26 (19.0) | 17 (13.7) | |
| Morgue | 13 (9.5) | 25 (20.2) |
| Outcome . | Platelets (n = 137) . | No platelets (n = 124) . | P . |
|---|---|---|---|
| 24-h mortality, n (%) | 8 (5.8) | 21 (16.9) | <.01* |
| 30-d mortality, n (%) | 13 (9.5) | 25 (20.2) | <.01† |
| Time to death, median (IQR), h | 13.8 (0.9-69.5) | 0.6 (0.3-5.7) | .02‡ |
| Achieved hemostasis, n (%) | 130 (94.9) | 91 (73.4) | <.01* |
| Anatomic, median (IQR), min | 81.5 (46-135) | 59 (36-109) | .14§ |
| Hospital-free days, median (IQR) | 13 (0-22) | 15 (0-22) | .77§ |
| Ventilator-free days, median (IQR) | 28 (23-29) | 28 (9-29) | .03§ |
| ICU-free days, median (IQR) | 25 (15-27) | 25 (7-27) | .09§ |
| Disposition location, n (%) | .07‖ | ||
| Home | 65 (47.5) | 51 (41.1) | |
| Other¶ | 33 (24.1) | 31 (25.0) | |
| Remained hospitalized | 26 (19.0) | 17 (13.7) | |
| Morgue | 13 (9.5) | 25 (20.2) |
ICU, intensive care unit.
Calculated using the Cochran-Mantel-Haenszel test, adjusting for plasma transfused in the first 24 hours.
The stratified log-rank test was used to test treatment effect, controlling for plasma transfused in the first 24 h.
Wilcoxon rank sum test was used to compare time to death for patients who died. Because of small numbers, this comparison was not adjusted for plasma.
The van Elteren test was used to compare medians, adjusting for amount of plasma transfused in the first 24 hours.
Generalized logit mixed model was fitted to test treatment effect, including plasma transfused in the first 24 hours as random effect.
Includes long-term care facility, skilled nursing facility, rehabilitation facility, acute care hospital, assisted living, psychiatric facility, jail, and unknown.
Kaplan-Meier curves. Curves demonstrate cumulative incidence of death during the first 6 hours (A) and 30 days (B).
Kaplan-Meier curves. Curves demonstrate cumulative incidence of death during the first 6 hours (A) and 30 days (B).
Among those patients who died, significantly fewer patients who received platelets exsanguinated compared with those who did not (1.1% vs 10.1%; P < .01; Table 4). There were no differences in any other causes of death between groups, although the number of events in most other groups was small. When comparing the prespecified complication rates between groups, aside from the reduction in overall death in the platelet group as previously stated, the rate of systemic inflammatory response syndrome was higher in the patients who received platelets (65.0% vs 49.2%; P = .01; supplemental Table 1).
Cause of death by treatment group
| . | First 24 hours . | 30 days . | ||||
|---|---|---|---|---|---|---|
| Platelets (n = 137) . | No platelets (n = 124) . | P* . | Platelets (n = 137) . | No platelets (n = 124) . | P* . | |
| Total number of deaths | 8 | 21 | 13 | 25 | ||
| Cause of death, n (%)† | ||||||
| Exsanguination | 2 (1.5) | 16 (12.9) | <.01 | 2 (1.5) | 16 (12.9) | <.01 |
| Traumatic brain injury | 4 (2.9) | 5 (4.0) | .63 | 8 (5.8) | 9 (7.3) | .64 |
| Respiratory, pulmonary contusion, or tension pneumothorax | 0 (0) | 0 (0) | — | 1 (0.7) | 0 (0) | .32 |
| Multiple organ failure | 0 (0) | 0 (0) | — | 0 (0) | 1 (0.8) | .32 |
| Myocardial infarction | 1 (0.7) | 1 (0.8) | .94 | 1 (0.7) | 1 (0.8) | .94 |
| Pulmonary embolism | 0 (0) | 1 (0.8) | .32 | 0 (0) | 1 (0.8) | .32 |
| . | First 24 hours . | 30 days . | ||||
|---|---|---|---|---|---|---|
| Platelets (n = 137) . | No platelets (n = 124) . | P* . | Platelets (n = 137) . | No platelets (n = 124) . | P* . | |
| Total number of deaths | 8 | 21 | 13 | 25 | ||
| Cause of death, n (%)† | ||||||
| Exsanguination | 2 (1.5) | 16 (12.9) | <.01 | 2 (1.5) | 16 (12.9) | <.01 |
| Traumatic brain injury | 4 (2.9) | 5 (4.0) | .63 | 8 (5.8) | 9 (7.3) | .64 |
| Respiratory, pulmonary contusion, or tension pneumothorax | 0 (0) | 0 (0) | — | 1 (0.7) | 0 (0) | .32 |
| Multiple organ failure | 0 (0) | 0 (0) | — | 0 (0) | 1 (0.8) | .32 |
| Myocardial infarction | 1 (0.7) | 1 (0.8) | .94 | 1 (0.7) | 1 (0.8) | .94 |
| Pulmonary embolism | 0 (0) | 1 (0.8) | .32 | 0 (0) | 1 (0.8) | .32 |
P value was based on the Wald test for comparing 2 proportions.
Patients may have had >1 cause of death.
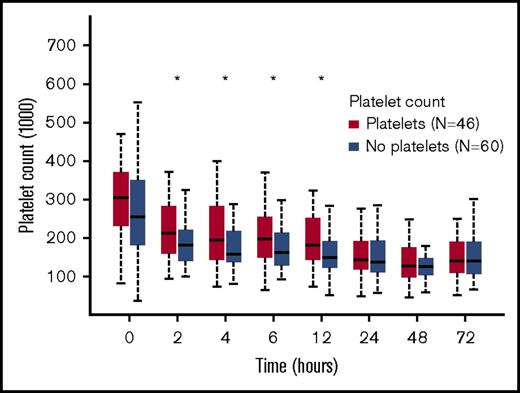
When determining the effects of platelet transfusion on platelet count, we needed to consider that many patients had received randomized treatment products, which included platelets in the 1:1:1 group, before the first blood draw used for analyzing platelet count. We therefore excluded patients who received randomized treatment blood products before the first blood sample collection to avoid falsely elevated baseline platelet counts in those who had already been transfused platelets. This resulted in a subgroup of 46 patients in the platelet cohort and 60 patients in the no-platelet cohort. This subgroup was generated only for analyzing platelet count, and all patients in this subgroup were included in the overall analysis. Although platelet counts dropped over time in all patients, those in the group receiving platelets had significantly higher platelet counts compared with the no-platelet group (P < .05 at 2, 4, 6, and 12 hours after admission; Figure 2).
Platelet counts over time. Counts (per microliter) measured by flow cytometric analysis (CD42b+ cells) over time among only those patients who did not receive prerandomized blood products before the 0-hour blood draw. Data are presented as box and whiskers plots.
Platelet counts over time. Counts (per microliter) measured by flow cytometric analysis (CD42b+ cells) over time among only those patients who did not receive prerandomized blood products before the 0-hour blood draw. Data are presented as box and whiskers plots.
Discussion
A definitive approach to platelet transfusion in trauma patients has been precluded by a lack of high-quality, randomized trial data demonstrating a clear effect on patient outcomes. In this subgroup analysis of the PROPPR randomized trial, we have shown that transfusion of platelets in bleeding patients is associated with improved early and late survival, improved hemostasis, and reduced number of deaths resulting from exsanguination, without an increase in significant inflammatory complications like acute respiratory distress syndrome, multiorgan failure, and acute kidney injury.
Platelets are critical components of the hemostatic system and are considered essential to effective clot formation and cessation of bleeding. Despite this, when, how, and whether to administer platelets during massive hemorrhage are poorly understood and constitute an ongoing discussion. The recent PROPPR trial data highlighted the benefit of higher plasma and platelet/RBC ratios, but distinguishing the relative benefit of 1 component over the other was, by design, not possible. This analysis shows that these same improvements in incidence of hemostasis and hemorrhaging death prevail, in addition to reduced early and late mortality, among patients who received platelets vs those who did not, while controlling for differences in plasma volume. Furthermore, our data support infusion of platelets as early as possible for a hemostatic resuscitation. Although we did not address the question of early vs late platelet administration through this study design, the median time to platelet infusion was 2 minutes after randomization, and a significant divergence in the mortality curve between the 2 cohorts occurred within the first 15 minutes after randomization. In addition, patients who received platelets did so first during the randomized treatment phase after a median of only 2 units of RBCs. Furthermore, given that the median time to death in those patients who did not receive platelets was 0.6 hours and that deaths occurring beyond 24 hours were due to causes other than hemorrhage, it can be concluded that the major benefit of platelet transfusions is the immediate management of exsanguination. Therefore, early platelet administration should be interpreted as urgently or as soon as possible.
In spite of data supporting the use of platelets after trauma and hemorrhage, many centers still administer platelets only after transfusion of ≥10 units of RBCs or when the platelet count has dropped below 100 × 109/L or 50 × 109/L.31-33 In this bleeding patient population, platelet counts never dropped below 100 × 109/L, and the median total number of RBC units transfused was 5. Therefore, these patients, despite their substantial bleeding, would never have achieved frequently used goal-directed critical thresholds for triggering platelet transfusion. Nonetheless, these data show a profound survival benefit when platelets were administered early and with platelet counts well within the normal range. The risk of complications such as transfusion-related acute lung injury has further precluded the use of platelets in the trauma population.34,35 However, receiving platelets was not associated with an increase in the incidence of acute lung injury or respiratory distress syndrome in this study, and in fact, we observed significantly more ventilator-free days in those who received platelets compared with those who did not.
In addition to the observed clinical benefits of platelet transfusions, this analysis also shows improvement in platelet count. In contrast, Vulliamy et al25 used data from a single center in the ACIT trial to show that platelet administration did not improve either platelet count or aggregation but did improve viscoelastic test parameters of fibrinolysis. Although this suggests that transfused platelets could still be beneficial after degranulation and release of procoagulant contents, even if not for their primary hemostatic capabilities, the study did not comment on clinical outcomes after platelet transfusion.
Several groups have reported aberrant platelet function after trauma; however, the mechanisms that drive this dysfunction are not well understood.12-14,36 Henriksen et al26 showed that although platelet aggregation function declined over time after admission in severely injured patients, it was further reduced in those patients receiving blood products, with platelet transfusion associated with the most dramatic reduction in function. One potential proposed mechanism for this is the known poor platelet function of stored platelets. Importantly, although in vitro laboratory tests of platelet aggregation may demonstrate a loss of platelet function after transfusion of platelets, the clinical outcome data presented here contradict this finding by showing that platelet transfusion was associated with improved hemostasis in vivo and survival. Because platelets have the shortest shelf-life of all transfused blood products and are the most frequently unavailable, efforts to mitigate storage lesion and extend shelf-life are important but must be balanced with clinical outcomes.
There are several limitations to this study. First, the 2 groups in our analysis were different with respect to plasma received because of the interventional design in PROPPR. The first cooler for the group receiving platelets (the 1:1:1 PROPPR study group) contained a unit of platelets and 3 more units of plasma. During the resuscitation phase, this group received the unit of platelets and a median of 1 additional unit of plasma. Therefore, these patients received extra plasma from the plasma transfusion plus the approximately 300 mL of plasma in the unit of platelets, and this additional plasma most likely had an effect on hemostasis. We controlled for these differences in plasma transfusion in the analysis; however, because this was not a study designed to examine the isolated effects of platelets, removing all bias was impossible. Nevertheless, although the difference in plasma between the 2 groups was statistically significant, the volume differences were of modest clinical significance and not likely to have contributed to the dramatic differences in outcomes observed here. Second, the patient population in this analysis was a subgroup of the PROPPR trial, and therefore, all patients enrolled were severely injured, had substantial bleeding, and were predicted to require a massive transfusion. Therefore, these data are not representative of the trauma population at large but rather a select group of critically ill patients, albeit those who might benefit most from a hemostatic resuscitation. Finally, the appropriate dose of platelets for trauma resuscitation is not known. The use of a unit of apheresis platelets as an adult dose was established in patients with cancer over decades of use and tested in the PLADO (Platelet Dose Trial) randomized controlled trial, but only in the context of prophylactic platelet transfusion to prevent World Health Organization grade 2 bleeding in hypoproliferative thrombocytopenia.37 The dose of 300+ billion platelets in such a unit is barely 16% of the total number of platelets normally in the body and was expected to raise the platelet concentration by 28 × 109/L.38 Nevertheless, the effect associated with this dose was clinically significant.
In conclusion, early platelet administration in severely injured and bleeding patients is associated with improved survival and hemostasis. At the time of platelet transfusions, median platelet counts were identical between groups (243 × 109/L), and no patients ever had platelet counts <100 × 109/L. We anticipate these findings will increase the early use of platelets in bleeding trauma patients, making efforts to address storage lesion and shelf-life limitations important. Such efforts will allow more platelets to be administered to bleeding patients earlier. Although we await a better alternative or improved storage conditions for platelet transfusion products that will further improve outcomes for bleeding patients, the platelets we have available now are good enough and should be infused first.
The full-text version of this article contains a data supplement.
Acknowledgments
The PROPPR trial was sponsored by the US National Institutes of Health, National Heart, Lung, and Blood Institute (U01HL077863), the US Department of Defense, and Defense Research and Development Canada in partnership with the Canadian Institutes for Health Research, Institute of Circulatory and Respiratory Health (CRR-120612).
Authorship
Contribution: J.C.C. designed the research, interpreted the data, and wrote the manuscript; X.Z. performed data analysis; E.E.F. assisted with data analysis and interpretation of the data; B.A.C., J.R.H., C.E.W., and M.A.S. assisted in study design and editing of the manuscript; and J.B.H. designed the research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the PROPPR Study Group appears in “Appendix.”
Correspondence: Jessica C. Cardenas, 6431 Fannin St, MSB 5.214, Houston, TX 77030; e-mail: jessica.c.cardenas@uth.tmc.edu.
Appendix: study group members
The members of the PROPPR Study Group are: John B. Holcomb, Charles E. Wade, Deborah J. del Junco, Erin E. Fox, Nena Matijevic, Jeanette Podbielski, and Angela M. Beeler (Clinical Coordinating Center); Barbara C. Tilley, Sarah Baraniuk, Hongjian Zhu, Joshua Nixon, Roann Seay, Savitri N. Appana, Hui Yang, and Michael O. Gonzalez (Data Coordinating Center); Lisa Baer, Yao-Wei Willa Wang, Brittany S. Hula, Elena Espino, An Nguyen, Nicholas Pawelczyk, Kisha D. Arora-Nutall, Rishika Sharma, Jessica C. Cardenas, Elaheh Rahbar, Tyrone Burnett Jr, and David Clark (Core Laboratory); Gerald van Belle, Susanne May, Brian Leroux, David Hoyt, Judy Powell, and Kellie Sheehan (Resuscitation Outcomes Consortium); Alan Hubbard and Adam P. Arkin (Systems Biology Committee); John R. Hess and Jeanne Callum (Transfusion Committee); and (in order of number of patients enrolled by clinical site) Bryan A. Cotton, Laura Vincent, Timothy Welch, Tiffany Poole, Evan G. Pivalizza, Sam D. Gumbert, Yu Bai, James J. McCarthy, Amy Noland, and Rhonda Hobbs (University of Texas Health Science Center at Houston); Eileen M. Bulger, Patricia Klotz, Lindsay Cattin, Keir J. Warner, Angela Wilson, David Boman, Nathan White, Andreas Grabinsky, and Jennifer A. Daniel-Johnson (University of Washington); Mitchell Jay Cohen, Rachael A. Callcut, Mary Nelson, Brittney Redick, Amanda Conroy, Marc P. Steurer, Preston C. Maxim, Eberhard Fiebig, Joanne Moore, and Eireen Mallari (University of California San Francisco); Peter Muskat, Jay A. Johannigman, Bryce R. H. Robinson, Richard D. Branson, Dina Gomaa, Christopher Barczak, Suzanne Bennett, Patricia M. Carey, Christopher N. Miller, Helen Hancock, and Carolina Rodriguez (University of Cincinnati); Kenji Inaba, Jay G. Zhu, Monica D. Wong, Michael Menchine, Kelly Katzberg, Sean O. Henderson, Rodney McKeever, Ira A. Shulman, Janice M. Nelson, Christopher W. Tuma, and Cheryl Y. Matsushita (University of Southern California); Thomas M. Scalea, Deborah M. Stein, Cynthia K. Shaffer, Christine Wade, Anthony V. Herrera, Seeta Kallam, Sarah E. Wade, Samuel M. Galvagno Jr, Magali J. Fontaine, Janice M. Hunt, and Rhonda K. Cooke (Shock, Trauma and Anesthesiology Research Organized Research Center, R. Adams Cowley Shock Trauma Center, University of Maryland Medical Center); Timothy C. Fabian, Jordan A. Weinberg, Martin A. Croce, Suzanne Wilson, Stephanie Panzer-Baggett, Lynda Waddle-Smith, and Sherri Flax (University of Tennessee Health Science Center Memphis); Karen J. Brasel, Pamela Walsh, David Milia, Allia Nelson, Olga Kaslow, Tom P. Aufderheide, Jerome L. Gottschall, and Erica Carpenter (Medical College of Wisconsin); Terence O’Keeffe, Laurel L. Rokowski, Kurt R. Denninghoff, Daniel T. Redford, Deborah J. Novak, and Susan Knoll (University of Arizona); Jeffrey D. Kerby, Jean-Francois Pittet, Patrick L. Bosarge, Albert T. Pierce, Carolyn R. Williams, Shannon W. Stephens, Henry E. Wang, and Marisa B. Marques (University of Alabama at Birmingham); Martin A. Schreiber, Jennifer M. Watters, Samantha J. Underwood, Tahnee Groat, Craig Newgard, Matthias Merkel, Richard M. Scanlan, and Beth Miller (Oregon Health and Science University); and Sandro Rizoli, Homer Tien, Barto Nascimento, Sandy Trpcic, Skeeta Sobrian-Couroux, Marciano Reis, Adic Pérez, Susan E. Belo, Lisa Merkley, and Connie Colavecchia (Sunnybrook Health Sciences Centre).