Background
Acute leukemias have an incidence rate of 75.3 per million people per year and represent 50.8% of childhood cancers in Mexico. Despite improved access to treatment through a fund for catastrophic illnesses for almost a decade, the population-based 5-year survival rate remains <50% for acute lymphoblastic leukemia (ALL) and 30.5% for acute myeloid leukemia (AML). At present, most pediatric hematology and oncology centers still evaluate response to therapy using morphology. This impairs the ability for correct and timely adjustment of treatment intensity and the disease-free and overall survival of Mexican children with acute leukemia.
The evaluation of minimal residual disease (MRD) by flow cytometry (FC) is now considered standard practice in high-income countries. However, the technology has only recently been made accessible in Mexico, including at our institution. Proper implementation of MRD by FC requires the use of standardized procedures as well as internal and external quality controls to ensure the accuracy and reliability of the results. These validation steps are important because the results directly affect treatment decisions.
Goals
To implement and validate MRD by FC at our center with support from an experienced center (Dana-Farber Cancer Institute [DFCI]/Boston Children’s Hospital [BCH]).
To become a self-sufficient center for reliable MRD determination by FC for pediatric acute leukemia in Mexico.
To become a national reference center and support other centers in correct diagnosis and MRD determination by FC, as well as in capacity building and education on these subjects.
Methods
Patients: Consecutive patients with a suspected new diagnosis of acute leukemia presenting to the Hospital Infantil Teletón de Oncología (HITO) from December 2015 to March 2017 were included.
Sample collection: Bone marrow samples were obtained at the time points established in the respective treatment protocols: DFCI 05-001 for B- and T-cell ALL, COG AALL1122 for Philadelphia-positive ALL patients, Interfant 06 for patients <1 year of age, and AML02 for patients with AML. No additional procedures were performed for the sole purpose of obtaining samples for this project. One EDTA tube with bone marrow (3 mL) and 1 unstained morphology slide were collected by HITO staff following institutional procedures and sent to BCH with a clinical patient summary and project front sheet for review and processing.
Processing: Samples were collected and processed at HITO first, and then sent to BCH for prospective, real-time, simultaneous evaluation. At HITO, we used a Gallios-model flow cytometer (Beckman-Coulter) with 8 fluorescences.
Shipping: Samples were sent the same day of collection and planned to arrive in Boston within the first 48 hours of collection for adequate processing.
Validation process: Plots and interpretation were exchanged between institutions. The HITO team always provided a result and interpretation first, and BCH provided their interpretation 3 to 5 days thereafter. Urgent treatment decisions were made using HITO results. HITO managed case logs and oversight of concordance. Difficult cases as well as discordant cases were discussed by e-mail and teleconferencing. HITO sequentially improved tests based on recommendations, from the sample handling to the analysis algorithm, interpretation, and structure of the final report.
Results
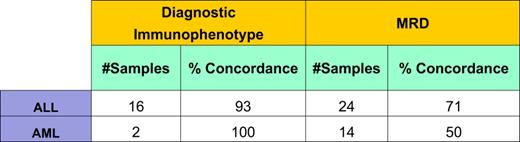
1) Concordance rate: A total of 59 samples from 20 patients were obtained. Three (5%) samples were inadequate for processing and excluded from the quality improvement project. The overall concordance for ALL and AML was 0.56 using Cohen’s κ index. Other concordance rates are reported below (Table 1). A noteworthy improvement in concordance was observed after standardization of antibody panels and the analytic strategy (Table 2).
1.1) Evaluation of discordant cases:
Inadequate sample: Samples shipped to BCH had to be ≥2 mL in volume, free of clots, transported at a temperature of 4 to 8°C, and stored for ≤48 hours. In 5% of cases, MRD could not be validated at BCH using the sample provided. All 5% were among the first samples collected and had debris as a result of delayed delivery. We adjusted bone marrow sample collection days and times and HITO laboratory processing to ensure samples could be sent out the same day they were collected and arrive in Boston on a business day. After these adjustments, all the samples could be processed and validated.
Different multiparameter antibody panel and different analytic strategies: The antibody panel standardized with support from BCH for ALL was: CD81, CD20, CD10, CD45, CD19, CD58, CD34, and CD38; for AML, it was: CD117, CD34, and the most significant markers of the immunophenotype.
Difficult cases: Three patients with a Ph-positive ALL diagnosis and 1 patient with M7 AML.
a) The 3 patients with Ph-positive ALL had many hematogones at day 32, the established time point for MRD evaluation. Trouble-shooting these cases helped establish an analysis strategy using CD81 to separate blasts from hematogones.
b) The AML M7 case helped us to define our analysis graphs with CD117 vs CD34 + myeloid antigens and thus rule out the possibility of observing false positive cells.
2) Clinical impact of the MRD validation project: Treatment plan reassignments occurred for 7 of the 20 patients studied (35% of patients studied).
ALL patients: Review of FC diagnostic panels motivated 1 diagnostic correction (from biphenotypic B/T- to B-cell ALL). The reassignment led to a clinically significant change in treatment strategy and prognosis. Review of end-of-induction MRD results motivated patient risk reassignment for 1 ALL patient from standard risk to very high risk, therefore leading to timely and appropriate intensification of therapy. For 3 patients with Ph-positive ALL diagnoses, the end-of-induction MRD results motivated changes in the flow cytometry panel; by the second time point of evaluation, the results were concordant. Had discordance not been identified and the panel not adjusted, results at the second time point could have led to unwarranted intensification of treatment.
AML patients: Review of MRD results motivated reassignment of risk classification for 2 patients with AML from high risk to standard risk; this eliminated the indication for hematopoietic stem cell transplantation in the first remission and therefore spared patients an unwarranted intensification of treatment.
Conclusions
External validation of diagnostic and MRD FC results is a crucial step during the implementation of these techniques in-house. The presence of hematogones poses a major challenge and risk during MRD implementation. The validation steps allowed for establishing MRD antibody panels and refining analytic strategy for successful distinction between blasts and hematogones.
In the absence of validation steps, up to one-third of patients run the risk of misclassification. Validation steps allowed timely and correct risk-group reassignment in 35% of patients and avoided overtreatment in 30% of patients. Considering the recognized power of FC MRD as a prognostic indicator and the expected increase in attempts of in-house implementation globally, our results document how technical standardization of the test is challenging but essential.
Authorship
Conflict of interest disclosure: No competing financial interests declared.
Correspondence: M. Hernández, Department of Pediatric Oncology, Hospital Infantil Teletón de Oncología, Querétaro, Mexico; e-mail: hernandez@hospitalteleton.org.mx.