Key Points
Coexisting psychiatric comorbidities are associated with greater increased health care utilization and cost of care in patients with MM.
Clinical complications of MM are seen more frequently in patients with coexisting psychiatric conditions.
Abstract
Approximately one third of cancer patients suffer from comorbid mood disorders that are associated with increased cost and poorer outcomes. The majority of patients with multiple myeloma (MM) are treated with corticosteroids; as many as three fourths of those taking corticosteroids develop neuropsychiatric complications, likely increasing morbidity and cost of care. MM patients diagnosed between 1991 and 2010 and reported in the Surveillance Epidemiology, and End Results-Medicare database were characterized as MM-Only, MM+Psychiatric (any psychiatric condition, preexisting or post-MM), or MM+Depression (depression as the only psychiatric diagnosis, preexisting or post-MM). Differences in demographic characteristics, occurrence of clinical myeloma-defining events (MDEs), health care utilization (inpatient, outpatient, ambulatory claims), and cost of care during the first 6 months of MM diagnosis were analyzed. Psychiatric comorbidities were reported more frequently in females, and racial minorities had lower rates of psychiatric comorbidities. All clinical MDEs were more common in the MM+Psychiatric and MM+Depression groups; within them, the majority were more common in patients diagnosed with the psychiatric condition or depression after MM compared with it being a preexisting condition. Health care utilization in all treatment settings was higher in those with psychiatric comorbidities. Cost of care within the first 6 months after MM diagnosis was significantly higher in the MM+Psychiatric and MM+Depression groups. This increase in cost was more pronounced for patients from racial minorities diagnosed with a psychiatric condition, including depression. Psychiatric comorbidities significantly impact the clinical presentations, health care utilization, and cost among patients with MM. These findings need to be addressed for improved survivorship of MM patients.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, with ∼30 000 new patients diagnosed annually.1 Management of MM has significantly improved over recent years, with better outcomes.2,3 With increased survival, many patients would experience common psychiatric issues that occur in up to 25% of cancer patients.4-6 MM patients have several factors that increase their risk for psychiatric diagnoses, including the median age at diagnosis (69 years), when other coexisting comorbidities can contribute to psychiatric conditions.1,7,8 Furthermore, nearly all MM patients are prescribed long-term systemic steroids, which are reported to cause a number of neuropsychiatric effects, including mood symptoms, psychosis, delirium, cognitive impairment, and sleep disorders, in nearly 60% of patients receiving them.4,9,10 To date, studies have not systematically explored the health care impact of psychiatric comorbidities among MM patients.
Cost of cancer care has increased considerably over time and is projected to increase much more in the future.11-14 Although novel therapeutic agents are considered the major cause of the increasing cost of cancer care, studies have also shown that caring for cancer patients with depression and other psychiatric conditions is associated with significantly higher cost and increased health care utilization.15,16 Due to the overlap of cancer diagnosis, near universal utilization of systemic steroids for prolonged periods of time, and an older population with frequent comorbidities, MM is a uniquely suited population for studying the impact of psychiatric conditions on cost of care and health care utilization. We performed a comprehensive population-based analysis to understand the true nature of these interactions so that policy and therapeutic decisions may be informed and the financial impact of psychiatric comorbidities on MM care can be addressed.
Methods
Data source
We used data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database with data linkage to Medicare claims (SEER-Medicare, linkage completed in 2013).17,18 The Medicare files used included the Patient Entitlement and Diagnosis Summary File, the Outpatient file (institutional Medicare Part B claims), the Medicare Provider Analysis and Review file (inpatient Medicare Part A claims), the National Claims History file (provider Medicare Part B claims), Durable Medical Equipment files, and Medicare Part D file (prescription drug coverage for beneficiaries who purchase the benefit; ∼60% of the beneficiaries).19
Study population and variables
Incident MM cases reported to the SEER registry during years the 1991 to 2010, defined by the International Classification of Diseases (ICD) O-3 code (version released 18 September 2015) of 9732/3 (MM) and microscopic confirmation from positive histology, cytology, or other microscopic method (diagnostic confirmation code 1-4), were identified. Autopsy-only cases were excluded. Only patients with full Medicare coverage (12 months of coverage each year) beginning 1 year prior to diagnosis through the end of follow-up were included in the analysis (until the end of 2012). Total Medicare claim amounts were summed for all patients for inpatient and outpatient visits within the first 6 months of MM care, because a period of 6 months reflects definition of an episode of care in the Centers for Medicare & Medicaid Services’ Oncology Care Model (episode-based payment model).20 Similar data were collected on MM patients with ICD-9 codes for any psychiatric disorder (supplemental Table 1) and separately, depression, because depression was the most common psychiatric diagnosis in our study cohort (supplemental Table 2). A patient was flagged with any of the respective conditions if the code appeared once in an inpatient setting or twice in an outpatient setting. A similar approach to identify psychiatric comorbidity has been used in the published literature.19 Comparisons were made among MM patients without any psychiatric diagnosis (MM-Only), those with MM and any psychiatric diagnosis (MM+Psychiatric), and those with MM and depression as the only psychiatric diagnosis (MM+Depression). Furthermore, we compared patients who were diagnosed with any psychiatric condition within 12 months prior to or 6 months after receiving the diagnosis of MM. We examined care data related to clinical myeloma-defining events (MDEs)21,22 (hypercalcemia, ICD-9 275.42; renal dysfunction, ICD-9 585.6 and 586; anemia, ICD-9 280.0-285.9; bone fractures, ICD-9 733.10-733.19; and dialysis, V45.11) by diagnosis code for that clinical encounter among patients in the MM-Only, MM+Psychiatric, and MM+Depression groups >30 days after their initial diagnosis of MM. These conditions developing within the first 30 days after MM diagnosis were not included because it could not be determined whether they were related to MM or acute adverse events from the antimyeloma treatment or were potentially preexisting and independent and were diagnosed simultaneously with the MM. The North American Association of Central Cancer Registries Hispanic/Latino Algorithm (NAACCR Race & Ethnicity Work Group, 2011)23 was used for grouping patients into 5 mutually exclusive racial-ethnic groups (races): non-Hispanic White (white), non-Hispanic African American (African American), non-Hispanic Asian/Pacific Islander (Asian), Hispanic, and Native American. Because of small numbers, Native Americans were not included separately in the statistical analysis. Cases with missing information about race were excluded from the analysis.
Statistical analysis
Data were descriptively summarized using frequencies and proportions for categorical variables and median and interquartile ranges for continuous variables. A χ2/Fisher's exact test was used for categorical variables (year, sex, race, clinical MDE), and a Wilcoxon test was used for continuous variables (age, Charlson comorbidity index [CCI] at diagnosis). Univariate and multivariate logistic regression models (adjusted for age, year, sex, race, and CCI) were performed to determine whether there were associations among patients in each of the diagnosis groups and health care claims (inpatient, ambulatory, emergency). Medicare insurance claims adjusted for inflation to the year 2013 for health care received within the first 6 months of MM diagnosis were summed and categorized into inpatient, outpatient, and drug charges. Associations among costs of care after MM diagnosis for MM-Only, MM+Psychiatric, and MM+Depression groups were assessed using univariate and multivariate proportional odds models. A variable for total, inpatient, and outpatient costs for the first 6 months of care was categorized into 11 categories: no charge and deciles. The same adjustment terms described previously were used in the multivariate models.
Results
Study cohort
A total of 47 608 unique incident cases of MM was identified in the SEER-Medicare database, diagnosed between 1991 and 2010. After excluding cases without full Medicare coverage, as defined above, 36 007 patients were included in the analysis. Median age at diagnosis was 76 years (interquartile range [IQR], 70-81; range, 18-104) with a median follow-up of 1.8 years. The study cohort had 52.1% males (n = 18 776) and 47.9% females (n = 17 231), with the majority being white (72%), followed by African American (16.2%), Hispanic (7.3%), Asian (4.1%), and others (0.4%). Median CCI was 1 for the study cohort. Clinical MDEs noted >30 days from the diagnosis of MM included anemia in 33.6%, bone fractures in 28.8%, renal dysfunction in 26.2%, hypercalcemia in 17.6%, and dialysis in 2.9% of patients. Selected baseline characteristics for all patients included in the analysis are summarized in Table 1.
Baseline characteristics of the study population
| Characteristic . | MM-Only (n = 20 839) . | MM+Psychiatric (n = 15 168) . | Total (N = 36 007) . |
|---|---|---|---|
| Age at diagnosis, median (range), y | 76 (70-81) | 76 (70-82) | 76 (70-81) |
| Year of diagnosis, n (%) | |||
| 1991-1995 | 3 663 (17.6) | 1 624 (10.7) | 5 287 (14.7) |
| 1996-2000 | 4 288 (20.6) | 2 862 (18.9) | 7 150 (19.9) |
| 2001-2005 | 6 929 (33.2) | 5 859 (38.6) | 12 788 (35.5) |
| 2006-2010 | 5 959 (28.6) | 4 823 (31.8) | 10 782 (29.9) |
| Sex, n (%) | |||
| Female | 9 476 (45.5) | 7 755 (51.1) | 17 231 (47.9) |
| Male | 11 363 (54.5) | 7 413 (48.9) | 18 776 (52.1) |
| Race/ethnicity, n (%) | |||
| Data missing | 104 | 61 | 165 |
| Non-Hispanic white | 14 699 (70.9) | 11 124 (73.6) | 25 823 (72.1) |
| Hispanic | 1 664 (8.0) | 960 (6.4) | 2 624 (7.3) |
| Non-Hispanic African American | 3 249 (15.7) | 2 540 (16.8) | 5 789 (16.2) |
| Non-Hispanic Asian | 1 055 (5.1) | 431 (2.9) | 1 486 (4.1) |
| Other | 68 (0.3) | 52 (0.3) | 120 (0.3) |
| CCI, median (range) | 0 (0-2) | 1 (0-3) | 1 (0-2) |
| Characteristic . | MM-Only (n = 20 839) . | MM+Psychiatric (n = 15 168) . | Total (N = 36 007) . |
|---|---|---|---|
| Age at diagnosis, median (range), y | 76 (70-81) | 76 (70-82) | 76 (70-81) |
| Year of diagnosis, n (%) | |||
| 1991-1995 | 3 663 (17.6) | 1 624 (10.7) | 5 287 (14.7) |
| 1996-2000 | 4 288 (20.6) | 2 862 (18.9) | 7 150 (19.9) |
| 2001-2005 | 6 929 (33.2) | 5 859 (38.6) | 12 788 (35.5) |
| 2006-2010 | 5 959 (28.6) | 4 823 (31.8) | 10 782 (29.9) |
| Sex, n (%) | |||
| Female | 9 476 (45.5) | 7 755 (51.1) | 17 231 (47.9) |
| Male | 11 363 (54.5) | 7 413 (48.9) | 18 776 (52.1) |
| Race/ethnicity, n (%) | |||
| Data missing | 104 | 61 | 165 |
| Non-Hispanic white | 14 699 (70.9) | 11 124 (73.6) | 25 823 (72.1) |
| Hispanic | 1 664 (8.0) | 960 (6.4) | 2 624 (7.3) |
| Non-Hispanic African American | 3 249 (15.7) | 2 540 (16.8) | 5 789 (16.2) |
| Non-Hispanic Asian | 1 055 (5.1) | 431 (2.9) | 1 486 (4.1) |
| Other | 68 (0.3) | 52 (0.3) | 120 (0.3) |
| CCI, median (range) | 0 (0-2) | 1 (0-3) | 1 (0-2) |
Psychiatric comorbidities
MM-Only vs MM+Psychiatric.
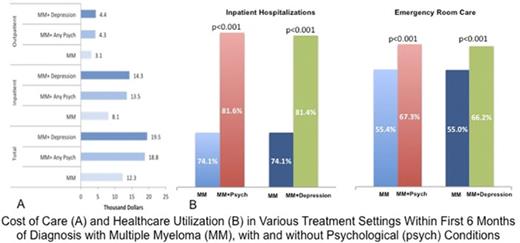
Of the 36 007 patients included in the analysis, 15 168 (42.1%) were in the MM+Psychiatric group, and 20 839 were in the MM-Only group. Median age for both groups was 76 years (IQR, 70-82). There was a significant difference in sex distribution between the 2 groups; the majority (54.5%) of MM-Only patients were males, whereas 51.1% of MM+Psychiatric patients were females (P < .001). The racial distribution of patients with any psychiatric comorbidity was significantly different (P < .001), with 43% of whites, 44% of African Americans, 38% of Hispanics, and 29% of Asians in the MM+Psychiatric group. Patients with a psychiatric diagnosis with MM had a higher median CCI of 1 compared with those with MM-Only, who had a median CCI of 0 (P < .001). All clinical MDEs reported >30 days after MM diagnosis were significantly more frequent in the MM+Psychiatric group (Table 2; Figure 1). We divided MM+Psychiatric patients into those who had the psychiatric diagnosis prior to (n = 9355) or after (n = 5813) the diagnosis of MM. Those with a preexisting psychiatric diagnosis were older (median age, 77 years; IQR, 71-82) compared with those with a psychiatric diagnosis after MM (median age, 75 years; IQR, 69-80; P < .001). A higher number of females (52.7%) had preexisting psychiatric conditions, whereas more males (51.5%) were diagnosed with any psychiatric disorder after MM (P < .001). There was no significant difference with regard to race for patients who had a psychiatric diagnosis prior to or after the MM diagnosis (P = .325). CCI was significantly higher in those with a preexisting psychiatric diagnosis (median 2) compared with those diagnosed after MM (median 0; P < .001). With the exception of anemia, all other clinical MDEs were seen in a significantly higher proportion of patients with a psychiatric diagnosis after MM rather than those with preexisting psychiatric diagnoses (P < .001) (Table 2).
Presence of MDEs at any time >30 days after receiving the diagnosis of MM
| Clinical MDE . | MM-Only (n = 20 839) . | MM+Psychiatric (n = 15 168) . | MM-Only (n = 20 839) . | MM+Depression (n = 8421) . | Preexisting psych. diagnosis (n = 9355) . | Psych. diagnosis after MM (n = 5813) . | Preexisting depression diagnosis (n = 4546) . | Depression diagnosis after MM (n = 3875) . |
|---|---|---|---|---|---|---|---|---|
| Hypercalcemia | 2 851 (13.7) | 3 487 (23.0)* | 2 851 (13.7) | 1967 (23.4)* | 2083 (22.3) | 1404 (24.2)* | 1002 (22.0) | 965 (24.9)* |
| Renal dysfunction | 4 580 (22.0) | 4 851 (32.0)* | 4 580 (22.0) | 2772 (32.9)* | 2943 (31.5) | 1908 (32.8)* | 1417 (31.2) | 1355 (35.0)* |
| Anemia | 5 626 (27.0) | 6 456 (42.6)* | 5 626 (27.0) | 3776 (44.8)* | 3984 (42.6) | 2472 (42.5) | 1941 (42.7) | 1835 (47.4)* |
| Bone fractures | 4 928 (23.6) | 5 449 (35.9)* | 4 928 (23.6) | 3230 (38.4)* | 3108 (33.2) | 2341 (40.3)* | 1564 (34.4) | 1666 (43.0)* |
| Renal dialysis | 468 (2.2) | 587 (3.9)* | 468 (2.2) | 346 (4.1)* | 345 (3.7) | 242 (4.2)* | 186 (4.1) | 160 (4.1) |
| Clinical MDE . | MM-Only (n = 20 839) . | MM+Psychiatric (n = 15 168) . | MM-Only (n = 20 839) . | MM+Depression (n = 8421) . | Preexisting psych. diagnosis (n = 9355) . | Psych. diagnosis after MM (n = 5813) . | Preexisting depression diagnosis (n = 4546) . | Depression diagnosis after MM (n = 3875) . |
|---|---|---|---|---|---|---|---|---|
| Hypercalcemia | 2 851 (13.7) | 3 487 (23.0)* | 2 851 (13.7) | 1967 (23.4)* | 2083 (22.3) | 1404 (24.2)* | 1002 (22.0) | 965 (24.9)* |
| Renal dysfunction | 4 580 (22.0) | 4 851 (32.0)* | 4 580 (22.0) | 2772 (32.9)* | 2943 (31.5) | 1908 (32.8)* | 1417 (31.2) | 1355 (35.0)* |
| Anemia | 5 626 (27.0) | 6 456 (42.6)* | 5 626 (27.0) | 3776 (44.8)* | 3984 (42.6) | 2472 (42.5) | 1941 (42.7) | 1835 (47.4)* |
| Bone fractures | 4 928 (23.6) | 5 449 (35.9)* | 4 928 (23.6) | 3230 (38.4)* | 3108 (33.2) | 2341 (40.3)* | 1564 (34.4) | 1666 (43.0)* |
| Renal dialysis | 468 (2.2) | 587 (3.9)* | 468 (2.2) | 346 (4.1)* | 345 (3.7) | 242 (4.2)* | 186 (4.1) | 160 (4.1) |
All data are n (%).
Psych., psychiatric.
Logistic regression P < .001.
Incidence of MDEs in psychiatric conditions. Increased incidence of MDEs (hypercalcemia [HC], renal dysfunction [RD], anemia [A], bone fractures [BF], and dialysis [D]) occurring any time >30 days after receiving the diagnosis of MM in the MM+Psychiatric group vs the MM-Only group (upper panel) and in the MM+Depression group vs the MM-Only group (lower panel).
Incidence of MDEs in psychiatric conditions. Increased incidence of MDEs (hypercalcemia [HC], renal dysfunction [RD], anemia [A], bone fractures [BF], and dialysis [D]) occurring any time >30 days after receiving the diagnosis of MM in the MM+Psychiatric group vs the MM-Only group (upper panel) and in the MM+Depression group vs the MM-Only group (lower panel).
MM-Only vs MM+Depression.
This comparison included 20 839 MM-Only and 8421 (23.4%) MM+Depression patients. Median age in both groups was 76 years (IQR, 70-81). There was a significant difference in sex distribution between the 2 groups with majority (54.5%) of MM-Only patients being males while 57.3% of MM+Depression patients being females (P < .001). Similar to the MM+Psychiatric group analysis, whites were the most numerous race overall (72.4%) followed by African Americans (15.3%), Hispanics (7.6%), Asians (4.4%) and others (0.3%), but again, the racial distribution of patients with depression was significantly different (P < .001) with 30% whites, 27% African Americans, 25% Hispanics, and only 18% Asians in the MM+Depression group. Patients with MM+Depression had a higher CCI (median 2) as compared with those with MM-Only (median 0; P < .001). Similar to the MM+Psychiatric analysis, all clinical MDEs were noted to be significantly more frequently reported in the MM+Depression group as compared with MM-Only (Table 2; Figure 1). We divided MM+Depression patients into those with preexisting depression (n = 4,546) and those with depression noted after the diagnosis of MM (n = 3,875). Those with preexisting depression were older (median, 77 years; IQR, 71-82) compared with patients with depression diagnosed after MM (median, 75 years; IQR 69-80; P < .001). Females had a much higher rate of depression in the preexisting (60.5%) and post-MM (53.5%) psychiatric diagnosis groups (P < .001). CCI was higher in those with preexisting depression (median, 2) compared with those diagnosed with depression after MM diagnosis (median, 1; P < .001). With the exception of kidney dialysis, all clinical MDEs were more commonly seen in patients with depression diagnosed after MM compared with those with preexisting depression (Table 2).
Health care utilization
Health care utilization, as measured by the number of patients undergoing inpatient, emergency department, or ambulatory care among the 3 patient groups, showed that utilization in all of these health care categories was higher in the MM+Psychiatric and MM+Depression groups compared with MM-Only patients (P < .001) (Table 3). In the MM+Psychiatric and MM+Depression groups, the hazard ratios for health care utilization were most prominent in inpatient hospitalizations and were least pronounced in the ambulatory care category compared with the MM-Only group (Table 3).
Health care utilization measured by inpatient hospitalizations, emergency department care and ambulatory care for MM-Only, MM+Psychiatric, and MM+Depression patients
| Health care setting . | MM-Only . | MM+Psychiatric . | OR (95% CI) . | MM-Only . | MM+Depression . | OR (95% CI) . |
|---|---|---|---|---|---|---|
| Inpatient | ||||||
| No. with condition | 26 652 | 9355 | 1.48 (1.39-1.57)* | 26 652 | 4546 | 1.41 (1.31-1.53)* |
| No. with inpatient care (%) | 19 728 (74.0) | 7632 (81.6) | 19 728 (74) | 3696 (81.3) | ||
| Emergency | ||||||
| No. with condition | 26 652 | 9355 | 1.48 (1.41-1.56)* | 26 652 | 4546 | 1.37 (1.28-1.47)* |
| No. with ED care (%) | 14 740 (55.3) | 6290 (67.2) | 14 740 (55.3) | 3006 (66.1) | ||
| Ambulatory | ||||||
| No. with condition | 26 652 | 9355 | 1.25 (1.18-1.31)* | 26 652 | 4546 | 1.22 (1.14-1.30)* |
| No. with ambulatory care (%) | 11 236 (42.2) | 4116 (44.0) | 11 236 (42.2) | 1967 (43.3) |
| Health care setting . | MM-Only . | MM+Psychiatric . | OR (95% CI) . | MM-Only . | MM+Depression . | OR (95% CI) . |
|---|---|---|---|---|---|---|
| Inpatient | ||||||
| No. with condition | 26 652 | 9355 | 1.48 (1.39-1.57)* | 26 652 | 4546 | 1.41 (1.31-1.53)* |
| No. with inpatient care (%) | 19 728 (74.0) | 7632 (81.6) | 19 728 (74) | 3696 (81.3) | ||
| Emergency | ||||||
| No. with condition | 26 652 | 9355 | 1.48 (1.41-1.56)* | 26 652 | 4546 | 1.37 (1.28-1.47)* |
| No. with ED care (%) | 14 740 (55.3) | 6290 (67.2) | 14 740 (55.3) | 3006 (66.1) | ||
| Ambulatory | ||||||
| No. with condition | 26 652 | 9355 | 1.25 (1.18-1.31)* | 26 652 | 4546 | 1.22 (1.14-1.30)* |
| No. with ambulatory care (%) | 11 236 (42.2) | 4116 (44.0) | 11 236 (42.2) | 1967 (43.3) |
CI, confidence interval; ED, emergency department; OR, odds ratio.
P < .001; adjusted for age, calendar year, sex, race, and CCI.
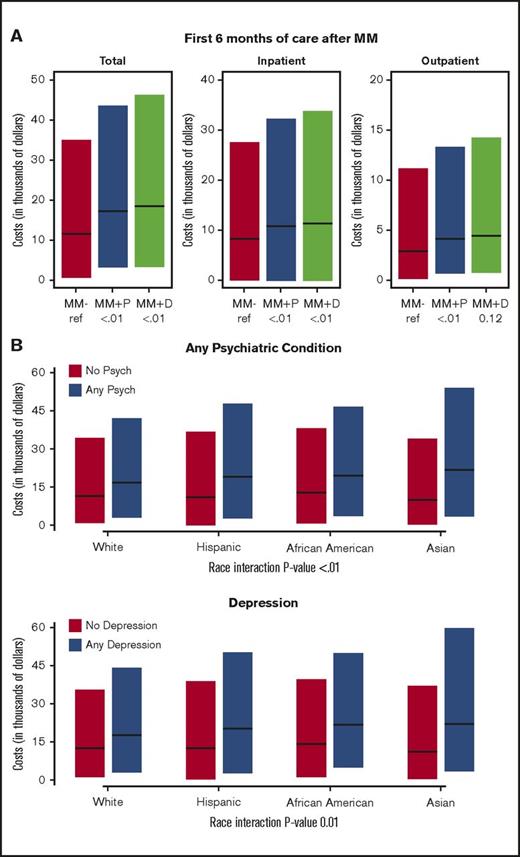
Cost of care
Patients in the MM+Psychiatric and MM+Depression groups had higher costs of care than MM-Only patients (Table 4; Figure 2A). During the first 6 months of treatment after MM diagnosis, MM+Psychiatric patients had a median cost of care of $18 700 compared with $12 300 for MM-Only patients (multivariate odds ratio, 1.25; 95% CI, 1.20-1.31, P < .001). The median cost of care over the first 6 months after MM diagnosis was even higher for MM+Depression patients ($19 500). Similar trends were noted when the cost of care for inpatient and outpatient care was evaluated separately, with the cost being highest for MM+Depression, followed by the MM+Psychiatric and then the MM-Only groups. All of the separate analyses were significant (P < 0.001), with the exception of that for outpatient care cost between MM-Only and MM+Depression groups (multivariate odds ratio, 1.05; 95% CI, 0.99-1.11; P = .123).
Association of cost of care with psychiatric conditions during the first 6 months of care after MM diagnosis in various health care settings
| Health care setting and associated cost . | MM-Only . | MM+Psychiatric . | OR (95% CI) . | MM-Only . | MM+Depression . | OR (95% CI) . |
|---|---|---|---|---|---|---|
| Total | ||||||
| Number | 22 497 | 8590 | 1.25 (1.20, 1.31)* | 22 497 | 4195 | 1.23 (1.16, 1.30)* |
| Mean (SD)† | 29.7 (56.2) | 36.6 (58.9) | 29.7 (56.2) | 37.4 (62.3) | ||
| Median (IQR)† | 12.3 (0.9-36.4) | 18.7 (4.2-45.8) | 12.3 (0.9-36.4) | 19.5 (4.1-46.8) | ||
| Inpatient | ||||||
| Number | 18 503 | 7052 | 1.29 (1.23, 1.36)* | 18 503 | 3390 | 1.33 (1.24, 1.42)* |
| Mean (SD)† | 24.0 (53.6) | 29.9 (56.0) | 24.0 (53.6) | 31.0 (60.5) | ||
| Median (IQR)† | 8.0 (0.0-27.3) | 13.5 (0.0-35.3) | 8.0 (0.0-27.3) | 14.3 (0.0-36.7) | ||
| Outpatient | ||||||
| Number | 20 733 | 8298 | 1.09 (1.04, 1.14)* | 20 733 | 4061 | 1.05 (0.99, 1.11) |
| Mean (SD)† | 10.8 (23.4) | 12.5 (24.0) | 10.8 (23.4) | 12.8 (25.0) | ||
| Median (IQR)† | 3.1 (0.2-11.5) | 4.3 (0.9-13.8) | 3.1 (0.2-11.5) | 4.4 (0.9-14.0) |
| Health care setting and associated cost . | MM-Only . | MM+Psychiatric . | OR (95% CI) . | MM-Only . | MM+Depression . | OR (95% CI) . |
|---|---|---|---|---|---|---|
| Total | ||||||
| Number | 22 497 | 8590 | 1.25 (1.20, 1.31)* | 22 497 | 4195 | 1.23 (1.16, 1.30)* |
| Mean (SD)† | 29.7 (56.2) | 36.6 (58.9) | 29.7 (56.2) | 37.4 (62.3) | ||
| Median (IQR)† | 12.3 (0.9-36.4) | 18.7 (4.2-45.8) | 12.3 (0.9-36.4) | 19.5 (4.1-46.8) | ||
| Inpatient | ||||||
| Number | 18 503 | 7052 | 1.29 (1.23, 1.36)* | 18 503 | 3390 | 1.33 (1.24, 1.42)* |
| Mean (SD)† | 24.0 (53.6) | 29.9 (56.0) | 24.0 (53.6) | 31.0 (60.5) | ||
| Median (IQR)† | 8.0 (0.0-27.3) | 13.5 (0.0-35.3) | 8.0 (0.0-27.3) | 14.3 (0.0-36.7) | ||
| Outpatient | ||||||
| Number | 20 733 | 8298 | 1.09 (1.04, 1.14)* | 20 733 | 4061 | 1.05 (0.99, 1.11) |
| Mean (SD)† | 10.8 (23.4) | 12.5 (24.0) | 10.8 (23.4) | 12.8 (25.0) | ||
| Median (IQR)† | 3.1 (0.2-11.5) | 4.3 (0.9-13.8) | 3.1 (0.2-11.5) | 4.4 (0.9-14.0) |
SD, standard deviation.
P < .001; adjusted for age, calendar year, sex, race, and CCI.
Thousands of dollars.
Cost of multiple myeloma care with coexisting psychiatric comorbidities. (A) Total cost of care broken down by inpatient and outpatient cost for the first 6 months of care after the diagnosis of MM in MM-Only (MM-), MM+Psychiatric (MM+P), and MM+Depression (MM+D) groups. (B) Total cost of care during the first 6 months after MM diagnosis for patients with any psychiatric condition (MM+Psychiatric) vs no psychiatric condition (MM-Only) (upper panel) and for patients with depression (MM+Depression) vs no depression (MM-Only) by patient race (white, Hispanic, African American, and Asian).
Cost of multiple myeloma care with coexisting psychiatric comorbidities. (A) Total cost of care broken down by inpatient and outpatient cost for the first 6 months of care after the diagnosis of MM in MM-Only (MM-), MM+Psychiatric (MM+P), and MM+Depression (MM+D) groups. (B) Total cost of care during the first 6 months after MM diagnosis for patients with any psychiatric condition (MM+Psychiatric) vs no psychiatric condition (MM-Only) (upper panel) and for patients with depression (MM+Depression) vs no depression (MM-Only) by patient race (white, Hispanic, African American, and Asian).
We also assessed any associations between the cost of care (total Medicare claims) during the first 6 months after MM diagnosis and patient race. Although patients of all races had a higher cost of care within the MM+Psychiatric and MM+Depression groups (Figure 2B), there was a stronger association for higher costs in racial minorities compared with whites, with the highest in Asians, followed by Hispanics and then African Americans (Table 5).
Associations among psychological conditions and total Medicare claims during the first 6 months after MM diagnosis by race
| Condition . | White . | Hispanic . | African American . | Asian . | Interaction P* . |
|---|---|---|---|---|---|
| Any psychiatric condition | <.001 | ||||
| MM-Only | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| MM+Psychiatric | 1.20 (1.14-1.27) | 1.59 (1.33-1.90) | 1.26 (1.13-1.41) | 1.89 (1.47-2.43) | |
| Depression | .014 | ||||
| MM-Only | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| MM+Depression | 1.19 (1.11-1.27) | 1.43 (1.14-1.80) | 1.24 (1.05-1.45) | 1.90 (1.35-2.67) |
| Condition . | White . | Hispanic . | African American . | Asian . | Interaction P* . |
|---|---|---|---|---|---|
| Any psychiatric condition | <.001 | ||||
| MM-Only | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| MM+Psychiatric | 1.20 (1.14-1.27) | 1.59 (1.33-1.90) | 1.26 (1.13-1.41) | 1.89 (1.47-2.43) | |
| Depression | .014 | ||||
| MM-Only | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| MM+Depression | 1.19 (1.11-1.27) | 1.43 (1.14-1.80) | 1.24 (1.05-1.45) | 1.90 (1.35-2.67) |
All data are OR (95% CI).
Proportional odds models are adjusted for age, calendar year, sex, and CCI. Interaction P value tests whether there is a significant interaction between race and the specified condition.
Discussion
There have been substantial improvements in management strategies for MM, resulting in declining death rates.1-3 With these advances, the focus is moving to chronic and comorbid health issues that may affect patient quality of life, general well-being, and survivorship among MM patients.24,25 An important component of survivorship is psychosocial well-being. One in 3 patients with cancer suffer from comorbid psychiatric disorders.26-28 However, treating all types of cancer patients, a very heterogeneous group, as a single cohort limits the generalizability to specific populations, such as MM patients. Although some initial data are available,15,16 appropriately powered studies, especially across specific cancer types, are lacking.
We included >36 000 patients in our analysis, the largest of its kind in patients with any single malignancy diagnosis. We noted a psychiatric diagnosis in ∼42% of patients with MM, a number that is significantly higher than previous literature pertaining to cancer patients in general.26,27 This could be secondary, at least in part, to the effect of systemic steroids, because we also noted that 16% of patients overall had a psychiatric diagnosis after the diagnosis of MM. Indeed, MM is a representative cancer diagnosis, with >90% patients treated with long-term systemic steroids, possibly the highest utilization of steroids in any cancer diagnosis.9,10 One study specifically looking at the prevalence of various symptoms in patients with MM reported depression in 23.4% of patients and anxiety in 35.7% of patients.29 Other, not yet published analyses have reported the prevalence of depression and anxiety in MM patients ranging from 14% to 37%.30-32 This was significantly less than our study and could be secondary to a smaller sample size in that study, as well as ours being a population-based study, which is more representative of the true prevalence. This same study also reported fatigue in nearly 99%, pain in 59%, and neuropathy symptoms in 53% of patients with MM.29 These chronic comorbidities could be a part of a psychosomatic symptom complex, with interplay among the effects of the underlying malignancy, its treatment, and preexisting or incident psychiatric conditions.
Because depression was the most common psychiatric condition noted in our dataset, it was considered separately in our analysis. Patients with depression had a significantly higher CCI, suggesting the global impact of depression on an individual’s health.7 Although overall psychiatric diagnoses after MM were more commonly seen in males, depression was more common in females prior to or after the diagnosis of MM, similar to a higher prevalence of depression in females in the general population.33 However, its impact on outcomes in MM patients was mostly similar to all other psychiatric diagnoses.
Clinical MDEs were more common after the diagnosis of MM in patients with psychiatric conditions. These were more common in patients developing the psychiatric condition after MM diagnosis compared with patients with preexisting psychiatric conditions. This could be secondary to the negative effects of certain psychiatric medications on physiological aspects of the patient, including bone density and hematopoiesis.34,35 Although we did not assess medication compliance, it is possible that the development of psychiatric comorbidities after the MM diagnosis could lead to behavioral factors resulting in poor compliance with anti-MM treatment, leading to more organ damage and poor MM control. At the same time, the presence of advanced MM with complications could give rise to psychiatric comorbidities, because we noted a higher incidence of clinical MDEs in patients with incident psychiatric conditions after MM diagnosis. Nevertheless, ours is a unique finding, because this is the first study to link physical signs noted in patients with MM with psychiatric comorbidities. Although it is known that psychiatric comorbidities, especially depression, can lead to other somatic symptoms, the increased incidence of physical signs is very significant. It is possible that appropriately controlling the psychiatric condition may help to limit the manifestations related to the underlying cancer as well, by improving the overall health of the patient.
Although psychiatric conditions are associated with poorer quality of life and shorter survival in cancer patients,36 their link with health care utilization and medical expenditures has been explored in relatively few studies.15,16 We noted a clear association between the presence of psychiatric comorbidities and an increased utilization of all aspects of health care for MM patients. This becomes significant, considering the strain on the current health care system in view of the population increase, improved survival, and longevity from cancer diagnoses in general and an increasingly diverse patient population with variable diagnostic and management challenges. Similar to our analysis in MM patients, Han et al noted a greater impact on health care utilization and expenditures in the inpatient and emergency care settings compared with ambulatory care for all cancer patients.15 They noted significantly increased medical expenditures in cancer survivors with serious psychological distress compared with cancer survivors or noncancer patients without such distress.15 However, compared with our analysis, their study included a much smaller number of cancer patients and did not focus on a single cancer diagnosis. Investigation within a single cancer diagnosis, as in our analysis, helps to identify and understand more specific factors that may be at play (eg, the impact of steroids).
We noted that a lower proportion of racial minorities, especially Hispanics and Asians, have a psychiatric diagnosis, whereas African Americans and whites had comparable numbers. This is similar to reports from the general United States population, where studies show that members of minority groups have either lower or equivalent rates of mental disorders compared with whites, although such data are not available for specific cancer diagnoses.37,38 Prior studies exploring racial disparities in psychiatric care have suggested problems with access, inaccurate diagnoses, inadequate medication prescribing, and monitoring in racial minorities.39,40 Specifically, decreased access to adequate MM care for patients of racial minorities has been reported in several studies.41-46 Although these studies have explored differences in access to care and the financial benefits of eliminating disparities, to our knowledge, no prior study has demonstrated an interaction between cost of care and race in cancer patients with psychiatric comorbidities.
A limitation of our study is that we used billing data to identify psychiatric comorbidities. This could lead to ascertainment bias, because it is dependent, at least in part, on patients soliciting the psychiatric conditions themselves, in addition to the clinician's assessment, leading to assigning of the diagnosis and its ICD-9 code. We used a similar approach as Himelhoch et al in identifying psychiatric comorbidities from claims-based databases.19 However, our study looked at all of the psychiatric comorbidities rather than narrowly focusing on depression syndrome, thus capturing the global impact of psychiatric conditions on health care in MM. We did attempt a separate analysis for other subcategories of psychiatric diagnoses (eg, bipolar disorder, schizophrenia, and other psychotic disorders), but the patient numbers for various prespecified categories, including health care settings and race/ethnicity, were too small to have any meaningful statistical value.
In conclusion, this study shows the high rate of psychiatric problems in MM patients. With these problems, health care utilization and the cost of care increase. Although national entities and accreditation bodies emphasize screening and identifying and addressing the psychological needs of cancer patients, more needs to be done.47-49 Improved understanding of the true prevalence of psychiatric comorbidities in cancer patients, as well as their impact on health care utilization and cost of cancer care, is imperative in developing targeted mitigation strategies.
Presented in part at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
The full-text version of this article contains a data supplement.
Authorship
Contribution: S.N., R.D.F., and S. Ames were involved in study design; S. Ailawadhi was involved in data collection; S.N., R.D.F., V.R., S. Ahmed, T.R., A.S., T.S., and W.T. were involved in data analysis; S.N., R.D.F., M.S., M.A., K.B., S. Ahmed, A.C.-K., and S. Ailawadhi wrote the manuscript; and the final manuscript was reviewed and approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sikander Ailawadhi, Division of Hematology-Oncology, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224; e-mail: ailawadhi.sikander@mayo.edu.

![Figure 1. Incidence of MDEs in psychiatric conditions. Increased incidence of MDEs (hypercalcemia [HC], renal dysfunction [RD], anemia [A], bone fractures [BF], and dialysis [D]) occurring any time >30 days after receiving the diagnosis of MM in the MM+Psychiatric group vs the MM-Only group (upper panel) and in the MM+Depression group vs the MM-Only group (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/10/10.1182_bloodadvances.2018016717/3/m_advances016717f1.jpeg?Expires=1770204837&Signature=OT5x1IH43y5Yz3IpWfbO2hG2Kr24WWTEJG1OC8iWA2juBFeTnKw7hZWv4Z1Ylbf4BXlBbaLWrNUXLTkjtWLBBK2LCOWT6DHrNOB9hIQ3fKiTjowCqC2r~EDIM-vYvL0jTlulAv~mJnJDok7pauT3EG~NgMLTM5XwgcPqMovjTIg~TnoTiWxEu430R2CYrG8q79x7MEQhW0d1sg-F999nkuVePCQQNxRh7bhuwAaTWTB7uEf5YzYLhVSRSZn4rA8Xf9~cGtHMNwFMwXDB-VuXWTl9HI1V3VWW95cR75E7rJbkLCuCYni~3uQwIa27SNp6caEllxIxBMnIQS9txIuB~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)