Key Points
HDAC inhibitors might induce ciHHV-6 reactivation.
In ciHHV-6 HSCT recipients posttransplant viral load can estimate persistent host chimerism when the donor is ciHHV-6 negative.
Introduction
Human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation (HSCT), in particular of the HHV-6B subtype, has been associated with numerous posttransplant complications including encephalitis, acute graft-versus-host-disease, delayed engraftment, fever, and rash, although a causative relationship and negative impact on HSCT outcomes has not been consistently demonstrated.1-4 In contrast to most herpesviruses, HHV-6 has the unique property of establishing lifelong latency following primary infection by integrating in chromosomal telomeres.5,6 Notably, HHV-6 integration in the gametes of a host may result in progeny who carry a copy of integrated HHV-6 genome in all nucleated cells, a phenomenon known as chromosomally integrated HHV-6 (ciHHV-6).7 The prevalence of ciHHV-6 is estimated to be ∼1%7,8 and follows Mendelian inheritance.9 Although the implications of ciHHV-6 in healthy subjects and HSCT recipients,8,10 as well as the capacity of ciHHV-6 for viral reactivation,6,7,11 are under active investigation, the constitutively high HHV-6 viral load levels associated with ciHHV-612 can complicate the care of HSCT patients with ciHHV-6 by leading to misdiagnosis and unnecessary treatment.13
Case description
We present the case of a 54-year-old female who was diagnosed with post–polycythemia vera acute myeloid leukemia (AML) with FLT3 internal tandem duplication mutation. The patient received induction chemotherapy with daunorubicin and cytarabine, but bone marrow assessment on day 32 postinduction revealed persistent AML. She subsequently received reinduction with vorinostat, idarubicin, and cytarabine, based on high efficacy of this regimen in FLT3+ AML.14 Repeat bone marrow assessment 14 days after reinduction revealed chemoablation, and the plan was to proceed to HSCT from her HLA-matched brother.
Eighteen days after starting reinduction, the patient developed high-grade fevers despite remaining on broad-spectrum antibacterials and antifungal prophylaxis. Extensive infectious workup was negative. The fevers lasted for 8 days, did not respond to antibiotic manipulation, and were succeeded by a maculopapular rash resembling viral exanthem. Notably, quantitative whole blood (WB) HHV-6 polymerase chain reaction (PCR) at that time showed markedly elevated HHV-6 viral load of 559 × 103 copies/mL, and foscarnet was initiated. The skin rash rapidly resolved, and the HHV-6 viral load gradually decreased.
Methods
WB and plasma HHV-6 viral load were tested with a quantitative real-time PCR assay at a commercial laboratory (lower detection limit 500 copies/mL). WB ciHHV-6 testing was performed at the University of Washington by a ratio-based PCR assay using RPP30 as the reference gene for normalization of HHV-6 viral copy number. This assay was the predecessor of the recently developed digital droplet PCR assay for ciHHV-6 detection.15 Qualitative HHV-6 real-time PCR assay on nail clippings was performed by Coppe Laboratories. Peripheral blood (PB) chimerism was monitored with interphase fluorescence in situ hybridization (FISH) for the sex chromosomes because of the sex-discordant donor.
Results and discussion
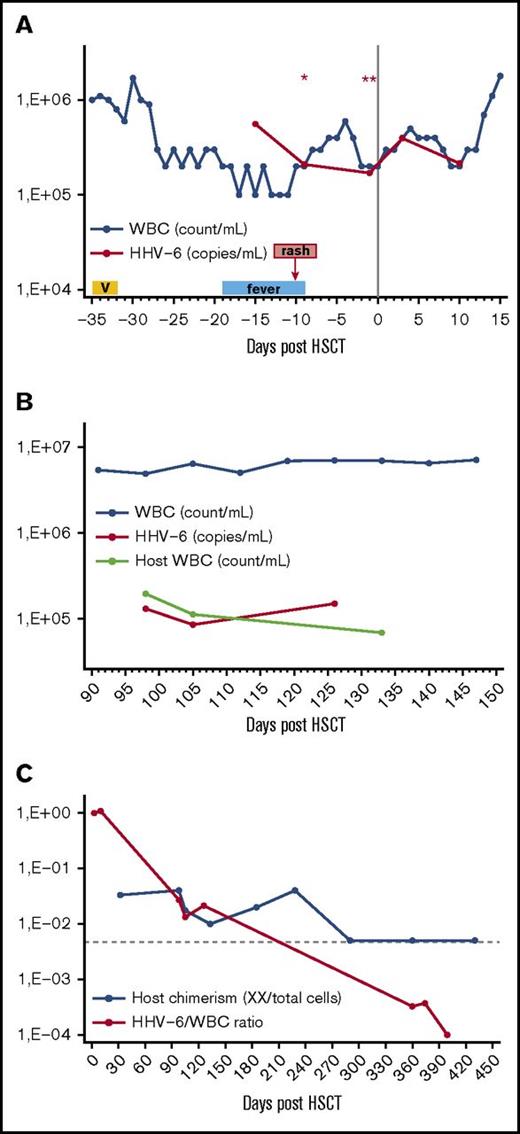
Because of the very high initial HHV-6 viremia, patient samples were also tested with a ratio-based HHV-6 PCR assay to evaluate for possible ciHHV-6. On 2 serial measurements, the ratio of HHV-6 viral copies per cell was ∼1 (1.12 and 0.9), supporting the diagnosis of ciHHV-6 with 1 viral genome copy integrated per nucleated cell. Subsequently, foscarnet was discontinued, and the patient proceeded to HSCT from her HLA-matched brother. The patient had persistent HHV-6 viremia for more than a year after HSCT, but without evidence of HHV-6-related manifestations or organ disease. The interpretation was confounded by the fact that the patient had ciHHV-6, whereas the ciHHV-6 status of her donor was unknown. In this case, the combined study of HHV-6 viral load kinetics, white blood cell (WBC) count, and PB chimerism studies at various pre- and posttransplant intervals highlighted several important points relevant to the implications and management of ciHHV-6 in the setting of HSCT (Figure 1; Table 1).
Association between HHV-6 viral load, WBC, and host chimerism at various time points before and after HSCT. (A) Pre- and early posttransplant until donor engraftment (when all circulating cells were of host origin), the HHV-6 viral load (copies per mL) was approximately equal to WBC (count per mL), except for the initial episode of HHV-6 viremia (day −15) after reinduction with a vorinostat (V)–containing regimen. Presence of ciHHV-6 in the host was confirmed with a ratio-based PCR on 2 separated time points (days −9 and −1), with an HHV-6 viral copies per cell genome ratio of ∼1, indicating that the source of HHV-6 viremia was circulating host cells with ciHHV-6. The only exception was day −15 when HHV-6 viral load (copies per mL) >> WBC (count per mL) in combination with fever and rash, suggesting presence of HHV-6 reactivation from ciHHV-6 state, possibly induced by exposure to a histone deacetylase (HDAC) inhibitor. (Results of ratio-based PCR assay for the detection of ciHHV-6: *1.12, **0.9.) (B) After donor engraftment and before full donor chimerism was achieved, the HHV-6 viral load (copies per mL) was much lower than WBC (count per mL). We reasoned that the donor did not have ciHHV-6 and that circulating nucleated cells of host origin with ciHHV-6 were the source of ongoing HHV-6 viremia during this time. Indeed, we observed that host WBC count (count per mL), which was calculated by multiplying PB host chimerism as measured by interphase FISH by the total WBC count obtained from same-day hemograms, approximated the HHV-6 viral load (copies per mL). (C) The calculated HHV-6 viral load to total WBC ratio can be used to estimate persistent host chimerism when the HSCT recipient, but not donor, has ciHHV-6. In the late posttransplant period, the HHV-6 viral load to WBC count ratio had greater sensitivity for the detection of host microchimerism compared with interphase FISH. Specifically, because the sensitivity of the FISH chimerism assay depends on the number of cells analyzed, 1 to 2 log more cells would have been needed to detect host microchimerism at that time. (The horizontal interrupted line represents the detection limit of 200-cell interphase FISH assay.)
Association between HHV-6 viral load, WBC, and host chimerism at various time points before and after HSCT. (A) Pre- and early posttransplant until donor engraftment (when all circulating cells were of host origin), the HHV-6 viral load (copies per mL) was approximately equal to WBC (count per mL), except for the initial episode of HHV-6 viremia (day −15) after reinduction with a vorinostat (V)–containing regimen. Presence of ciHHV-6 in the host was confirmed with a ratio-based PCR on 2 separated time points (days −9 and −1), with an HHV-6 viral copies per cell genome ratio of ∼1, indicating that the source of HHV-6 viremia was circulating host cells with ciHHV-6. The only exception was day −15 when HHV-6 viral load (copies per mL) >> WBC (count per mL) in combination with fever and rash, suggesting presence of HHV-6 reactivation from ciHHV-6 state, possibly induced by exposure to a histone deacetylase (HDAC) inhibitor. (Results of ratio-based PCR assay for the detection of ciHHV-6: *1.12, **0.9.) (B) After donor engraftment and before full donor chimerism was achieved, the HHV-6 viral load (copies per mL) was much lower than WBC (count per mL). We reasoned that the donor did not have ciHHV-6 and that circulating nucleated cells of host origin with ciHHV-6 were the source of ongoing HHV-6 viremia during this time. Indeed, we observed that host WBC count (count per mL), which was calculated by multiplying PB host chimerism as measured by interphase FISH by the total WBC count obtained from same-day hemograms, approximated the HHV-6 viral load (copies per mL). (C) The calculated HHV-6 viral load to total WBC ratio can be used to estimate persistent host chimerism when the HSCT recipient, but not donor, has ciHHV-6. In the late posttransplant period, the HHV-6 viral load to WBC count ratio had greater sensitivity for the detection of host microchimerism compared with interphase FISH. Specifically, because the sensitivity of the FISH chimerism assay depends on the number of cells analyzed, 1 to 2 log more cells would have been needed to detect host microchimerism at that time. (The horizontal interrupted line represents the detection limit of 200-cell interphase FISH assay.)
Kinetics of WBC, HHV-6 viral load, and PB host chimerism
| . | Days post-HSCT . | WBC count, k/mL . | HHV-6 viral load, ×103 copies/mL WB . | PB chimerism by FISH, XX cells/total cells (% host chimerism) . | HHV-6 viral load (×103 copies/mL WB)/WBC count (k/mL) . |
|---|---|---|---|---|---|
| Peritransplant period* | −15 | 100 | 559 | 5.59 | |
| −9 | 200 | 210 | 1.05 | ||
| −1 | 200 | 170 | 0.85 | ||
| 3 | 400 | 393 | 0.98 | ||
| 10 | 200 | 216 | 1.08 | ||
| Early posttransplant period† | 32 | 3800 | 10/300 (3.3) | ||
| 98 | 4900 | 131 | 8/200 (4) | 0.027 | |
| 105 | 6400 | 85 | 7/400 (1.7) | 0.013 | |
| 126 | 7000 | 150 | 0.021 | ||
| 133 | 6900 | 4/200 (2) | |||
| Late posttransplant period‡ | 290 | 6600 | 0/200 (0) | ||
| 360 | 5000 | 1.6 | 0/200 (0) | 0.0003 | |
| 374 | 4000 | 1.4 | 0.0004 | ||
| 399 | 5100 | <0.5 | <0.0001 | ||
| 430 | 3900 | 0/200 (0) |
| . | Days post-HSCT . | WBC count, k/mL . | HHV-6 viral load, ×103 copies/mL WB . | PB chimerism by FISH, XX cells/total cells (% host chimerism) . | HHV-6 viral load (×103 copies/mL WB)/WBC count (k/mL) . |
|---|---|---|---|---|---|
| Peritransplant period* | −15 | 100 | 559 | 5.59 | |
| −9 | 200 | 210 | 1.05 | ||
| −1 | 200 | 170 | 0.85 | ||
| 3 | 400 | 393 | 0.98 | ||
| 10 | 200 | 216 | 1.08 | ||
| Early posttransplant period† | 32 | 3800 | 10/300 (3.3) | ||
| 98 | 4900 | 131 | 8/200 (4) | 0.027 | |
| 105 | 6400 | 85 | 7/400 (1.7) | 0.013 | |
| 126 | 7000 | 150 | 0.021 | ||
| 133 | 6900 | 4/200 (2) | |||
| Late posttransplant period‡ | 290 | 6600 | 0/200 (0) | ||
| 360 | 5000 | 1.6 | 0/200 (0) | 0.0003 | |
| 374 | 4000 | 1.4 | 0.0004 | ||
| 399 | 5100 | <0.5 | <0.0001 | ||
| 430 | 3900 | 0/200 (0) |
Peritransplant period is defined as the interval between pretransplant and early post-HSCT before donor myeloid engraftment.
Early posttransplant period is defined as the post-HSCT interval after donor myeloid engraftment and before full donor PB chimerism was established.
Late posttransplant period is defined as the post-HSCT interval after full donor PB chimerism.
Pre- and early posttransplant before donor engraftment (Figure 1A), the patient had high-grade HHV-6 viremia, and the HHV-6 viral load approximated the WBC count resulting in a ratio of HHV-6 (copies per mL WB) to WBC (count per mL) of ∼1, suggesting that circulating cells of host origin, each carrying 1 copy of viral genome, were the source of the HHV-6 viremia. In marked contrast, at the time of initial HHV-6 viremia (day −15), which was associated with fever and rash, the HHV-6 to WBC ratio was much greater than 1, indicating an additional source of HHV-6 viral copies. Several reports have now demonstrated or suggested viral reactivation of ciHHV-6.6,11,16,17 Moreover, the HDAC inhibitor trichostatin-A has been shown to facilitate reactivation of ciHHV-6 in vitro,6 while other drugs with HDAC properties have been implicated in in vivo reactivation of HHV-6.7 In our case, the combination of a clinically compatible syndrome 2 weeks after exposure to vorinostat together with a markedly higher than expected HHV-6 viral load provide compelling evidence for the first report of in vivo viral reactivation from ciHHV-6 state possibly provoked by exposure to an HDAC inhibitor, corroborating earlier in vitro data.6 The relevance of the present report is based on the increasing use of HDAC inhibitors in patients with hematologic malignancies, who are often immunocompromised, and aims to raise awareness of a previously unrecognized but potentially significant side effect of this drug class.
In the early posttransplant period after myeloid engraftment but before full donor PB chimerism was established (Figure 1B), the patient had ongoing HHV-6 viremia but the HHV-6 viral load was much lower than the WBC count. At that time we hypothesized that the donor did not have ciHHV-6 and that residual circulating nucleated cells of host origin with ciHHV-6 were the source of HHV-6 viremia. In support of this hypothesis, during this period the calculated number of host WBC (count per mL) was approximately equal to the HHV-6 viral load (copies per mL) (Figure 1B), and the ratio of HHV-6 viral load to total WBC count approximated the residual PB host chimerism as measured by FISH (Table 1). Moreover, plasma HHV-6 viral load at that time was only 2239 copies per mL arguing against an active HHV-6 infection, as such low plasma levels can be expected in ciHHV-6 patients because of cell breakdown between sample procurement and processing.5,12
In the late posttransplant period after full donor PB chimerism was established, the patient had ongoing but low-level HHV-6 viremia (Table 1). We hypothesized that nucleated cells of host origin with ciHHV-6 were still the source of HHV-6 viremia at that time but could not be detected by the chimerism assay because of their very low frequency. In support of this hypothesis, the HHV-6 to WBC ratio (which was previously shown to correlate with host chimerism) was much lower than the detection limit of the 200- to 400-cell interphase FISH assay (Figure 1C). Indeed, 1 to 2 logs more cells would have been needed for the FISH assay at that time to be able to detect such low-level persistent donor microchimerism. Finally, at day +399 after HSCT, the patient’s HHV-6 viral load became undetectable, providing evidence that the donor was negative for ciHHV-6 and that host nucleated cells were no longer present in the circulation. Notably, after clearance of the HHV-6 viremia, qualitative PCR testing on the patient’s nail clippings confirmed the presence of ciHHV-6B viral genome in nonhematopoietic host tissues.
Our findings are in accord with other case reports of ciHHV-6 HSCT recipients from non-ciHHV-6 donors showing gradual decrease of the high HHV-6 viral load following donor engraftment and increasing donor chimerism.18-22 Similarly, we also observed ongoing low-level HHV-6 viremia after conversion to complete donor chimerism, which others have attributed to intermittent release of HHV-6 genome from nonhematopoietic tissues of ciHHV-6 recipients.5,19,21 In contrast, we show that ongoing microchimerism of recipient nucleated cells continues to be the most likely source of HHV-6 viremia in this setting but cannot be detected because of the limitations of the standard chimerism assay.
Together our observations in this unusual case provide guidance that when ciHHV-6 is suspected in HSCT recipients, the diagnosis should be confirmed by digital droplet PCR and/or by testing for the presence of ciHHV-6 in nonhematopoietic host tissues. Moreover, when the patient, but not the donor, is ciHHV-6 positive, the posttransplant HHV-6 viral load can serve as an indicator of persistent host chimerism with a greater sensitivity compared with FISH.
Acknowledgments
The authors thank the University of Washington for their assistance with the PCR assay for detection of ciHHV-6.
This work was supported by an HHV-6 Foundation Pilot Grant, and by the National Institutes of Health, National Cancer Institute (grants RO1CA183605-01 and RO1CA183605S1) (V.A.B.).
Authorship
Contribution: I.P., M.M., D.A., and V.A.B. were involved in patient care; C.B. performed FISH studies for analysis of chimerism; I.P. and V.A.B. conceived the research, collected data, and wrote the manuscript; and all authors have read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vassiliki A. Boussiotis, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Dana 513, Boston, MA 02215; e-mail: vboussio@bidmc.harvard.edu.