Key Points
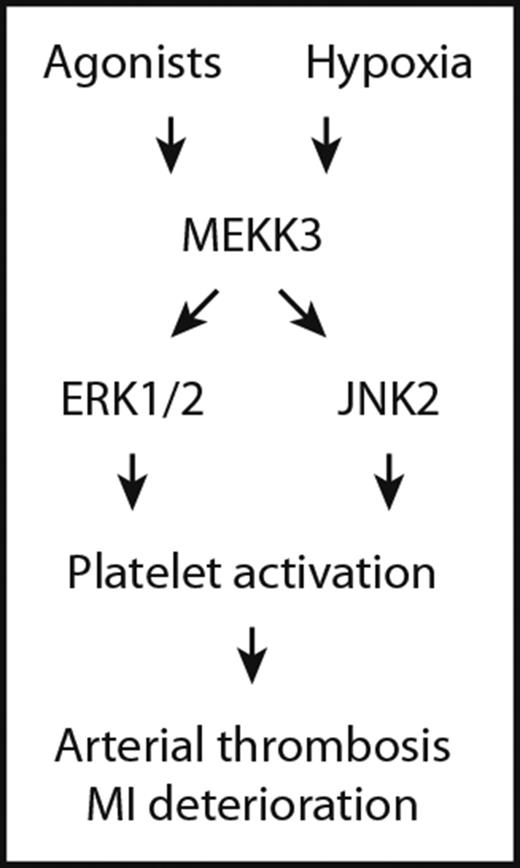
MEKK3 regulates platelet activation through ERK1/2 and JNK2.
MEKK3−/− mice are protected from microthrombosis and myocardial infarct expansion post-MI.
Abstract
MAPKs play important roles in platelet activation. However, the molecular mechanisms by which MAPKs are regulated in platelets remain largely unknown. Real-time polymerase chain reaction and western blot data showed that MEKK3, a key MAP3K family member, was expressed in human and mouse platelets. Then, megakaryocyte/platelet-specific MEKK3-deletion (MEKK3−/−) mice were developed to elucidate the platelet-related function(s) of MEKK3. We found that agonist-induced aggregation and degranulation were reduced in MEKK3−/− platelets in vitro. MEKK3 deficiency significantly impaired integrin αIIbβ3–mediated inside-out signaling but did not affect the outside-in signaling. At the molecular level, MEKK3 deficiency led to severely impaired activation of extracellular signal–regulated kinases 1/2 (ERK1/2) and c-Jun NH2-terminal kinase 2 but not p38 or ERK5. In vivo, MEKK3−/− mice showed delayed thrombus formation following FeCl3-induced carotid artery injury. Interestingly, the tail bleeding time was normal in MEKK3−/− mice. Moreover, MEKK3−/− mice had fewer microthrombi, reduced myocardial infarction (MI) size, and improved post-MI heart function in a mouse model of MI. These results suggest that MEKK3 plays important roles in platelet MAPK activation and may be used as a new effective target for antithrombosis and prevention of MI expansion.
Introduction
Platelet activation and accumulation at sites of vascular injury play critical roles in arterial thrombosis and are the primary pathogenic mechanisms underlying acute coronary syndromes.1,2 Antiplatelet drugs, including aspirin and clopidogrel, are effective in reducing the mortality and morbidity of acute coronary syndromes. However, reports of drug resistance and severe side effects, such as life-threatening bleeding, have been increasing in recent years.3-6 A better understanding of the molecular mechanisms leading to platelet activation, especially under pathological conditions, is essential for the development of new therapeutics.
MAPKs constitute a family of serine/threonine protein kinases that convert extracellular stimuli into a wide range of cellular responses. All eukaryotic cells possess multiple MAPK pathways that coordinately regulate gene expression, mitosis, metabolism, motility, survival, apoptosis, and differentiation. Conventional MAPKs include the extracellular signal-regulated kinases (ERK)1/2, p38 (α, β, γ, δ), JNK1/2/3, and big MAPK (ERK5). Previous studies using specific inhibitors or knockout mice showed that these 4 MAPKs were important regulators of platelet activation.7-11 However, precisely how these MAPKs are activated in platelets remains largely unclear.
MAPKs are activated through a 3-tiered kinase cascade that includes a MAPK, a MAPK kinase (MAP2K), and a MAP2K kinase (MAP3K).12 MAP3K phosphorylates and activates a MAP2K, which then stimulates MAPK activity through dual phosphorylation on Thr and Tyr residues within a conserved Thr–X–Tyr motif located in the activation loop of the kinase.13 MEKK3, a member of the MAP3K family, is an important activator of MAPK pathways.14,15 Although MEKK3 plays essential roles in cardiovascular development16 and immune responses,17 its functions in platelet activation and thrombosis remain unknown. Here, using megakaryocyte/platelet-specific MEKK3-deletion (MEKK3−/−) mice, we unravel an undocumented role for MEKK3 in platelet activation, thrombosis, and myocardial infarct expansion. Our study may provide evidence for applying MEKK3 to antiplatelet therapies.
Methods
Materials
Adenosine diphosphate (ADP), fibrinogen (Fg), and the thromboxane A2 (TxA2) stable analog U46619 were purchased from Sigma-Aldrich (St. Louis, MO). Thrombin was obtained from Enzyme Research Laboratories (South Bend, IN), and collagen was from CHRONO-LOG (Havertown, PA). Mouse anti-MEKK3, fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD62P, and phycoerythrin (PE)-conjugated hamster anti-mouse β3 antibodies were from BD Biosciences (San Jose, CA). Rabbit anti-MLK3, anti–phospho-ERK1/2, anti–phospho-p38, anti–phospho-JNK, anti–phospho-ERK5, and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were from Cell Signaling Technology (Danvers, MA). Rabbit anti-MEKK5, anti–B-Raf, and anti–C-Raf antibodies were from Bioworld Technology (Nanjing, China). Rabbit anti–A-Raf antibody was from Santa Cruz Biotechnology (Dallas, TX). FITC-conjugated rat anti-mouse GPVI antibody (JAQ1) and PE-conjugated rat anti-mouse GPIb monoclonal antibody were from Emfret Analytics (Würzburg, Germany). FITC- and PE-conjugated rat IgG controls were from BioLegend (San Diego, CA). Alexa Fluor 647–conjugated Fg was from Life Technologies (Gaithersburg, MD). Anti-CD42C antibody was from Lifespan Biosciences (Seattle, WA). Secondary horseradish peroxidase–conjugated antibodies were from Jackson ImmunoResearch (West Grove, PA). SuperSignal chemiluminescent substrate was from EMD Millipore (Boston, MA). The inhibitors SP600125 and U0126 were from Selleck Chemicals (Houston, TX).
Mice
MEKK3-floxed mice (MEKK3f/f) on the C57BL/6 genetic background were described previously.17 To delete MEKK3 specifically in platelets, MEKK3f/f mice were crossed with PF4-Cre–transgenic mice18 to obtain MEKK3f/fPF4-Cre+MEKK3−/− mice. Mice were genotyped by polymerase chain reaction (PCR), and MEKK3 deficiency in platelets was confirmed by western blotting. The Shanghai Jiao Tong University School of Medicine Animal Care and Use Committee approved the animal research.
FeCl3-induced carotid artery injury model
A murine FeCl3-induced carotid artery injury thrombosis model was created as described previously.19 Monitoring of carotid artery blood flow was initiated at the time of FeCl3 treatment and maintained continuously for 10 min. Carotid artery blood flow <0.06 mL/min was scored as occlusion, and the time to first occlusion was determined.
Tail-bleeding assay
Mice were anesthetized and maintained with 2% isoflurane. A 2-mm portion of the tail tip was excised and submerged in 0.9% NaCl at 37°C. Bleeding was followed visually, and time to stable cessation of bleeding (no rebleeding within 1 min) was recorded.
Myocardial infarction model
Chronic myocardial infarction (MI) was induced by permanent ligation of the left anterior descending (LAD) coronary artery. The anesthetized mouse was placed on a heating pad and intubated endotracheally for mechanical ventilation (inspiratory tidal volume of 250 μL at 130 breaths per minute). A left thoracotomy was performed in the fourth intercostal space. The mouse heart was exposed, and the LAD coronary artery was ligated 2 mm from its ostial origin with a 7-0 silk suture. Regional ischemia was confirmed by electrocardiography changes (ST elevation). A sham operation involved the same procedure without ligation.
Echocardiography
Mice were anesthetized and maintained with 1%-2% isoflurane in 95% oxygen. Echocardiography was performed using a Vevo 2100 echocardiography machine (VisualSonics, Toronto, ON, Canada) and a linear-array 30-MHz transducer (MS-550D). Left ventricular (LV) systolic and diastolic measurements were captured in M-mode from the parasternal short axis.
LV infarct assessment
Hearts were harvested after perfusion and fixation in 4% paraformaldehyde on day 7 post-MI. Parasternal short-axis sections were cut before mounting and staining with Masson’s trichrome reagent. Slides were analyzed and photographed using an Olympus light microscope (model IX71). The infarct area (green collagen staining for scar tissue) is expressed as a percentage of the LV surface area, as determined using ImageJ software (National Institutes of Health, Bethesda, MD).
Platelet preparation, aggregation, and ATP secretion
Human and mouse washed platelets were prepared as described previously,19,20 and inhibitors were incubated with the platelets for 3 minutes before stimulation. Adenosine triphosphate (ATP) secretion was measured using CHRONO-LUME reagent (CHRONO-LOG) according to the manufacturer’s protocol.
CD62P exposure and Fg-binding assay
Washed MEKK3f/f and MEKK3−/− platelets (3 × 107/mL) were incubated for 20 min at room temperature with 40 μg/mL Alexa Fluor 647–conjugated Fg and thrombin, ADP, U46619, and collagen in a final volume of 50 μL of Tyrode’s buffer containing 1 mmol/L CaCl2. Fg binding was measured using a flow cytometer (CytoFLEX S; Beckman Coulter). For the CD62P-exposure assay, washed platelets (3 × 107/mL) were incubated with 5 μg/mL FITC-conjugated rat anti-mouse CD62P antibody and thrombin, ADP, U46619, and collagen in a final volume of 50 μL of Tyrode’s buffer for 20 min at room temperature.
Platelet spreading on immobilized Fg
Analysis of platelet spreading on immobilized Fg was done as described previously.19,20 Platelets were stained with rhodamine-conjugated phalloidin and visualized with an upright fluorescent AXIO ScopeA1 microscope (Zeiss Group, Jena, Germany) equipped with a 100×/1.30 oil objective lens, an X-Cite 120Q light source (EXFO, Mississauga, ON, Canada), and a digital camera. Up to 4 images were chosen at random per experiment and analyzed under blind conditions. The platelet surface area was analyzed using ImageJ software.
Clot retraction
Clot retraction using mouse platelets was determined as described previously.19,20 Clot size was quantified from photographs using ImageJ software. Retraction is expressed as a ratio (1 − [final clot size/initial clot size]).
Measurement of MAP3K expression levels in human and mouse platelets
Total mRNA was extracted from washed human or mouse platelets (n = 3 for each group). The expression levels of MEKK1-8, MLK1-3, MEKK12, MEKK13, MLK4, TaoK1-3, A-Raf, B-Raf, and C-Raf in human and mouse platelets were measured using an ABI 7500 real-time quantitative PCR system (Applied Biosystems, Foster City, CA). Relative mRNA expression levels were normalized to the GAPDH expression level.
Western blotting
For detection of target proteins by immunoblotting, platelets were processed and developed as described previously.20 After detection of target proteins, the membranes were stripped and incubated with an anti-GAPDH antibody to determine the amount of protein present in each lane.
Statistical analysis
Statistical analyses were performed using Prism 5 (GraphPad). The Student t test (mean and standard deviation [SD]) and 1-way ANOVA were used to determine statistical significance between 2 groups and among multiple groups, respectively. P < .05 was considered statistically significant.
Results
MAP3K expression profiles in human and mouse platelets
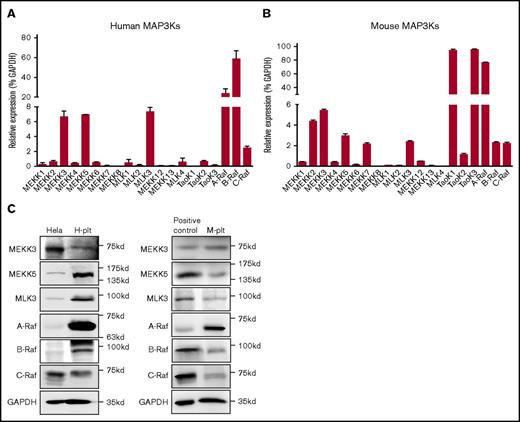
In mammals, ≥20 MAP3K family members have been characterized, including MEKK1-8, MEKK12, MEKK13, MLK1-4, TAOK1-3, A-Raf, B-Raf, and C-Raf. The expression of the 20 MAP3K family members was detected by real-time quantitative PCR (Figure 1A-B) and confirmed using western blotting (Figure 1C). In addition to MEKK5 and Raf, which have been reported to exist in platelets,21,22 MEKK3 was expressed at a relatively high level in human and mouse platelets. As a leukocyte marker, CD53 mRNA was barely detectable in the human or mouse samples, demonstrating that there was no contamination of leukocytes in the platelet mRNA samples (supplemental Figure 1A).
MAP3K expression profiles in human and mouse platelets. Relative mRNA levels of MAP3Ks in human platelets (H-plt) (n = 3) (A) and in mouse platelets (M-plt) (n = 3) (B). Data are mean ± SD. (C) Expression of MEKK3, MEKK5, MLK3, A-Raf, B-Raf, and C-Raf in H-plt and M-plt was detected by western blot. Proteins extracted from HeLa cells were used as positive controls for human MAP3K detection. NIH 3T3 cells were used as positive controls for mouse MLK3, A-Raf, and C-Raf detection; mouse heart tissue was used as positive controls for MEKK3 and MEKK5 detection; and mouse leukocytes were used as positive controls for B-Raf detection. GAPDH was used to verify equal loading.
MAP3K expression profiles in human and mouse platelets. Relative mRNA levels of MAP3Ks in human platelets (H-plt) (n = 3) (A) and in mouse platelets (M-plt) (n = 3) (B). Data are mean ± SD. (C) Expression of MEKK3, MEKK5, MLK3, A-Raf, B-Raf, and C-Raf in H-plt and M-plt was detected by western blot. Proteins extracted from HeLa cells were used as positive controls for human MAP3K detection. NIH 3T3 cells were used as positive controls for mouse MLK3, A-Raf, and C-Raf detection; mouse heart tissue was used as positive controls for MEKK3 and MEKK5 detection; and mouse leukocytes were used as positive controls for B-Raf detection. GAPDH was used to verify equal loading.
MEKK3 deficiency delayed arterial thrombosis but had no effect on platelet counts and hemostasis
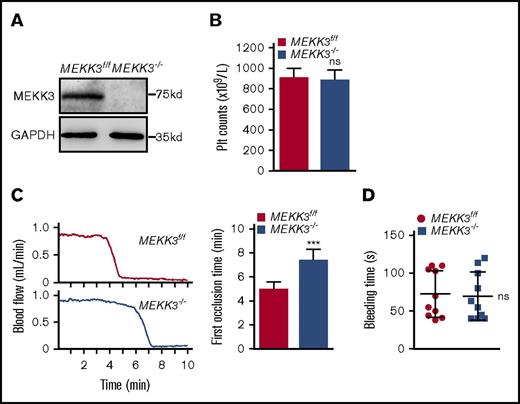
MEKK3 germline–knockout mice die at approximately embryonic day 11 due to the arrest of early embryonic angiogenesis.16 Therefore, MEKK3−/− mice were developed to study the functions of MEKK3 in platelet activation and thrombosis. Western blot results showed that MEKK3 was totally ablated in MEKK3−/− mouse platelets (Figure 2A). The peripheral platelet number was 907.5 ± 28.8 × 109/L in MEKK3f/f mice vs 885.2 ± 30.8 × 109/L in MEKK3−/− mice (Figure 2B). The data demonstrated that megakaryocyte/platelet depletion of MEKK3 had no effect on platelet formation, indicating that MEKK3 was not required for thrombopoiesis. Additionally, platelet size and morphology were unchanged in MEKK3−/− mice (supplemental Figure 1C-D).
MEKK3 deficiency delayed arterial thrombosis but had no effect on platelet counts or hemostasis. (A) Washed platelets were prepared from MEKK3f/f and MEKK3−/− mice. Western blot results show that MEKK3 was depleted in MEKK3−/− platelets. (B) Platelet counts in the peripheral blood of MEKK3f/f and MEKK3−/− mice (n = 10; mean ± SD; P > .05). (C) The mouse carotid artery was treated with 10% FeCl3 for 3 minutes, and blood flow traces were monitored (n = 10, mean ± SD). (D) Mouse tail-bleeding time was determined as the time to visual cessation of bleeding (n = 10, mean ± SD; P > .05). ***P < .001. ns, not significant.
MEKK3 deficiency delayed arterial thrombosis but had no effect on platelet counts or hemostasis. (A) Washed platelets were prepared from MEKK3f/f and MEKK3−/− mice. Western blot results show that MEKK3 was depleted in MEKK3−/− platelets. (B) Platelet counts in the peripheral blood of MEKK3f/f and MEKK3−/− mice (n = 10; mean ± SD; P > .05). (C) The mouse carotid artery was treated with 10% FeCl3 for 3 minutes, and blood flow traces were monitored (n = 10, mean ± SD). (D) Mouse tail-bleeding time was determined as the time to visual cessation of bleeding (n = 10, mean ± SD; P > .05). ***P < .001. ns, not significant.
To determine the role of MEKK3 in platelet activation in vivo, a FeCl3-induced carotid artery thrombosis model was used. The first occlusion time was delayed in MEKK3−/− mice induced by 7.5%, 10%, and 15% FeCl3 (Figure 2C; supplemental Figure 1F). This result demonstrated that MEKK3 played important roles in arterial thrombus formation. Interestingly, hemostasis was normal in MEKK3−/− mice evaluated by measuring tail-bleeding time (average bleeding time was 69.7 ± 10 seconds vs 72.6 ± 9.8 seconds in MEKK3f/f mice, Figure 2D). To further evaluate the roles of MEKK3 in platelet function under shear stress, a whole-blood perfusion was performed over a fibrillar collagen matrix at an arterial shear rate of 30 dyn/cm2 or an intravenous shear rate of 5 dyn/cm2. After 5 minutes, the fluorescence of aggregated MEKK3−/− platelets was 331 600 ± 201 300 in contrast to 1 125 000 ± 166 400 for MEKK3f/f platelets at a shear rate of 30 dyn/cm2. However, there was no significant difference between MEKK3f/f and MEKK3−/− platelets at a shear rate of 5 dyn/cm2 (supplemental Figure 1G). These data suggested that the role of platelet MEKK3 in thrombus formation was dependent on high shear stress.
MEKK3 regulated platelet activation through integrin αIIbβ3–mediated inside-out signaling
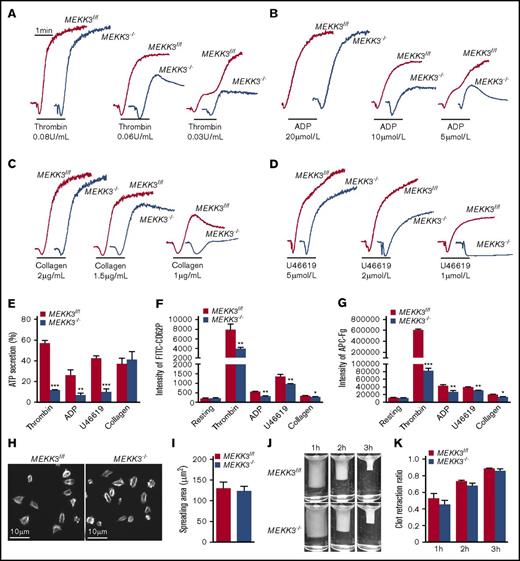
The role of MEKK3 in agonist-induced platelet aggregation was next investigated by stimulating MEKK3f/f and MEKK3−/− platelets with thrombin, ADP, collagen, and a TxA2 receptor agonist, U46619. Compared with MEKK3f/f platelets, the aggregation levels of MEKK3-deficient platelets were markedly reduced in response to low concentrations of thrombin, ADP, collagen, and U46619 (Figure 3A-D).
MEKK3 regulates platelet activation through integrin αIIbβ3–mediated inside-out signaling. Aggregation traces of washed MEKK3f/f and MEKK3−/− platelets treated with thrombin (A), ADP (B), collagen (C), or U46619 (D) were measured by aggregometry. (E) ATP secretion by MEKK3f/f and MEKK3−/− platelets induced by 0.06 U/mL thrombin, 10 µmol/L ADP, 2 µmol/L U46619, or 1.5 µg/mL collagen (n = 3, mean ± SD). (F) Expression of P-selectin in MEKK3f/f and MEKK3−/− platelets stimulated with 0.05 U/mL thrombin, 10 µmol/L ADP, 5 µmol/L U46619 or 2 µg/mL collagen was analyzed by flow cytometry. P-selectin expression was presented as mean fluorescence density (n = 3, mean ± SD). (G) Binding of Alexa Fluor 647–conjugated Fg to washed MEKK3f/f and MEKK3−/− platelets stimulated with 0.05 U/mL thrombin, 10 µmol/L ADP, 5 µmol/L U46619, or 2 µg/mL collagen. The results are expressed as mean fluorescence intensity (n = 3, mean ± SD). (H) Representative phalloidin staining and quantification of washed MEKK3f/f and MEKK3−/− platelets spreading on immobilized Fg for 90 minutes. (I) Quantification of the areas of spreading platelets in (H) (n = 4, mean ± SD; P > .05). (J) Representative pictures of the clot retraction of washed MEKK3f/f and MEKK3−/− platelets stimulated by 1 U/mL thrombin. (K) The 2-dimensional area of clots in panel J. Data are expressed as retraction ratios (n = 3, mean ± SD; P > .05). *P < .05, **P < .01, ***P < .001.
MEKK3 regulates platelet activation through integrin αIIbβ3–mediated inside-out signaling. Aggregation traces of washed MEKK3f/f and MEKK3−/− platelets treated with thrombin (A), ADP (B), collagen (C), or U46619 (D) were measured by aggregometry. (E) ATP secretion by MEKK3f/f and MEKK3−/− platelets induced by 0.06 U/mL thrombin, 10 µmol/L ADP, 2 µmol/L U46619, or 1.5 µg/mL collagen (n = 3, mean ± SD). (F) Expression of P-selectin in MEKK3f/f and MEKK3−/− platelets stimulated with 0.05 U/mL thrombin, 10 µmol/L ADP, 5 µmol/L U46619 or 2 µg/mL collagen was analyzed by flow cytometry. P-selectin expression was presented as mean fluorescence density (n = 3, mean ± SD). (G) Binding of Alexa Fluor 647–conjugated Fg to washed MEKK3f/f and MEKK3−/− platelets stimulated with 0.05 U/mL thrombin, 10 µmol/L ADP, 5 µmol/L U46619, or 2 µg/mL collagen. The results are expressed as mean fluorescence intensity (n = 3, mean ± SD). (H) Representative phalloidin staining and quantification of washed MEKK3f/f and MEKK3−/− platelets spreading on immobilized Fg for 90 minutes. (I) Quantification of the areas of spreading platelets in (H) (n = 4, mean ± SD; P > .05). (J) Representative pictures of the clot retraction of washed MEKK3f/f and MEKK3−/− platelets stimulated by 1 U/mL thrombin. (K) The 2-dimensional area of clots in panel J. Data are expressed as retraction ratios (n = 3, mean ± SD; P > .05). *P < .05, **P < .01, ***P < .001.
When stimulated by low concentrations of agonists, granule secretion plays an important role in platelet aggregation. Therefore, the role of MEKK3 in dense granule secretion was investigated by measuring ATP release, and its role in α-granule secretion was determined by detecting CD62P exposure using flow cytometry.23 In response to thrombin, ADP, and U46619, but not collagen, less ATP was released by MEKK3−/− platelets than by MEKK3f/f platelets (Figure 3E). CD62P exposure levels on the platelet surface were lower in MEKK3−/− platelets than in MEKK3f/f platelets stimulated with thrombin, ADP, U46619, and collagen (Figure 3F). Platelet aggregation by low doses of agonists also depends on TxA2 production. Thus, cPLA2 activation was determined in MEKK3f/f and MEKK3−/− platelets stimulated with thrombin, ADP, U46619, and collagen. The data in supplemental Figure 4B show that cPLA2 activation was not changed in MEKK3−/− platelets. These data suggest that MEKK3 plays important roles in agonist-induced platelet aggregation and granule secretion but not TxA2 production.
Integrin αIIbβ3–mediated bidirectional signaling is essential for platelet aggregation and stable thrombus formation.24,25 The function of MEKK3 in agonist-induced integrin αIIbβ3 activation was evaluated by measuring Alexa Fluor 647–conjugated Fg binding using flow cytometry. The results showed that Fg binding to MEKK3−/− platelets was much less than that to control platelets in response to thrombin, ADP, U46619, and collagen (Figure 3G).
Platelet spreading on immobilized Fg and clot retraction are dependent on cytoskeletal reorganization driven by integrin αIIbβ3–mediated outside-in signaling. The average size of spread platelets in MEKK3f/f mice was 128.9 ± 16 µm2 vs 123.9 ± 10.8 µm2 in MEKK3−/− mice. The average clot retraction ratio of platelet-rich plasma (PRP) containing MEKK3f/f platelets was 0.528 ± 0.031 vs 0.456 ± 0.024 in PRP containing MEKK3−/− platelets at 1 hour, PRP containing MEKK3f/f platelets was 0.735 ± 0.009 vs 0.686 ± 0.016 in PRP containing MEKK3−/− platelets at 2 hours, and PRP containing MEKK3f/f platelets was 0.890 ± 0.005 vs 0.867 ± 0.009 in PRP containing MEKK3−/− platelets at 3 hours. The results demonstrated that platelet spreading on immobilized Fg and clot retraction were not significantly affected by MEKK3 deficiency (Figure 3H-I), indicating that MEKK3 is mainly involved in integrin αIIbβ3–mediated inside-out signaling initiated by agonists but not outside-in signaling.
MEKK3 deficiency impaired the activation of ERK1/2 and JNK2
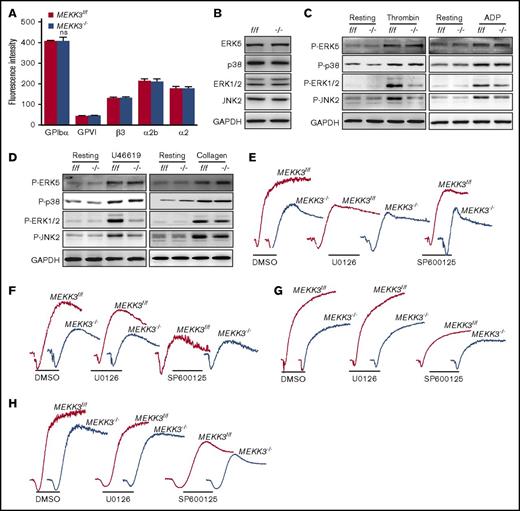
MEKK3 deficiency did not change the expression of GPVI, GPIb, integrin αIIbβ3, or integrin α2 on the platelet surface (Figure 4A), which are key receptors involved in platelet aggregation and thrombus formation.26-28 MEKK3 was reported to activate MAPKs.14-17,29 The activation of p38, ERK1/2, JNK1/2, and ERK5 in MEKK3f/f and MEKK3−/− platelets was determined. MEKK3 deficiency did not affect the activation of p38, JNK1, or ERK5, but it greatly impaired the activation of ERK1/2 and JNK2 induced by thrombin, ADP, U46619, or collagen (Figure 4C-D; supplemental Figure 4A-C). Because different MAPKs can be phosphorylated with different kinetics, the time course of p38 and ERK5 phosphorylation in agonist-stimulated platelets was investigated. The data in supplemental Figure 3A show that there was no significant difference between MEKK3f/f and MEKK3−/− platelets stimulated with thrombin, ADP, U46619, or collagen at 30, 60, and 90 seconds. These results suggest that ERK1/2 and JNK2, but not p38 and ERK5, are the major MEKK3 downstream signaling molecules in platelets activated by agonists.
MEKK3 regulates platelet activation through ERK1/2 and JNK2. (A) Expression levels of β3, GPIbα, GPVI, α2b, and α2 proteins in MEKK3f/f and MEKK3−/− platelets were detected using flow cytometry. The results are expressed as mean fluorescence intensity (n = 3, mean ± SD; P > .05). (B) Lysates of washed MEKK3f/f and MEKK3−/− platelets were probed for the expression of p38, ERK1/2, JNK, and ERK5 proteins. Washed MEKK3f/f and MEKK3−/− platelets were stimulated with 0.06 U/mL thrombin or 10 µmol/L ADP (C) or with 2 µmol/L U46619 or 1.5 μg/mL collagen (D) for 3 minutes, and the lysates were probed with phospho-specific antibodies for p38, ERK1/2, JNK, or ERK5 to quantify MAPK activation. GAPDH was used to verify equal loading. Aggregation of washed MEKK3f/f and MEKK3−/− mouse platelets in response to 0.05 U/mL thrombin (E), 10 µM ADP (F), 1.5 µg/mL collagen (G), and 2 µM U46619 (H) in the presence of 5 µM JNK inhibitor SP600125 or 5 µM MEK1/2 inhibitor U0126. At least 3 independent experiments were performed.
MEKK3 regulates platelet activation through ERK1/2 and JNK2. (A) Expression levels of β3, GPIbα, GPVI, α2b, and α2 proteins in MEKK3f/f and MEKK3−/− platelets were detected using flow cytometry. The results are expressed as mean fluorescence intensity (n = 3, mean ± SD; P > .05). (B) Lysates of washed MEKK3f/f and MEKK3−/− platelets were probed for the expression of p38, ERK1/2, JNK, and ERK5 proteins. Washed MEKK3f/f and MEKK3−/− platelets were stimulated with 0.06 U/mL thrombin or 10 µmol/L ADP (C) or with 2 µmol/L U46619 or 1.5 μg/mL collagen (D) for 3 minutes, and the lysates were probed with phospho-specific antibodies for p38, ERK1/2, JNK, or ERK5 to quantify MAPK activation. GAPDH was used to verify equal loading. Aggregation of washed MEKK3f/f and MEKK3−/− mouse platelets in response to 0.05 U/mL thrombin (E), 10 µM ADP (F), 1.5 µg/mL collagen (G), and 2 µM U46619 (H) in the presence of 5 µM JNK inhibitor SP600125 or 5 µM MEK1/2 inhibitor U0126. At least 3 independent experiments were performed.
The JNK inhibitor SP600125 inhibited MEKK3f/f platelet aggregation and CD62P exposure, but it attenuated MEKK3−/− platelet aggregation and CD62P exposure only slightly in response to ADP, collagen, and U46619. In contrast, the ERK1/2 upstream kinase MEK1/2 inhibitor U0126 suppressed MEKK3f/f platelet aggregation and CD62P exposure to similar levels as those of MEKK3−/− platelets under thrombin stimulation (Figure 4E-H; supplemental Figure 4D-E). These results demonstrate that JNK2 is a major downstream effector of MEKK3 in ADP-, collagen-, and U46619-induced platelet aggregation. However, ERK1/2 are the major signaling molecules downstream of MEKK3 in thrombin-induced platelet aggregation.
Platelet MEKK3 deficiency reduced microvascular obstruction and prevented MI expansion
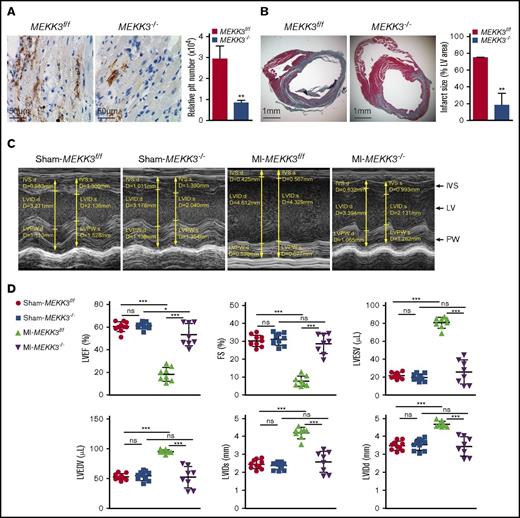
In MI, following the major vessel occlusion, the lower microvascular in the peri-infarct tissue might occlude under ischemic circumstances, which contributes to infarct expansion and a continued decline in heart function.11 To evaluate the contribution of platelet MEKK3 in ischemia-induced microthrombosis and myocardial infarct expansion, a mouse MI model was used. In this model, the LAD coronary artery was permanently ligated. LAD coronary artery ligation induces transmural ischemia, as shown by ST segment elevation on electrocardiography in both groups of mice (data not shown). Histologic data showed that fewer microthrombi were found in MEKK3−/− hearts by immunostaining of a platelet-specific marker, CD42C (average CD42+ area in MEKK3f/f hearts was 29240 ± 6265 pixels vs 8305 ± 2173 pixels in MEKK3−/− hearts; Figure 5A). Additionally, the infarct area spread far beyond the anterior wall of the left ventricle in MEKK3f/f mice, whereas the expansion area was reduced in MEKK3−/− mice (74.83% ± 0.29% vs 18.15% ± 14.32%, respectively, Figure 5B).
Platelet MEKK3 deficiency reduced microvascular obstruction and infarct expansion post-MI. (A) CD42C staining of hearts from MEKK3f/f and MEKK3−/− mice on day 3 following ligation of the LAD artery (original magnification ×400). Relative platelet (plt) number (pixels) in 4 random fields per experiment was quantified (mean ± SD). (B) Masson’s trichrome staining in parasternal short-axis sections of hearts from MEKK3f/f and MEKK3−/− mice on day 7 post-LAD coronary artery ligation (original magnification ×20). Masson’s trichrome–stained areas were measured. Infarct size is expressed as a percentage of the LV area (n = 3, mean ± SD). (C) Representative M-mode echocardiograms for MEKK3f/f and MEKK3−/− mice on day 7 post–LAD coronary artery ligation. (D) Echocardiography quantification of LVEF, LVFS, LVESV, LVEDV, LVIDs, and LVIDd in MEKK3f/f and MEKK3−/− mice on day 7 post–LAD coronary artery ligation (n = 8-10, mean ± SD). *P < .05, **P < .01, ***P < .001. IVS, interventricular septum; PW, posterior wall.
Platelet MEKK3 deficiency reduced microvascular obstruction and infarct expansion post-MI. (A) CD42C staining of hearts from MEKK3f/f and MEKK3−/− mice on day 3 following ligation of the LAD artery (original magnification ×400). Relative platelet (plt) number (pixels) in 4 random fields per experiment was quantified (mean ± SD). (B) Masson’s trichrome staining in parasternal short-axis sections of hearts from MEKK3f/f and MEKK3−/− mice on day 7 post-LAD coronary artery ligation (original magnification ×20). Masson’s trichrome–stained areas were measured. Infarct size is expressed as a percentage of the LV area (n = 3, mean ± SD). (C) Representative M-mode echocardiograms for MEKK3f/f and MEKK3−/− mice on day 7 post–LAD coronary artery ligation. (D) Echocardiography quantification of LVEF, LVFS, LVESV, LVEDV, LVIDs, and LVIDd in MEKK3f/f and MEKK3−/− mice on day 7 post–LAD coronary artery ligation (n = 8-10, mean ± SD). *P < .05, **P < .01, ***P < .001. IVS, interventricular septum; PW, posterior wall.
Cardiac function of MEKK3f/f and MEKK3−/− mice was assessed by echocardiography 7 days after LAD coronary artery ligation. There was no difference in cardiac function between the sham MEKK3f/f and MEKK3−/− mice. However, hearts from MEKK3f/f mice were more dilated, congested, and remodeled compared with MEKK3−/− hearts following LAD coronary artery ligation (Figure 5C). MEKK3−/− mice had improved heart function post-MI, including improved LV ejection fraction (LVEF), improved LV fractional shortening (LVFS), a less dilated LV cavity, and lower LV volume indices (LV end-diastolic volume [LVEDV], LV end-systolic volume [LVESV], LV internal dimension-diastole [LVIDd], and LV internal dimension-systole [LVIDs]). Specifically, LVEF, LVFS, LVEDV, LVESV, LVIDd, and LVIDs in MEKK3f/f mice post-MI were 18.33% ± 2.16%, 7.67% ± 1%, 95.06 ± 1.14 μL, 80.68 ± 2.18 μL, 4.7 ± 0.06 mm, and 4.21 ± 0.11 mm, respectively, vs 53.29% ± 3.49%, 28.66% ± 1.96%, 52.57 ± 6.34 μL, 25.61 ± 4.74 μL, 3.47 ± 0.19 mm, and 2.61 ± 0.21 mm, respectively, in MEKK3−/− mice (Figure 5D).
Discussion
The activation of MAPK signal transduction pathways is the major intracellular event in cells in response to environmental stress. Platelet activation during vascular injury is actually a stress-response process. Thus, it is not surprising that MAPKs play indispensable roles in platelet activation. Although MAP3Ks are the essential upstream kinases for MAPK activation, few studies have been carried out to elucidate their roles in platelet activation. Naik et al recently reported that MEKK5, a MAP3K family member, was responsible for platelet p38/cPLA2 activation and granule secretion.21 Also, Kamiyama et al further reported that MEKK5/p38 facilitates tumor metastasis through phosphorylation of the ADP receptor P2Y12 in platelets.30 Although these 2 studies clearly showed the roles of MEKK5 in platelet activation and thrombus formation, MEKK5-deficient mice with prolonged bleeding times limited its application as a viable antiplatelet target. In this study, we found that another MAP3K family member (MEKK3) is highly expressed in human and mouse platelets. Platelet-specific MEKK3 deficiency impaired arterial thrombus formation but did not cause bleeding disorders in mice, suggesting that MEKK3 is an effective antithrombosis target with minimal bleeding risks. MEKK3-specific inhibitors are worthy of development for antithrombosis applications. Because MEKK3 expression is not restricted to platelets, normal physiological function may also be disturbed by MEKK3 inhibitors. However, developing drug-delivery systems may cause the antithrombotic drug to specifically target platelets, avoiding the possible adverse effects.
In the classical MAP3K–MAP2K–MAPK signaling axis, MAP2Ks display very little activity on substrates other than their cognate MAPKs. Thus, MAP2K–MAPKs are well-known linear pathways, including MEK1/2–ERK1/2, MKK3/4/6–p38, MKK4/7–JNKs, and MEK5–ERK5. However, the specificity of MAP3Ks for MAP2K–MAPK activation is tissue or stimulus dependent. MEKK3 has been shown to bind directly to MEK5 and mediate growth factor–induced ERK5 activation in HeLa cells.29 A study also showed that MEKK3 regulates T-cell receptor–mediated ERK1/2, p38, and JNK MAPK activation in CD4 T cells.15 In our study, we found that MEKK3 is responsible for activation of ERK1/2 and JNK2, but not p38, ERK5, or JNK1, in platelets. Adam et al claimed that JNK1 plays important roles in platelet activation and proposed that JNK2 also contributes to platelet aggregation, based on the observation of further inhibitory effects of the JNK inhibitor SP600125 on the aggregation of JNK1-deficient platelets.10 Although the upstream kinases for JNK1 and JNK2 are supposed to be common,31 interestingly, we found that JNK2, but not JNK1, could be activated by MEKK3 in platelets in response to all agonists. The mechanism underlying selective activation of JNK2 by MEKK3 in platelets requires further study. MEKK3 deficiency partially blocked agonist-induced activation of ERK1/2 and JNK2, also implying that other MAP3Ks may play complementary roles in MEKK3-mediated ERK1/2 and JNK2 activation in platelets. The expression profiles of the entire MAP3K family showed that Rafs and MLK3 also are expressed at relatively high levels in human and mouse platelets. Raf–MEK1/2–ERK1/2 has been shown to be a classical pathway for ERK1/2 activation in various physiological and pathological processes32 ; however Nadal-Wollbold et al reported that the activation of platelet ERK2 was independent of Raf.33 Also, our recent studies demonstrated that Raf was responsible for platelet ERK5 activation.34 MLK3 has been reported to activate JNKs and regulate apoptosis and dorsal closure during embryonic morphogenesis,35,36 suggesting that MLK3 might play a synergistic role in MEKK3-mediated JNK2 activation in platelets. However, because the role of MLK3 in platelet activation is unclear, further studies are required.
When the vessels occluded in MI patients, platelets were rapidly activated under the ischemic stress and contributed to ongoing physiological processes, including microthrombosis and myocardial infarct expansion through mediating inflammation and secretion of matrix metalloproteinases.11 Microthrombosis is a common cause of the no-reflow phenomenon, which leads to defective myocardial reperfusion after reperfusion therapy, including thrombolytic or mechanical interventions.37 Patients with myocardial infarct expansion have poorer exercise tolerance, more symptoms of congestive heart failure, and greater early and late mortality than those without expansion.38,39 Excitingly, using a chronic MI model, we found that MEKK3 deficiency significantly impaired microthrombosis at the edge of the infarct area, alleviated infarct expansion from the ischemic region, and improved heart function post-MI. The above data indicated that platelet MEKK3 serves as an effective target to prevent infarct expansion in MI patients.
In conclusion, our study reveals an important role for MEKK3 in the activation of the ERK1/2 and JNK2 pathways during platelet activation, which contributes to arterial thrombus formation and the infarct expansion post-MI. Of greater significance is that MEKK3 deficiency had no effect on normal hemostasis, suggesting that MEKK3 may serve as an effective and safe drug target for antiplatelet therapy.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81600104 [X.F.]; 81525001, 91739302, and 81721004 [J.L.]; and 31470845 and 81430033 [B.S.]) and the Shanghai Science and Technology Commission (13JC1404700) (B.S.).
Authorship
Contribution: J.L., B.S., and X.F. designed the experiments, analyzed data, and wrote the manuscript; X.F., C.W., P.S., W.G., J.G., Y.G., W.Y., N.W., and Y.W. performed the experiments; and Y.X., X.C., L.Z., and K.W. assisted with the experiments.
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Correspondence: Junling Liu, Room 212, Building 7, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: liujl@shsmu.edu.cn; and Bing Su, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: bingsu@sjtu.edu.cn.