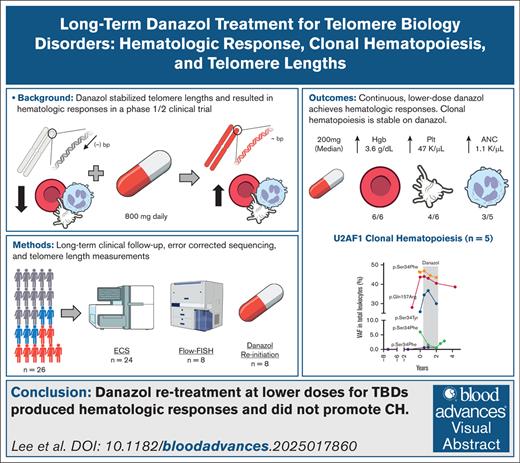
Visual Abstract
TO THE EDITOR:
Telomere biology disorders (TBD) are a group of inherited bone marrow failure syndromes caused by pathogenic germ line variants (PGV) in telomere-related genes and characterized by abnormal telomere maintenance and a clinically heterogeneous phenotype, including bone marrow failure, pulmonary and liver disease, and predisposition to hematologic and solid malignancies.1-3 Treatment options are limited, and include supportive care, androgens, and/or hematopoietic stem cell transplant. In our prospective phase 1/2 study (ClinicalTrials.gov identifier: NCT01441037), danazol improved the hematologic parameters and stabilized telomere attrition in patients with TBD. Telomere length (TL) measurement was performed by real-time quantitative polymerase chain reaction.4 Inclusion required age-adjusted TL ≤1st percentile and/or identified mutations in telomere maintenance plus 1 cytopenia and/or pulmonary fibrosis (supplemental Table 1). Of 26 evaluable patients, most harbored PGV in known TBD-related genes: TERT (n = 9), TERC (n = 7), DKC1 (n = 2), or RTEL1 (n = 1), and 7 had no identified PGV. Clinical phenotypes included bone marrow failure (n = 24), pulmonary fibrosis (n = 13), liver fibrosis (n = 6), myelodysplastic syndrome (MDS) (n = 2), and previously diagnosed, inactive solid cancers (n = 2; supplemental Tables 2 and 3). Patients were treated with danazol, 800 mg daily, for 2 years or until the study was closed for efficacy, with a primary biologic end point of telomere attrition. Secondary end points included hematologic response (3 and 6 months and yearly thereafter), loss of hematologic response, hematologic malignancy development, pulmonary function decline (using pulmonary function tests), and overall survival (OS).
We report long-term follow-up of secondary end points, retrospective analysis of TL through flow-fluorescence in situ hybridization (FISH), and dynamics of clonal hematopoiesis during danazol therapy. The TL of lymphocytes was measured in all available samples using flow-FISH at either baseline, after danazol discontinuation, or yearly thereafter (RepeatDx, Vancouver, Canada). Delta TL was defined as the difference between measured and expected TL of age-matched controls in kilobases. Error-corrected sequencing with a customized panel at a minimum variant allele frequency of 0.5% was performed in available total DNA samples at different time points (supplemental Table 4).5 Event-free survival (EFS) was defined as requiring hematopoietic stem cell transplant, lung transplant, liver transplant, or death. Loss of hematologic response, EFS, and OS were determined by Kaplan-Meier analysis (supplemental Table 2). All patients provided informed consent in accordance with the Declaration of Helsinki under this National Institutes of Health Institutional Review Board–approved protocol. Patients, once off study, were followed long-term as part of standard clinical care or on a natural history study (ClinicalTrials.gov identifier: NCT05012111).
Median follow-up time to last clinical evaluation was 3.3 years (range, 0.3-12.6 years). Half the cohort (n = 13) had at least 1 posttrial follow-up visit (Figure 1A). After danazol discontinuation, median time to loss of hematologic response in 12 evaluable patients was 1.6 years (Figure 1B). Median EFS was 5.7 years (supplemental Figure 1A). Median OS from enrollment was 7.7 years, with 62% (n = 16) of the patients known to have died at last follow-up (Figure 1C) with infection as the most common known cause of death (60%, n = 6/10; supplemental Table 5). There were no significant EFS or OS differences by PGV (supplemental Figure 1B-E). Eight patients restarted danazol posttrial at a median dose of 200 mg (range, 75-600 mg), with second hematologic response in 86% (n = 6/7; Figure 1D; supplemental Figure 2); median increase in hemoglobin, platelet, and neutrophil levels was 3.6 g/dL (n = 6/6), 47 × 103/μL (n = 4/6), and 1.1 × 103/μL (n = 3/5), respectively. Liver enzymes and lipid profiles did not require modification of danazol therapy (supplemental Figure 3); unique patient number (UPN)-5 had fluctuating alanine aminotransferase/aspartate aminotransferase elevations on and off danazol, hyperlipidemia, and eventually developed a small hepatic adenoma requiring danazol cessation.
Long-term clinical outcomes of patients on danazol. (A) CONSORT diagram from initial enrollment on study, study completion, and post-protocol follow-up. One patient from the 27 enrolled was deemed nonevaluable due to subsequent Fanconi anemia diagnosis. (B) Time to loss of hematologic response after danazol discontinuation per protocol. (C) OS of the patients on the danazol study with long-term follow-up. (D) A swimmer diagram indicating hematologic response (blue) or lack of hematologic response (red) to danazol (green line overlayed). A gray bar indicates hematologic response is nonevaluable, such as if there was remote follow-up or post-hematopoietic stem cell transplant (HSCT). Events indicated are chromosomal abnormalities, MDS/myelodysplastic neoplasm development, HSCT, liver transplant, lung transplant, adverse events, and death.
Long-term clinical outcomes of patients on danazol. (A) CONSORT diagram from initial enrollment on study, study completion, and post-protocol follow-up. One patient from the 27 enrolled was deemed nonevaluable due to subsequent Fanconi anemia diagnosis. (B) Time to loss of hematologic response after danazol discontinuation per protocol. (C) OS of the patients on the danazol study with long-term follow-up. (D) A swimmer diagram indicating hematologic response (blue) or lack of hematologic response (red) to danazol (green line overlayed). A gray bar indicates hematologic response is nonevaluable, such as if there was remote follow-up or post-hematopoietic stem cell transplant (HSCT). Events indicated are chromosomal abnormalities, MDS/myelodysplastic neoplasm development, HSCT, liver transplant, lung transplant, adverse events, and death.
Extrahematologic manifestations of TBD include pulmonary disease and cancer. Forced vital capacity was stable while on the drug but declined after danazol cessation (P = .04; supplemental Figure 4). Decline in diffusing lung capacity of the lung for carbon monoxide before danazol treatment stabilized while on therapy and remained as such after drug withdrawal (P = .01; supplemental Figures 4 and 5). No new solid malignancies developed on trial. Two patients had previously reported isolated cytogenetic abnormalities without morphologic MDS,4 trisomy 21, and +1q, and they did not develop further cytogenetic or morphologic changes (Figure 2A). One TERT-mutated patient (UPN-10), who had MDS with multilineage dysplasia at enrollment, progressed to acute myeloid leukemia (AML) 3 years after study completion. One patient (UPN-23), without a detectable TBD-related germ line variant, developed MDS with multilineage dysplasia with monosomy 7 at 24 months (Figure 2A; supplemental Table 6); this patient also developed a small paroxysmal nocturnal hemoglobinuria clone, and our error-corrected sequencing later showed BCOR and PIGA mutations, suggesting that this patient likely had immune aplastic anemia (AA; supplemental Table 7) and highlighting the ongoing difficulty in discerning inherited bone marrow failure syndromes from immune AA.
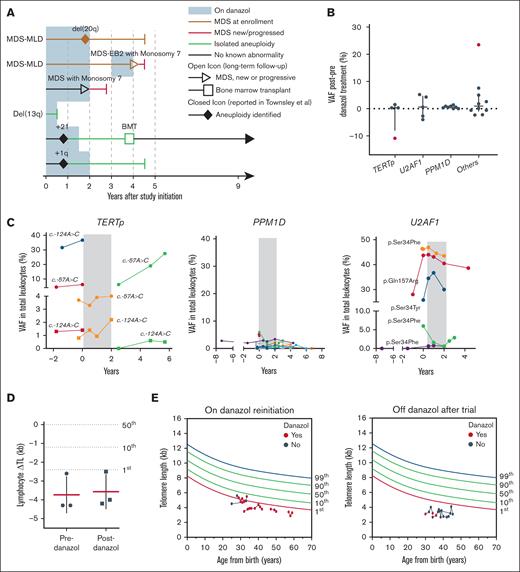
Myeloid malignancies, clonal dynamics, and TLs. (A) A swimmer diagram highlighting long-term follow-up of patients with chromosomal abnormalities and/or myeloid malignancies. (B) Differences of VAF of somatic mutations after the completion of danazol therapy compared to baseline in PPM1D, TERTp, U2AF1, and other genes (RUNX1, ASLX1, BCOR, GATA2, SETBP1, ZRSR2, ETV6, and PHF6). Positive values represent variants that expanded posttherapy, whereas negative values represent variants that decreased in size. Outliers in red represent a BCOR variant that highly expanded after danazol therapy but eventually decreased in size after drug discontinuation and a TERTp variant that decreased in size (supplemental Figure 6). (C) Clonal dynamics of TERTp, PPM1D (all exon 6 truncations), and U2AF1 p.Ser34Phe somatic mutations in available samples from therapy initiation. The time of danazol treatment is indicated in gray. Each line color represents a single patient. (D) Average ΔTL before and after danazol treatment on study. Mean and standard deviation are marked. (E) TL of lymphocytes measured using flow-FISH after the trial for patients for whom danazol was reinitiated (left panel) or who were continued off-therapy (right panel). BMT, bone marrow transplant; del, deletion; EB2, excess blasts 2; MLD, multilineage dysplasia; ΔTL, delta TL; VAF, variant allele frequency.
Myeloid malignancies, clonal dynamics, and TLs. (A) A swimmer diagram highlighting long-term follow-up of patients with chromosomal abnormalities and/or myeloid malignancies. (B) Differences of VAF of somatic mutations after the completion of danazol therapy compared to baseline in PPM1D, TERTp, U2AF1, and other genes (RUNX1, ASLX1, BCOR, GATA2, SETBP1, ZRSR2, ETV6, and PHF6). Positive values represent variants that expanded posttherapy, whereas negative values represent variants that decreased in size. Outliers in red represent a BCOR variant that highly expanded after danazol therapy but eventually decreased in size after drug discontinuation and a TERTp variant that decreased in size (supplemental Figure 6). (C) Clonal dynamics of TERTp, PPM1D (all exon 6 truncations), and U2AF1 p.Ser34Phe somatic mutations in available samples from therapy initiation. The time of danazol treatment is indicated in gray. Each line color represents a single patient. (D) Average ΔTL before and after danazol treatment on study. Mean and standard deviation are marked. (E) TL of lymphocytes measured using flow-FISH after the trial for patients for whom danazol was reinitiated (left panel) or who were continued off-therapy (right panel). BMT, bone marrow transplant; del, deletion; EB2, excess blasts 2; MLD, multilineage dysplasia; ΔTL, delta TL; VAF, variant allele frequency.
A major concern with danazol treatment is the potential for selection/expansion of premalignant hematopoietic stem and progenitor clones. At baseline, 63% (15/24) of patients had somatic mutations, most commonly in the TERT promoter (TERTp; n = 7), PPM1D (n = 6), U2AF1 (n = 4), and other MDS-related genes (n = 8) (supplemental Figure 6; supplemental Table 7), consistent with our recent, multi-institutional report.5 There was no difference in hematologic response at 6 months in those with or without TERTp somatic mutations (P = .63). There was no marked expansion of preexistent clones or acquisition of novel ones after therapy (Figure 2B). Long-term clonal dynamics were largely stable, regardless of mutated gene (U2AF1, PPM1D, or TERTp; Figure 2C) and clinical MDS diagnosis (supplemental Figure 7). In 1 patient with MDS (UPN-10) who ultimately developed AML, evolution coincided with the acquisition of monosomy 7 and a KRAS mutation in a driver hematopoietic stem cell clone harboring chromosome 1q gain (Chr1q+), U2AF1Q157R, ASXL1, and SETBP1 mutations that were stable for many years (supplemental Figure 7). Chr1q+ is a premalignant clonal change in patients with TBD linked to latent MDS/AML development via linear second hits.5 In the patient with likely immune AA (UPN-23), new PIGA, BCOR, and ASXL1 clones emerged, as previously reported in this disease (supplemental Figure 7).6,7 These 2 events likely represent the natural history of the underlying TBD and immune-mediated AA, respectively, rather than an androgen effect.
The TL dynamics of patients with TBD on androgens have been variable in reports,8-10 with some showing no effect and others indicating stabilization or elongation of TLs. Most recently, a prospective trial using nandrolone showed TL elongation using flow-FISH.8,10 The TLs of lymphocytes retrospectively measured in stored samples collected at trial enrollment and 2 years posttherapy from 2 patients were stable (Figure 2D). Serial TL measurements of lymphocytes from 8 patients monitored post-trial were stable, regardless of reintroduction on low-dose danazol treatment in the patients (Figure 2E; supplemental Figure 8). No significant TL elongation was observed in this limited number of samples.
In summary, most patients required continuous administration of danazol to improve hematologic parameters, which worked at lower doses than mandated by protocol. Long-term analysis showed no evidence that danazol induces clonal hematopoiesis or malignant evolution, and TL, as assessed by flow-FISH, was stable over time. The longitudinal analysis of TL using flow-FISH and pulmonary end points were limited due to cohort size. However, our currently enrolling low-dose danazol protocol (ClinicalTrials.gov identifier: NCT03312400) will assess TLs measured via flow-FISH and should provide more definitive conclusions.
Acknowledgments: This research is supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, under the National Institutes of Health (NIH).
The contributions of the NIH authors are considered works of the US Government. The findings and conclusions presented in this article are those of the authors and do not necessarily reflect the views of the NIH or the US Department of Health and Human Services.
Contribution: N.C.L., F.G.-R., and E.M.G. designed the research; N.S.Y., B.A.P., F.G.-R., and E.M.G. guided the research; T.M., J.L., N.S.Y., B.A.P., and E.M.G. provided patient-associated resources and/or patients samples for the study; G.A. provided telomere length analysis; S.P. created the oncoprint; R.S. and C.O.W. performed the statistical analysis; S.K., D.Q.R., and L.A., collected the samples and helped with the data analysis; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: G.A. is an employee of RepeatDx, a clinical laboratory specializing in telomere length measurement services. The remaining authors declare no competing financial interests.
Correspondence: Nicholas C. Lee, National Heart, Lung, and Blood Institute, Building 10-CRC, Room 4-5140, 10 Center Dr, Bethesda, MD 20892-1202; email: nicholas.lee3@nih.gov; and Emma M. Groarke, National Heart, Lung, and Blood Institute, Building 10-CRC, Room 4-5140, 10 Center Dr, Bethesda, MD 20892-1202; email: emma.groarke@nih.gov.
References
Author notes
Deidentified data will be provided to qualified researchers from the corresponding authors, Nicholas C. Lee (nicholas.lee3@nih.gov) and Emma M. Groarke (emma.groarke@nih.gov), on request.
The full-text version of this article contains a data supplement.