Key Points
NK cell natural cytotoxicity and antibody-dependent cellular cytotoxicity of patients with systemic mastocytosis are normal.
Trispecific killer engagers (161533 TriKE) target NK cells from normal donors and systemic mastocytosis patients to kill mast cells.
Introduction
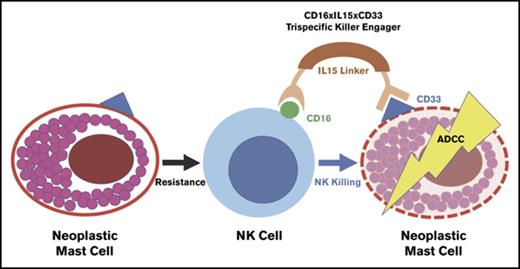
Systemic mastocytosis (SM) invariably involves the bone marrow (BM) and is categorized into indolent, smoldering, and advanced forms, which have poor prognostics and include aggressive SM, SM with an associated hematologic neoplasm (AHN), and mast cell leukemia.1,2 Given the lack of established standard therapy for advanced systemic mastocytosis (advSM), novel treatments are needed. We reported that neoplastic mast cells (MCs) were persistent after haploidentical natural killer (NK) cell therapy, and that NK cells poorly targeted ROSAKIT D816V and HMC-1.1 neoplastic MC lines.3 Our group has described a trispecific killer engager (TriKE) that amplifies NK cell–mediated killing of CD33+ myeloid targets by splicing together a single-chain variable fragment (scFv) against CD16, an scFv against CD33, and interleukin-15 (IL-15) inserted between the 2 as a linker (termed 161533 TriKE). The goal of this study was to demonstrate that the 161533 TriKE can trigger NK cell activation against neoplastic MCs expressing CD33 as a promising therapeutic strategy in SM.
Methods
Patients’ cells and cell lines
BM and peripheral blood samples were collected from SM patients at University of Minnesota and Stanford University after written informed consent was obtained for relevant local studies approved by each institution’s Human Subjects in Research Committee according the Declaration of Helsinki. Memorial Blood Centers (Minneapolis, MN) provided healthy donor peripheral blood mononuclear cells (PBMCs) after written informed consent. PBMCs were collected, and NK cells were enriched as described previously.4 HL-60, K562,5 HMC-1.1, HMC-1.2, and ROSAKIT D816V cell lines were maintained in culture as described.6,7
Functional assays
Fluorochrome-conjugated antibodies were from BD Bioscience (anti-CD3), Biolegend (anti-CD117, CD34, CD45, CD2, CD25, CD30, CD56, CD16, CD57, CD158a, CD158b, NKB1, NKG2A, CD107a, tumor necrosis factor-α [TNF-α], interferon-γ [IFN-γ]), and the CellTrace Violet Cell Proliferation Dye kit and the Live/Dead Fixable Aqua Dead Cell Stain Kit were from Invitrogen. Flow cytometry and analysis were as described.4 PBMCs were treated at 30 nmol/L of 161533 TriKE or anti-CD16 scFv as indicated. rhIL-15 was diluted to provide equivalent biological activity to the IL-15 linker of 161533 TriKE. CD107a, TNF-α, and IFN-γ were determined as previously described.8 ROSAKIT D816V MCs were labeled with CellTrace Violet Cell Proliferation Dye (Invitrogen), incubated with NK cells for 4 hours, and counted with flow cytometer for the killing assay. NK cell–mediated cytotoxicity of ROSAKIT D816V cells was also assessed in real time over a 24-hour period using an IncuCyte Live Cell Analysis System (Essen BioScience) as previously described.9
Statistical analysis
Graphpad Prism software was used for statistical analysis and figure preparation. One-way analysis of variance was used for multiple comparisons. Bars in figures indicate mean ± standard error of the mean. Statistical significance is indicated by *P ≤ .05; **P < .01; ***P < .001; ****P < .0001.
Results and discussion
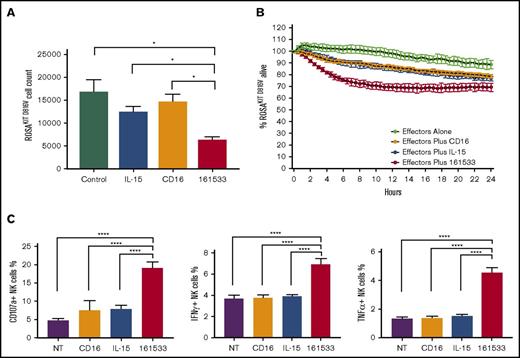
The central question to be tested is whether NK cells could be used to treat SM. Because CD33 is highly expressed on neoplastic MCs from SM patients,10 compared with normal MCs,11,12 we hypothesized that a 161533 TriKE molecule could be used to specifically drive NK cell killing of neoplastic MCs. When compared with other MC lines, high CD33 expression was observed on ROSAKIT D816V MCs, which contain the KIT D816V mutation found in >80% of SM patients, making this a good cell line model (supplemental Figure 1A).2 In a flow cytometry–based killing assay, the 161533 TriKE induced better NK cell–mediated killing of ROSAKIT D816V MCs when compared to controls (62.8% reduction in MC count vs 26.3% reduction in the rhIL-15 group, and 12.5% reduction in the anti-CD16 scFv group compared to no treatment group) (Figure 1A). Kinetics of ROSAKIT D816V cell killing measured by IncuCyte imaging confirmed effective NK cell–mediated cytotoxicity induced by 161533 TriKE when compared with controls (Figure 1B). Next, targeting of primary patient SM cells by normal NK cells was assessed. Eight SM patients were analyzed (supplemental Table 1). The median proportion of MCs in BM samples, identified as CD45+CD117highCD34− cells, was 0.62% (0.08-8.79) (supplemental Figure 1B). The MC proportion tended to be lower in BM samples of indolent SM patients as compared with advSM, but CD33 and other SM-associated markers (CD25/CD30/CD2) were highly expressed (supplemental Figure 1C). An scFv against CD16 and exogenous rhIL-15 alone did not induce NK cell activation, but 161533 TriKE treatment showed robust function with increased NK cell degranulation (CD107a) and inflammatory cytokine (IFN-γ and TNF-α) production (Figure 1C), demonstrating that allogeneic NK cells, when appropriately targeted by the 161533 TriKE, can be triggered to eliminate neoplastic MC targets.
NK cell killing assay targeting ROSAKIT D816Vcell line and NK cell activation assay targeting primary BM samples. (A) Normal donor NK cell–mediated killing of ROSAKIT D816V cells was evaluated in a 4-hour assay by measuring the number of remaining live ROSAKIT D816V cells using flow cytometry. (B) ROSAKIT D816V cell killing kinetics was measured by an IncuCyte imaging by evaluating the proportion of remaining live cells (representative of 3 studies). (C) Normal donor NK cell activation was measured against primary SM patient BM samples in the presence of indicted treatments with expression of CD107a, IFN-γ, and TNF-α production. *P ≤ .05; ****P < .0001. 161533, 161533 TriKE; CD16, anti-CD16 scFv; NT, no treatment.
NK cell killing assay targeting ROSAKIT D816Vcell line and NK cell activation assay targeting primary BM samples. (A) Normal donor NK cell–mediated killing of ROSAKIT D816V cells was evaluated in a 4-hour assay by measuring the number of remaining live ROSAKIT D816V cells using flow cytometry. (B) ROSAKIT D816V cell killing kinetics was measured by an IncuCyte imaging by evaluating the proportion of remaining live cells (representative of 3 studies). (C) Normal donor NK cell activation was measured against primary SM patient BM samples in the presence of indicted treatments with expression of CD107a, IFN-γ, and TNF-α production. *P ≤ .05; ****P < .0001. 161533, 161533 TriKE; CD16, anti-CD16 scFv; NT, no treatment.
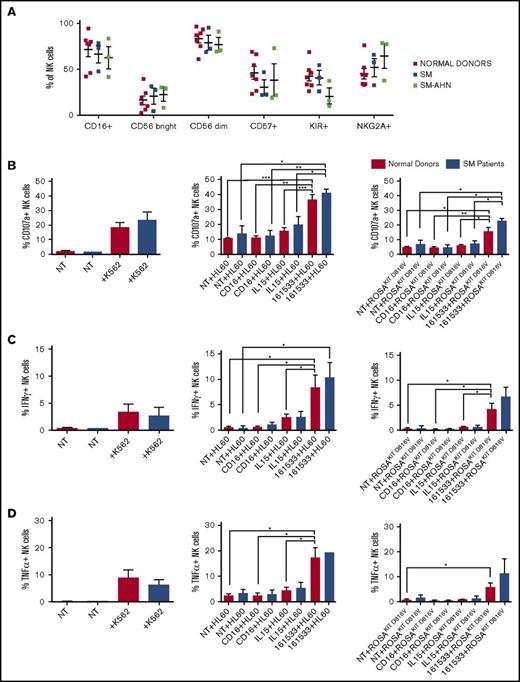
To gauge whether the 161533 TriKE could also engage endogenous NK cells, SM patient peripheral blood was evaluated. NK cells from SM patients showed no significant differences compared with normal donor expression of CD16, CD56bright/dim, CD57, KIR, and NKG2A (Figure 2A). The proportion of NK cells in the lymphocyte population was similar between SM patients (8.6% ± 3.1%) and normal donors (7.8% ± 2.4%), whereas the absolute NK cell count was low (63.2/μL [33.4-345.6]) compared with that reported in normal donors.13 No significant differences were noted between SM and SM-AHN NK cells in our small cohort. SM patient NK cells exhibited normal natural cytotoxicity against K562 target cells (Figure 2B-D left) but poor targeting of HL-60 and ROSAKIT D816Vwith anti-CD16 ligation alone or rhIL-15. In contrast, antibody-dependent cell-mediated cytotoxicity with the 161533 TriKE induced NK cell activation against both HL-60 and ROSAKIT D816V cells (Figure 2B-D center and right). These data imply that endogenous SM patient NK cells can respond to neoplastic MC targets when activated by a 161533 TriKE to promote antibody-dependent cell-mediated cytotoxicity.
NK cell immunophenotyping and activation assay of SM patients. (A) Six SM patients with AHN (n = 3) and without AHN (n = 3) were compared with 7 normal donors by flow cytometry. (B) CD107a, (C) IFN-γ, and (D) TNF-α expression of NK cells was measured against K562 (left), HL-60 (center), and ROSAKIT D816V (right) target cells with noted treatment after 4-hour incubation. *P ≤ .05; **P < .01; ***P < .001. Red bars, normal donors; blue bars, SM patients.
NK cell immunophenotyping and activation assay of SM patients. (A) Six SM patients with AHN (n = 3) and without AHN (n = 3) were compared with 7 normal donors by flow cytometry. (B) CD107a, (C) IFN-γ, and (D) TNF-α expression of NK cells was measured against K562 (left), HL-60 (center), and ROSAKIT D816V (right) target cells with noted treatment after 4-hour incubation. *P ≤ .05; **P < .01; ***P < .001. Red bars, normal donors; blue bars, SM patients.
Treatment options for advSM patients are limited.14-16 Targeting CD33 might be a good therapeutic option. Gemtuzumab ozogamicin demonstrated clinical activity in a patient with refractory advSM17 with in vitro antiproliferative effects in neoplastic MCs.12 The present study revealed robust ability of a 161533 TriKE to drive both endogenous NK cells from SM patients and healthy allogeneic donor NK cells to target neoplastic MCs. Implementation of the 161533 TriKE in a therapeutic setting of SM would largely be based on the competency and number of the patient’s NK cells. A first-in-human trial, planned to open by the end of 2018, will evaluate the safety of the 161533 TriKE (on endogenous NK cells) on CD33+ myeloid malignancies, including advSM patients, containing ≥25 NK cells per microliter of blood.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Joseph Butterfield at Mayo Clinic, Rochester, MN for providing HMC-1.1 and -1.2 cell lines, and the Flow Cytometry Core at the University of Minnesota for their excellent technical support and services.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute T32 training grant T32HL007062 (H.D.Y.), National Cancer Institute grants P01 CA111412 (M.F. and J.S.M.), P01 CA65493 (M.F. and J.S.M.), and US Department of Defense grant CA150085 (M.F.).
Authorship
Contribution: H.D.Y., M.F., C.U., and J.S.M. designed the study, analyzed data, and wrote the manuscript; J.G. and C.U. enrolled patients with SM, collected BM and peripheral blood samples, and wrote the manuscript; M.F., D.A.V., and J.S.M. constructed the 161533 TriKE; M.A. provided the ROSAKIT D816V cell line and edited the manuscript; P.H. performed functional assays; and S.C. provided data on samples and wrote the manuscript.
Conflict-of-interest disclosure: J.S.M. and D.A.V. serve on the Scientific Advisory Board of GT Biopharma and have received research funds and clinical trial support from these relationships. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict-of-interest policies. M.F. has received research funds from GT Biopharma. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, Division of Hematology, Oncology, and Transplantation, University of Minnesota, 420 Delaware St SE, Mayo Mail Code 806, Minneapolis, MN 55455; e-mail: mille011@umn.edu.