Key Points
Childhood B-ALL patients, including those with VHR features, had favorable outcomes on DFCI 05-001 risk-stratified therapy.
IKZF1 deletion was an independent predictor of inferior outcome, including among patients with low end-induction MRD.
Abstract
Dana-Farber Cancer Institute (DFCI) ALL Consortium Protocol 05-001 tested a new risk stratification system in children and adolescents with newly diagnosed acute lymphoblastic leukemia (ALL). At study entry, B-ALL patients were classified as standard risk (SR) or high risk (HR) based on age, white blood cell (WBC) count, and central nervous system status. After achieving complete remission (CR), patients with high end-induction minimal residual disease (MRD) (≥10−3 by polymerase chain reaction analysis of patient-specific antigen receptor rearrangements) and/or adverse cytogenetics (KMT2A rearrangement or hypodiploidy) were reclassified as very high risk (VHR) and received intensified therapy. IKZF1 deletion status was retrospectively evaluated by multiplex ligation-dependent probe amplification. Between 2005 and 2011, 678 Philadelphia chromosome-negative B-ALL patients aged 1 to 18 years enrolled; 651 achieved CR and 648 received a final risk group. Among all 678 patients, 5-year event-free survival (EFS) was 87% (95% confidence interval [CI], 84-89) and overall survival 93% (95% CI, 90-94). Five-year disease-free survival of SR patients (N = 407) was 94% (95% CI, 91-96), HR (N = 176) was 84% (95% CI, 77-88), and VHR (N = 65) was 79% (95% CI, 67-87). IKZF1 deletion was present in 62 of 385 (16%) assessed patients and was associated with inferior 5-year EFS (63%; 95% CI, 49%-74% vs 88%; 95% CI, 84%-91%; P < .001), and higher 5-year cumulative incidence of relapse, including among those with low MRD (24% vs 8%, P = .001). In multivariable analysis, age ≥15 years, WBC ≥50 × 109/L, IKZF1 deletion, and MRD ≥10−4 was each associated with inferior outcome. In conclusion, risk-stratified therapy on DFCI 05-001 resulted in favorable outcomes for B-ALL patients, including those with VHR features. IKZF1 deletion was an independent predictor of inferior outcome. This trial was registered at www.clinicaltrials.gov as #NCT00400946.
Introduction
Despite advances in the treatment of childhood acute lymphoblastic leukemia (ALL), as many as 20% of children and adolescents with newly diagnosed ALL experience relapse. Efforts to improve outcomes have included the stratification of treatment intensity based upon prognostic factors, including presenting age, white blood cell (WBC) count, immunophenotype, and central nervous system (CNS) status at diagnosis.1 In addition to these features, biologic features of the leukemia have been shown to have prognostic significance. For instance, rearrangements of the KMT2A (MLL) gene and low hypodiploidy have each been associated with inferior outcome.2-4 Early response to initial therapy is also an important and independent predictor of outcome in childhood B-ALL. High levels of minimal residual disease (MRD) at the end of 4 weeks of multiagent induction chemotherapy are associated with an increased risk of subsequent relapse in children with B-ALL.5-7
On Dana-Farber Cancer Institute (DFCI) protocol 05-001, we incorporated end-induction MRD response into risk stratification for patients with Philadelphia chromosome (Ph)–negative (P−) B-ALL, and piloted an intensified regimen for patients with very high risk (VHR) features, defined as high end-induction MRD and/or adverse cytogenetics (KMT2A rearrangements or hypodiploidy). In addition, we assessed the prognostic impact of IKZF1 gene deletion, an abnormality that has been identified in ∼15% of Ph− childhood B-ALL patients, and is associated with an inferior outcome.8-10 We present here the outcome for patients with Ph− B-ALL treated on DFCI 05-001.
Patients and methods
Patients
Patients aged 1 to 18 years with newly diagnosed ALL (excluding mature B-cell ALL) were eligible for enrollment on DFCI 05-001 from participating institutions (supplemental Table 1). The institutional review board of each participating institution approved the protocol before enrolling patients. Informed consent was obtained from parents/guardians before study enrollment and initiation of therapy.
Risk groups, cytogenetics, and MRD assessment
At study entry, patients were assigned an initial risk group, standard risk (SR) or high risk (HR), based on age, presenting WBC count, and CNS status. Patients were classified as initial HR if they demonstrated age ≥10 years, WBC ≥50 × 109/L, or diagnostic cerebrospinal fluid sample with lymphoblasts and ≥5 WBC/high-powered field (CNS-3.) All other patients were classified as Initial SR.
All patients were screened for cytogenetic abnormalities by karyotype, fluorescence in situ hybridization, and/or polymerase chain reaction (PCR); adverse cytogenetics were defined as the presence of KMT2A rearrangement (any fusion partner) or hypodiploidy (<45 chromosomes). MRD was assessed in those who achieved morphologic complete remission (CR) at the end of the first month of treatment (day 32) by real-time quantitative PCR analysis of patient-specific antigen receptor gene rearrangements as previously described.5 Results were reported as the ratio of copy numbers of target gene:glyceraldehyde-3-phosphate dehydrogenase; a ratio of ≥10−3 (≥.001) was considered high end-induction MRD. The final risk group was assigned based on initial risk group, cytogenetics, and MRD results. Patients with indeterminate MRD results were assigned a final risk group based on initial risk group and cytogenetic findings. Patients with KMT2A rearrangements, hypodiploidy, and/or high end-induction MRD were assigned to the VHR group, regardless of initial risk group. The final risk group for patients lacking any VHR feature was the same as the initial risk group. Patients with BCR-ABL1-positivity (Ph+) were removed from trial during induction and enrolled on other clinical trials or were treated on the HR arm with imatinib until proceeding to hematopoietic stem cell transplant (HSCT) in first CR.
Therapy
DFCI 05-001 therapy is summarized in supplemental Table 2 and supplemental Figure 1. All patients received a 4-week multiagent Induction phase, including 60 mg/m2 of doxorubicin. Remission assessment was performed on day 32. CR was defined as <5% marrow blasts with peripheral blood count recovery, evidence of normal hematopoiesis, and absence of extramedullary disease. Patients with persistent morphologic leukemia (≥5% blasts in marrow) at end-induction were classified as induction failure and removed from study to receive alternative therapy (and considered events at time 0 in outcome analyses). Patients achieving CR proceeded to the 3-week Consolidation IA phase. Final risk group was assigned by the end of Consolidation IA. SR and HR patients proceeded to the CNS phase, followed by Consolidation II and Continuation phases.
VHR patients received intensified treatment with 2 additional 21-day chemotherapy cycles, Consolidation IB, and Consolidation IC beginning week 7 of therapy. Consolidation IB included cyclophosphamide (1000 mg/m2 on day 1), low-dose cytarabine (75 mg/m2 per day, days 2-5 and 9-12), 6-mercaptopurine (50 mg/m2 per day, days 1-14), and intrathecal methotrexate (day 1). Consolidation IC included cytarabine (2 g/m2 per dose IV every 12 hours for 4 doses starting day 1), etoposide (100 mg/m2 per day, days 3-5), dexamethasone (18 mg/m2 per day, days 1-5), and l-asparaginase beginning day 8. VHR therapy then followed the HR treatment arm beginning with the CNS phase. Cranial radiation was administered to VHR patients and to those presenting with WBC ≥100 × 109/L or CNS-3 status. Intensification for VHR patients did not include HSCT. Total planned treatment duration was 24 months in CR for all patients.
Between April 2005 and February 2010, patients achieving CR were eligible to participate in randomized assignment to intramuscular (IM) Escherichia coli asparaginase or IV pegaspargase for post-induction therapy, as previously reported.11 Patients who declined randomization were directly assigned to IM E coli asparaginase. After February 2010, when the randomization had met target accrual, patients were directly assigned to IM E coli asparaginase. Because there was no difference in outcome between native E colil-asparaginase and pegaspargase during the randomized portion of the trial,11 results for all Ph− B-ALL patients have been combined here.
IKZF1 deletion status
IKZF1 deletion status was retrospectively assessed in 385 patients with available samples (by multiplex ligation-dependent probe amplification [SALSA MLPA P202 IKZF1, MRC-Holland]) using DNA extracted from archived diagnostic blood or bone marrow samples.
Statistical methods
Descriptive statistics are presented as frequencies/percentages or medians/ranges. Fisher’s exact test and Wilcoxon rank-sum test compared patient characteristics by groups for categorical and continuous variables, respectively. Event-free survival (EFS) was defined as time from trial registration to relapse, subsequent malignant neoplasm (SMN), or death (whichever occurred first), and was censored at date of last known status. Induction failures and deaths were considered events at time 0. Disease-free survival (DFS), defined among patients achieving CR, was calculated as time from date of remission to first event, including relapse, SMN, or death, and censored at last known status. Overall survival (OS) was calculated from time of trial registration to death from any cause. EFS, DFS, and OS were estimated using the method of Kaplan and Meier and tested between groups with the log-rank test. EFS and DFS were modeled univariately and adjusted in multivariable models with the Cox proportional hazards model. Multivariable models were fit with the stepwise selection method considering patient characteristic (including presenting WBC, sex, age, Down syndrome) and cytogenetics (including hyperdiploidy with double trisomy, ETV6-RUNX1, KMT2A-rearranged, iAMP21, and TCF3-PBX1) with a significance level of 0.25 to be considered in the model and a significance level of 0.05 to remain in the model. DFS models included end-induction MRD at designated thresholds (10−3 or 10−4). IKZF1 deletion status was included in modeling where indicated. Cumulative incidence of relapse, with death as a competing risk, was estimated with the cuminc utility in the cmprsk12 package in R and tested using the Gray test, and is defined from date of remission. To explore refining initial risk classification, we used recursive partitioning in the R package rpart.13 The cutoffs for age and WBC at diagnosis that resulted in the largest difference in EFS were explored. P values are 2-sided: values <.05 were considered significant. No adjustments were made for performing multiple statistical tests. SAS, version 9.4, and R, version 3.4.3, were used for statistical analyses.
Results
Patients and risk group stratification
Between April 2005 and December 2011, 697 B-ALL patients enrolled. Baseline characteristics are presented in Table 1. Of 697 total patients, 19 (3%) were Ph+. For the 678 (97%) Ph− patients, median follow-up was 5.9 years.
Patient characteristics and 5-y EFS of children and adolescents with newly diagnosed Ph− B-ALL treated on DFCI ALL 05-001
| . | N (%) . | 5-y EFS (95% CI), % . | P . |
|---|---|---|---|
| Overall | 678 | 87 (84-89) | |
| Initial DFCI risk group | <.001 | ||
| Standard | 460 (68) | 91 (88-94) | |
| High | 218 (32) | 77 (71-82) | |
| Age at diagnosis, y | .002* | ||
| <10 | 531 (78) | 89 (86-91) | |
| ≥10 | 147 (22) | 79 (71-85) | |
| 10-<15 | 97 (14) | 85 (76-91) | |
| ≥15 | 50 (7) | 66 (51-78) | |
| WBC at diagnosis, × 109/L | <.001 | ||
| <50 | 578 (85) | 90 (87-92) | |
| ≥50 | 100 (15) | 70 (60-78) | |
| Sex | .51 | ||
| Male | 357 (53) | 86 (82-89) | |
| Female | 321 (47) | 87 (83-91) | |
| CNS status at diagnosis | .50 | ||
| CNS-1 | 540 (80) | 87 (84-90) | |
| CNS-2 | 90 (13) | 86 (77-92) | |
| CNS-3 | 6 (1) | 100 (-) | |
| Traumatic with blasts | 24 (3) | 75 (53-88) | |
| Traumatic without blasts | 18 (3) | 89 (62-97) | |
| Down syndrome | 25 (4) | 95 (68-99) | .19 |
| Cytogenetics | |||
| Hyperdiploidy (51-65 chr) | 198 (29) | 89 (84-93) | .11 |
| Trisomy chr 4 and 10 | 121 (18) | 92 (86-96) | .034 |
| No double trisomy | 77 (11) | 85 (74-91) | .74 |
| ETV6-RUNX1 | 154 (23) | 95 (90-98) | <.001 |
| Hypodiploidy (<45 chr) | 10 (1) | 80 (41-95) | .50 |
| KMT2A-rearranged | 12 (2) | 58 (27-80) | .001 |
| iAMP21 | 13 (2) | 67 (33-86) | .081 |
| TCF3-PBX1 | 23 (3) | 82 (59-93) | .39 |
| Normal karyotype | 128 (19) | 87 (79-92) | .54 |
| Final DFCI risk group† | <.001 | ||
| Standard | 407 (60) | 94 (91-96) | |
| High | 176 (26) | 84 (77-88) | |
| Very high | 65 (10) | 79 (67-87) | |
| End-induction (day 32) MRD† | <.001 | ||
| Low <10−3 | l488 | 91 (88-93) | |
| High ≥10−3 | 47 | 77 (62-87) | |
| Indeterminate/unknown‡ | 113 | 87 (78-92) |
| . | N (%) . | 5-y EFS (95% CI), % . | P . |
|---|---|---|---|
| Overall | 678 | 87 (84-89) | |
| Initial DFCI risk group | <.001 | ||
| Standard | 460 (68) | 91 (88-94) | |
| High | 218 (32) | 77 (71-82) | |
| Age at diagnosis, y | .002* | ||
| <10 | 531 (78) | 89 (86-91) | |
| ≥10 | 147 (22) | 79 (71-85) | |
| 10-<15 | 97 (14) | 85 (76-91) | |
| ≥15 | 50 (7) | 66 (51-78) | |
| WBC at diagnosis, × 109/L | <.001 | ||
| <50 | 578 (85) | 90 (87-92) | |
| ≥50 | 100 (15) | 70 (60-78) | |
| Sex | .51 | ||
| Male | 357 (53) | 86 (82-89) | |
| Female | 321 (47) | 87 (83-91) | |
| CNS status at diagnosis | .50 | ||
| CNS-1 | 540 (80) | 87 (84-90) | |
| CNS-2 | 90 (13) | 86 (77-92) | |
| CNS-3 | 6 (1) | 100 (-) | |
| Traumatic with blasts | 24 (3) | 75 (53-88) | |
| Traumatic without blasts | 18 (3) | 89 (62-97) | |
| Down syndrome | 25 (4) | 95 (68-99) | .19 |
| Cytogenetics | |||
| Hyperdiploidy (51-65 chr) | 198 (29) | 89 (84-93) | .11 |
| Trisomy chr 4 and 10 | 121 (18) | 92 (86-96) | .034 |
| No double trisomy | 77 (11) | 85 (74-91) | .74 |
| ETV6-RUNX1 | 154 (23) | 95 (90-98) | <.001 |
| Hypodiploidy (<45 chr) | 10 (1) | 80 (41-95) | .50 |
| KMT2A-rearranged | 12 (2) | 58 (27-80) | .001 |
| iAMP21 | 13 (2) | 67 (33-86) | .081 |
| TCF3-PBX1 | 23 (3) | 82 (59-93) | .39 |
| Normal karyotype | 128 (19) | 87 (79-92) | .54 |
| Final DFCI risk group† | <.001 | ||
| Standard | 407 (60) | 94 (91-96) | |
| High | 176 (26) | 84 (77-88) | |
| Very high | 65 (10) | 79 (67-87) | |
| End-induction (day 32) MRD† | <.001 | ||
| Low <10−3 | l488 | 91 (88-93) | |
| High ≥10−3 | 47 | 77 (62-87) | |
| Indeterminate/unknown‡ | 113 | 87 (78-92) |
Chr, chromosome.
P value reflects age with grouping of <10 and ≥10 y. For age grouping of <10, 10-<15, and ≥15 y, P < .001.
DFS reported for 648 patients with final DFCI risk group and MRD subgroups.
Characteristics of patients with indeterminate/unknown end-induction MRD: 72% <10 y of age at diagnosis, 57% male, 89% with WBC at diagnosis <50 × 109/L.
Of the 678 Ph− patients, 460 (68%) were classified as Initial SR and 218 (32%) as Initial HR (Figure 1). Four patients were not evaluable for induction response (2 withdrew consent before day 32, 2 did not have end-induction bone marrow assessment). Of the 674 evaluable patients, 651 (97%) achieved CR, including 451 (99%) Initial SR and 200 (92%) Initial HR patients. Ten patients had persistent leukemia at end-induction (2 Initial SR and 8 Initial HR). Thirteen patients died in Induction (4 Initial SR and 9 Initial HR) from infection (N = 10, 1 also with encephalopathy), CNS hemorrhage (N = 3, 1 also with infection), and acute respiratory distress syndrome (N = 1). Three patients who achieved CR withdrew consent within 16 days of documented CR and did not receive final risk group assignment.
B-ALL patient enrollment and DFCI 05-001 risk group stratification. *Changed to very high risk for the following: 28 high MRD only, 3 KMT2A-rearranged only, 5 hypodiploidy. †Changed to very high risk for the following: 17 high MRD only, 2 KMT2A-rearranged and high MRD, 5 KMT2A-rearranged only, 5 hypodiploidy.
B-ALL patient enrollment and DFCI 05-001 risk group stratification. *Changed to very high risk for the following: 28 high MRD only, 3 KMT2A-rearranged only, 5 hypodiploidy. †Changed to very high risk for the following: 17 high MRD only, 2 KMT2A-rearranged and high MRD, 5 KMT2A-rearranged only, 5 hypodiploidy.
Of the 648 patients who received final risk group assignment, 407 (63%) were classified as SR, 176 (27%) as HR, and 65 (10%) as VHR. Of 449 Initial SR patients with a final risk group, 407 (91%) remained SR, 6 (1%) changed to HR (5 because of CNS-3 status identified after study enrollment, 1 previously misclassified as SR with age ≥10 years), and 36 (8%) changed to VHR. Of the 199 Initial HR patients with a final risk group, 170 (85%) remained HR and 29 (15%) changed to VHR (Figure 1).
Reasons for assignment of the 65 patients to the VHR group were as follows: 45 (69%) had high end-induction MRD without adverse cytogenetic findings, including 28 Initial SR and 17 Initial HR patients (Figure 1); 10 had KMT2A rearrangement (5 low MRD, 2 high MRD, 3 indeterminate MRD); and 10 had hypodiploidy (7 low MRD, 3 indeterminate MRD). Features of VHR patients are presented in supplemental Table 3.
Survival outcomes in Ph− B-ALL patients
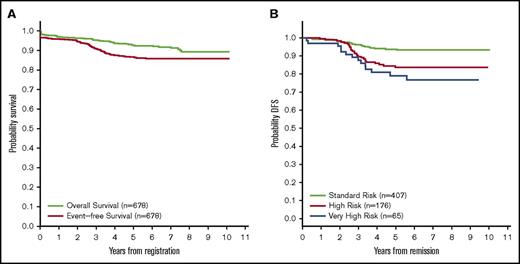
The 5-year EFS and OS for the 697 B-ALL patients on DFCI 05-001 were 86% (95% confidence interval [CI], 83-89) and 92% (95% CI, 90-94). Among the 678 Ph− patients, 5-year EFS was 87% (95% CI, 84-89) and OS was 93% (95% CI, 90-94) (Figure 2A). Postinduction events included 2 deaths in remission (sepsis/grade 3-4 neutropenia in a VHR patient with hypodiploidy and pneumonia in an SR patient). There were 2 SMN (both AML, 1 HR patient and 1 VHR patient with KMT2A rearrangement). The secondary AML in the VHR patient was also KMT2A rearranged, but had a different fusion partner. There were 62 total relapses. The 5-year DFS was 94% (95% CI, 91-96) for Final SR patients, 84% (95% CI, 77-88) for Final HR, and 79% (95% CI, 67-87) for Final VHR (Figure 2B). Sites and cumulative incidence of relapse based on final risk group are presented in supplemental Table 3 and supplemental Figure 2, respectively.
Kaplan-Meier curves reporting survival for Ph−B-ALL patients. (A) OS and EFS overall. (B) DFS by final risk group for patients achieving CR.
Kaplan-Meier curves reporting survival for Ph−B-ALL patients. (A) OS and EFS overall. (B) DFS by final risk group for patients achieving CR.
Among the 65 VHR patients, there were 12 relapses: 10 marrow only and 2 combined marrow/CNS. Of the 10 patients with hypodiploidy, 6 had <40 chromosomes (1 of whom relapsed and 1 died in remission) and 4 had 40 to 44 chromosomes (none with events). Five-year DFS for the 45 patients with high MRD and no known adverse cytogenetic features was 81% (95% CI, 65-90). The 5-year DFS was 70% (95% CI, 33-89) for KMT2A-rearranged patients and 80% (95% CI, 41-95) for those with hypodiploidy. There was no significant difference in DFS between VHR patients who were Initial SR (5-year DFS 77%; 95% CI, 60-88) vs Initial HR (82%; 95% CI, 62-92, P = .74).
Prognostic factors
The following features were each univariately associated with inferior EFS: Initial HR group, age at diagnosis ≥10 years, presenting WBC ≥50 × 109/L, and KMT2A rearrangement. There was a trend toward inferior outcome in patients with intrachromosomal amplification of RUNX1 (iAMP21) that did not reach statistical significance (P = .081). ETV6-RUNX1 and high hyperdiploidy with trisomies of chromosomes 4 and 10 were each significantly associated with favorable EFS. No significant associations between EFS and other factors, including sex, CNS status, and Down syndrome status, were observed. Final risk group was significantly associated with DFS (P < .001) overall; in pairwise comparisons, SR patients had significantly better DFS than HR (P < .001) and VHR patients (P < .001). A significant difference in DFS comparing HR and VHR patients was not detectable (P = .27). High end-induction MRD (≥10−3) was associated with inferior DFS (Table 1). In the subset of patients with determinate MRD and cytogenetics (n = 526), multivariable model selection indicated features associated with inferior DFS were high end-induction MRD (hazard ratio, 2.9; 95% CI, 1.5-5.7; P = .002), KMT2A rearrangement (hazard ratio, 4.3; 95% CI, 1.3-14.8; P = .019), and iAMP21 (hazard ratio, 5.3; 95% CI, 1.9-14.8; P = .002); high WBC count (≥50 × 109/L) was marginally significant (hazard ratio, 1.9; 95% CI, 1.0-3.7; P = .054).
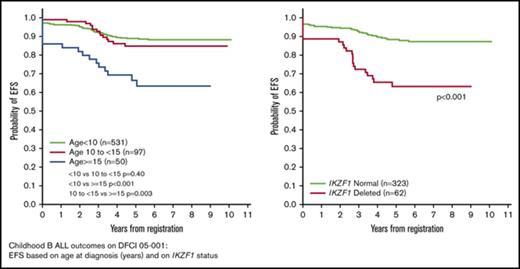
To refine risk classification for future trials, we explored the prognostic significance of alternative age and WBC count thresholds, alternative end-induction MRD levels, and IKZF1 deletion status. Recursive partitioning suggested that age ≥15 years resulted in the largest separation of EFS curves within Ph− B-ALL patients. Further analysis of outcome by age demonstrated that Ph− B-ALL patients aged 10 to 14.99 years had similar EFS to those <10 years of age, whereas those ≥15 years of age had significantly worse outcome (Table 1; Figure 3A). Recursive partitioning indicated that WBC ≥40 × 109/L resulted in the largest separation in EFS curves; however, results were similar compared with the trial-defined ≥50 × 109/L cutoff.
Kaplan-Meier curves reporting survival for Ph−B-ALL patients. (A) EFS by age at diagnosis (<10, 10-<15, and ≥15 years of age). (B) DFS based on end-induction MRD (very low [<10−4], intermediate low [10−3-10−4], and high [≥10−3]). (C) EFS based on IKZF1 deletion status. (D) DFS based on IKZF1 deletion status in patients with low end-induction MRD (<10−3).
Kaplan-Meier curves reporting survival for Ph−B-ALL patients. (A) EFS by age at diagnosis (<10, 10-<15, and ≥15 years of age). (B) DFS based on end-induction MRD (very low [<10−4], intermediate low [10−3-10−4], and high [≥10−3]). (C) EFS based on IKZF1 deletion status. (D) DFS based on IKZF1 deletion status in patients with low end-induction MRD (<10−3).
Detailed quantitative day 32 bone marrow MRD results were available for 514 patients. In exploratory analysis, MRD was recategorized as very low (<10−4), intermediate low (10−4-<10−3), and high (≥10−3). The recategorized MRD classification was significantly associated with DFS (Figure 3B). Pairwise comparisons indicated that inferior DFS was associated with intermediate (10−4-<10−3) compared with very low (<10−4) MRD (P = .006) and high (≥10−3) compared with very low MRD (P < .001). Of 46 relapses in the patient subset with detailed quantitative end-induction MRD, 35 (76%) occurred in those considered to have low MRD by protocol definition (<10−3); 12 (26%) occurred among patients with intermediate low end-induction MRD and 23 (50%) among those with very low MRD. Eleven (24%) of the total relapses occurred among high MRD patients (supplemental Table 4).
IKZF1 deletion status was assessed in all 385 Ph− patients with available samples. A total of 62 (16%) had IKZF1 deletion (24 were dominant negative, 32 expected null, and 6 other). Patients with samples for IKZF1 testing were more likely to have high diagnostic WBC (P < .001) and be considered Initial HR (P < .001) compared with those without samples (supplemental Table 5). Patient characteristics based on IKZF1 deletion status are presented in supplemental Table 6. IKZF1 deletions were more common in patients ≥10 years of age at diagnosis, with WBC ≥50 × 109/L, and in Hispanic patients. Patients with IKZF1 deletion experienced a higher rate of induction failure (8% vs 1%, P = .003) and a higher 5-year cumulative incidence of relapse (29% vs 8%, P < .001) compared with those without IKZF1 deletion. Five-year EFS was significantly lower in patients with IKZF1 deletion (63%; 95% CI, 49-74) among IKZF1 deleted vs 88% (95% CI, 84-91) for non-IKZF1 deleted patients, P < .001). There were no detectable differences in EFS based on type of IKZF1 deletion. Five-year OS was significantly lower in IKZF1 deleted vs non-IKZF1 deleted patients (79%; 95% CI, 67-88 vs 94%, 95% CI, 91-96; P < .001).
Of the 363 patients tested for IKZF1 achieving CR and continuing to postinduction therapy, 316 had evaluable end-induction MRD levels. Among the 294 patients with IKZF1 testing and trial-defined low end-induction MRD (<10−3), IKZF1 deletion was associated with a significantly higher 5-year cumulative incidence of relapse (24%; 95% CI, 12-39 vs 8%; 95% CI, 5-12%; without deletion, P = .001), and significantly worse 5-year DFS (75%; 95% CI, 58-86 vs 91%; 95% CI, 86-94; P = .002) (Figure 3D). Among the 236 patients with very low end-induction MRD (<10−4), IKZF1 deletion remained significantly associated with higher 5-year cumulative incidence of relapse (17.4%; 95% CI, 6.2-33.3 vs 6.5%; 95% CI, 3.5-10.8; P = .034) and worse 5-year DFS (82.6%; 95% CI, 63.1-92.4 vs 93.0%; 95% CI, 88.1-95.9; P = .043.) Among the 22 patients with high end-induction MRD (≥10−3), all of whom received intensified VHR therapy, 3 of 8 with IKZF1 deletion subsequently relapsed compared with 3 of 14 without IKZF1 deletion (P = .62).
In the subset of patients tested for IKZF1 (n = 385), multivariable Cox proportional hazards models for EFS were fit with the stepwise selection method considered including age, sex, presenting WBC, cytogenetic findings, and IKZF1 deletion. With the age cutoff ≥10 years, the final model indicated that IKZF1 deletion (P < .001) and high WBC (P < .001) were associated with inferior EFS. In modeling considering an age cutoff ≥15 years, IKZF1 deletion (P = .011), high WBC (P < .001), and older age (P = .003) were associated with inferior EFS. Multivariable Cox proportional hazards models for DFS were considered including age, sex, WBC, cytogenetic findings, end-induction MRD, and IKZF1 deletion (n = 316). When including the trial-defined MRD cutoff of 10−3 in model selection, IKZF1 deletion (P = .005) and WBC (P = .036) were significantly associated with DFS considering age cutoffs of 10 or 15 years. In the subset with detailed quantitative MRD (n = 306), when an MRD threshold of 10−4 was considered, WBC (P = .042) and MRD (P = .002) remained significant and IKZF1 deletion (P = .050) marginally significant (Table 2). Five-year DFS was 93% (95% CI, 88-96) for the 166 patients who lacked any of the following adverse prognostic factors: age at diagnosis ≥15 years, presenting WBC ≥50 × 109/L, end-induction MRD (≥10−4), IKZF1 deletion, KMT2A rearrangement, and iAMP21.
Multivariable hazard models for EFS and DFS among Ph− B-ALL patients
| . | Univariate . | Final multivariable models . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| EFS (10-y age threshold), N = 385 | ||||
| IKZF1, deleted vs nondeleted | 3.5 (2.1-6.0) | <.001 | 2.8 (1.6-4.6) | <.001 |
| WBC, ≥50 vs <50 × 109/L | 3.9 (2.4-6.6) | <.001 | 3.3 (1.9-5.6) | <.001 |
| EFS (15-y age threshold), N =385 | ||||
| IKZF1, deleted vs nondeleted | 3.5 (2.1-6.0) | <.001 | 2.2 (1.2-3.9) | .011 |
| Age, ≥15 y vs <15 y | 4.6 (2.5-8.8) | <.001 | 2.8 (1.4-5.7) | .003 |
| WBC, ≥50 vs <50 × 109/L | 3.9 (2.4-6.6) | <.001 | 3.1 (1.8-5.3) | <.001 |
| DFS, N = 526*† | ||||
| MRD, ≥10−3 vs <10−3 | 3.0 (1.5-5.8) | .001 | 2.9 (1.5-5.7) | .002 |
| KMT2A, rearranged vs not rearranged | 6.0 (1.9-19.3) | .003 | 4.3 (1.3-14.8) | .019 |
| iAMP21, present vs not present | 4.3 (1.6-12.1) | .005 | 5.3 (1.9-14.8) | .002 |
| WBC, ≥50 vs <50 × 109/L | 2.3 (1.2-4.2) | .011 | 1.9 (1.0-3.7) | .054 |
| DFS (10−3MRD threshold), N = 316*‡ | ||||
| IKZF1, deleted vs nondeleted | 3.1 (1.6-6.4) | .001 | 2.7 (1.3-5.6) | .005 |
| WBC, ≥50 vs <50 × 109/L | 2.5 (1.3-5.0) | .008 | 2.1 (1.0-4.2) | .036 |
| DFS (10−4MRD threshold), N = 306*§ | ||||
| IKZF1, deleted vs nondeleted | 2.9 (1.4-5.8) | .004 | 2.1 (1.0-4.4) | .050 |
| WBC, ≥50 vs <50 × 109/L | 2.7 (1.4-5.4) | .004 | 2.1 (1.0-4.2) | .042 |
| MRD, ≥10−4 vs <10−4 | 3.4 (1.7-6.6) | <.001 | 2.9 (1.5-5.7) | .002 |
| . | Univariate . | Final multivariable models . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| EFS (10-y age threshold), N = 385 | ||||
| IKZF1, deleted vs nondeleted | 3.5 (2.1-6.0) | <.001 | 2.8 (1.6-4.6) | <.001 |
| WBC, ≥50 vs <50 × 109/L | 3.9 (2.4-6.6) | <.001 | 3.3 (1.9-5.6) | <.001 |
| EFS (15-y age threshold), N =385 | ||||
| IKZF1, deleted vs nondeleted | 3.5 (2.1-6.0) | <.001 | 2.2 (1.2-3.9) | .011 |
| Age, ≥15 y vs <15 y | 4.6 (2.5-8.8) | <.001 | 2.8 (1.4-5.7) | .003 |
| WBC, ≥50 vs <50 × 109/L | 3.9 (2.4-6.6) | <.001 | 3.1 (1.8-5.3) | <.001 |
| DFS, N = 526*† | ||||
| MRD, ≥10−3 vs <10−3 | 3.0 (1.5-5.8) | .001 | 2.9 (1.5-5.7) | .002 |
| KMT2A, rearranged vs not rearranged | 6.0 (1.9-19.3) | .003 | 4.3 (1.3-14.8) | .019 |
| iAMP21, present vs not present | 4.3 (1.6-12.1) | .005 | 5.3 (1.9-14.8) | .002 |
| WBC, ≥50 vs <50 × 109/L | 2.3 (1.2-4.2) | .011 | 1.9 (1.0-3.7) | .054 |
| DFS (10−3MRD threshold), N = 316*‡ | ||||
| IKZF1, deleted vs nondeleted | 3.1 (1.6-6.4) | .001 | 2.7 (1.3-5.6) | .005 |
| WBC, ≥50 vs <50 × 109/L | 2.5 (1.3-5.0) | .008 | 2.1 (1.0-4.2) | .036 |
| DFS (10−4MRD threshold), N = 306*§ | ||||
| IKZF1, deleted vs nondeleted | 2.9 (1.4-5.8) | .004 | 2.1 (1.0-4.4) | .050 |
| WBC, ≥50 vs <50 × 109/L | 2.7 (1.4-5.4) | .004 | 2.1 (1.0-4.2) | .042 |
| MRD, ≥10−4 vs <10−4 | 3.4 (1.7-6.6) | <.001 | 2.9 (1.5-5.7) | .002 |
Final multivariable model results are displayed, with only statistically significant factors presented. All multivariable models were adjusted for baseline characteristics, cytogenetic findings, and end-induction MRD (in DFS models) as detailed in the “Patients and methods” section.
HR, hazard ratio.
Age was considered with a 10 y and 15 y of age threshold in each DFS model, with the same final model suggested with either threshold.
IKZF1 deletion status not considered in this model (considered in all other models).
Does not include indeterminate MRD.
Does not include indeterminate MRD and required detailed quantitative MRD results.
Discussion
We present outcomes for children and adolescents with Ph− B-ALL on DFCI 05-001, including those who received intensified therapy based on high end-induction MRD and/or HR cytogenetic features. The overall 5-year EFS and OS (87% and 93%, respectively) compared favorably with other contemporaneously conducted trials.14-19 Intensifying therapy for patients with VHR features (without HSCT) resulted in a relatively favorable DFS of 79%.
Previously published reports indicated that pediatric B-ALL patients with high end-induction MRD had significantly worse outcomes when treated with standard chemotherapy.5,6 The randomized United Kingdom ALL-2003 study demonstrated that intensification of therapy for non-HR patients with high end-induction MRD (>0.01% by PCR methodology) resulted in a superior EFS compared with those receiving standard therapy.18 In our study, we used a higher cutoff to define high MRD, with a resultant 5-year DFS of 81% for those considered VHR only on the basis of MRD (and without adverse cytogenetics). Intensification of therapy was not associated with excessive rates of toxic death or SMN, with a single remission death and a single case of subsequent AML within the VHR group. Despite the relatively favorable outcome of patients with high end-induction MRD, there remained greater likelihood of relapse, suggesting that intensification of therapy does not fully eliminate the prognostic impact of end-induction MRD status. However, the majority of relapses were observed in patients with low end-induction MRD, indicating that further refinement of prognostic features is needed to identify patients at risk for adverse outcome.
Analyses of prognostic factors on DFCI 05-001 identified potential changes in risk stratification to better identify higher and lower risk patients. Age has long been recognized as a significant predictor of outcome, with the traditional National Cancer Institute (NCI) risk group classification cutoff of 10 years of age at diagnosis used to designate age-based SR or HR groups.1,20 In our study, recursive partitioning suggested that age ≥15 years resulted in the largest separation of EFS; further analysis of outcome by age demonstrated that B-ALL patients aged 10 to 14.99 years had similar EFS to those <10 years of age, whereas those ≥15 years of age had significantly worse outcome. Age ≥15 years was statistically associated with EFS in multivariable modeling. High presenting WBC (≥50 × 109/L) remained a statistically significant predictor of EFS. Sex did not have prognostic significance, in keeping with our prior reports.21 KMT2A rearrangements and iAMP21 were each significantly associated with inferior outcomes. Others have shown that using HR rather than SR therapy in patients with iAMP21 may abrogate the adverse prognostic significance of iAMP21.22,23 Our outcomes for patients with hypodiploidy were relatively favorable4 ; however, interpretation is limited by small patient numbers. Definitions of hypodiploidy should also be considered when comparing with other trials. As reported by others, patients with ETV6-RUNX1 and high hyperdiploidy with trisomies of 4 and 10 achieved excellent outcomes.2,24,25
Our results also highlight the prognostic importance of IKZF1 deletion status among Ph− B-ALL patients.8-10 IKZF1 deletion was associated with higher rates of induction failure and of relapse and was a significant predictor of outcome on univariate and multivariable analyses. Importantly, among patients with low end-induction MRD, IKZF1 deletion was associated with inferior DFS. A limitation is that patients with samples available for IKZF1 testing were more likely to have HR characteristics, which could affect interpretation. Nonetheless, our results support that IKZF1 deletion status helps to identify patients at higher risk of relapse who do not have other HR features, and thus has importance in risk stratification.
IKZF1 deletion has been described to occur frequently in cases of Ph-like ALL, a poor-prognostic subset of B-ALL characterized by a gene expression profile similar to Ph+ ALL, but without the BCR-ABL1 fusion.9,26 Genetic alterations in Ph-like ALL have been shown to affect signaling pathways, including ABL class and JAK-STAT pathways, suggesting the potential for targeted interventions26 ; thus, screening for fusion status may identify patients who might benefit from targeted therapies such as tyrosine kinase inhibitors.27 We have previously reported that, for NCI HR B-ALL patients treated on DFCI 05-001, IKZF1 deletions retained significance as an adverse prognostic factor, even when controlling for the presence of a kinase-activating fusion,28 suggesting that testing for kinase fusions would enhance but not replace IKZF1 screening in future risk-adapted trials. In our current trial, DFCI protocol 16-001, we are prospectively screening for Ph-like lesions in all NCI HR B-ALL patients, as well as in other B-ALL patients with IKZF1 deletion and/or high end-consolidation MRD. Others have shown that deletions of the ERG gene may abrogate the adverse prognostic significance of IKZF1 deletion when they cooccur.29,30 ERG deletion status was not assessed in our cohort, but should be considered in future evaluations.
MRD is an important predictor of outcome, although other factors, including age, WBC, and IKZF1 status, retained significance as outcome predictors even when controlling for end-induction MRD. This finding may be due, in part, to the protocol-defined MRD cutoff (10−3) and because MRD was only assessed at 1 time point. For instance, patients with very low (<10−4) end-induction MRD had superior DFS compared with intermediate levels (between 10−4 and 10−3), identifying a subgroup of patients with particularly favorable DFS. Others have shown that MRD measured at a second time point in therapy, 10 to 12 weeks after the start of treatment, is an important predictor of outcome.7,14,31 The Italian Association of Pediatric Hematology Oncology-Berlin-Frankfurt-Munster ALL 2000 study used MRD assessment at 2 time points to stratify patients, with MRD assessment at days 33 and 78.14 Patients with high MRD (≥10−3) at the second, later, time point had poorer outcome, with intermediate outcomes observed among those with high end-induction but low second time point MRD. The Dutch Childhood Oncology Group also risk-stratified patients based on 2 MRD time points. Five-year EFS was 87%, with EFS of 88% in MRD medium-risk patients (high end-induction/low second time point MRD) who received intensification and maintenance therapy similar to DFCI 05-001 HR patients.31 This result suggests that, among the patients classified as VHR based on MRD on DFCI 05-001, incorporation of a second MRD time point may have discriminated between those who could be successfully treated as HR patients (with low second time point MRD) vs those requiring more intensified therapy.
On DFCI 05-001, cranial radiation was administered to VHR patients and to those presenting with WBC ≥100 × 109/L or CNS-3 status. On our current trial, only CNS-3 patients receive cranial radiation, based in part on results of a meta-analysis that indicated cranial radiation was without significant benefit for B-ALL patients without overt CNS disease at diagnosis.32
Results of DFCI 05-001 should be interpreted noting the limitation of patient numbers as exemplified by wide CI ranges in some subgroup analyses. However, the results of DFCI 05-001, as well as those reported by others, suggest that current risk classification could be refined to better identify patients at higher risk of relapse for whom intensified or novel therapies may be indicated, as well as those with very favorable expected outcomes, who potentially could be effectively treated with less intensive regimens. In our current trial, DFCI protocol 16-001, we are incorporating changes to risk stratification for B-ALL patients including the use of an age cutoff of 15 years to distinguish SR and HR patients, incorporation of prospectively determined IKZF1 deletion status, and assessment of MRD by next-generation sequencing assay including at a second, postinduction time point to identify VHR patients.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors express their gratitude to the patients and families who participated in this study and Annette Dalton, Jeffrey Kutok, Albert Moghrabi, Yvan Samson, and Cindy Schwartz for their contributions to this work.
This work was supported by a grant from the National Institutes of Health, National Cancer Institute (5P01CA068484) and Enzon Pharmaceuticals.
Authorship
Contribution: All authors designed research, performed research, analyzed and interpreted data, and contributed to the written manuscript; T.M.B. and K.E.S. performed the statistical analysis; M.H.H. performed minimal residual disease analysis and IKZF1 deletion analysis; M.H.H., J.E.O., B.L.A., U.H.A., L.A.C., P.D.C., K.M.K., C.L., J.-M.L., B.M., M.A.S., J.J.G.W., and L.B.S. collected data; and L.M.V. and L.B.S. wrote the manuscript with contributions from all coauthors.
Conflict-of-interest disclosure: B.L.A. has served on advisory boards for Sigma Tau Pharmaceuticals and advisory boards and speakers bureau for Jazz Pharmaceuticals. L.B.S. has served on advisory boards for Sigma Tau Pharmaceuticals, Jazz Pharmaceuticals, and Baxalta. The remaining authors declare no competing financial interests.
Correspondence: Lynda M. Vrooman, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: lynda_vrooman@dfci.harvard.edu.


![Figure 3. Kaplan-Meier curves reporting survival for Ph−B-ALL patients. (A) EFS by age at diagnosis (<10, 10-<15, and ≥15 years of age). (B) DFS based on end-induction MRD (very low [<10−4], intermediate low [10−3-10−4], and high [≥10−3]). (C) EFS based on IKZF1 deletion status. (D) DFS based on IKZF1 deletion status in patients with low end-induction MRD (<10−3).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/12/10.1182_bloodadvances.2018016584/3/m_advances016584f3.jpeg?Expires=1765888126&Signature=dSUZw-TrF7HPBbdiMlyXTXU7AGeOWwZISj-O~6ul7UHI9XVocaxAR6XA3pFbpGy~cKH9I29GbmKgyjoHVfmhyJZHpdvmRvZ4CshiIANRnBew7tKgtgYNnmoiZazQsG7EQlfEAp28Z6l0nXvDuKK4Sbmh5pVdyNsOdgcnEmTIQ252xjARy28rneJCTH1wicr5g1yHu-oS~TIcQnxfsj6TkwnGiZswpTChX75ncrqysxaNEV06I8tqUf7dFatJn5vUE43kccG-pBvE557gVZ6dBvJrdMYvKK6wCh93a3Bc6QGxAqnIqr0dYLbAzG7oapsbLH58cnO3pXyW5LUWYybtpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)