Background
Stem cell transplant centers in low- and middle-income countries have a lesser contribution in advancement through clinical research in the field of stem cell transplantation. Among the various reasons for this disproportion are limitations in access to technology and lack of training to acquire the demanding level of skills. We showcase our experience of establishing a stem cell research department at our hospital through training received from the American Society of Hematology Visitor Training Program (VTP).
Methods
In the year 2011, the presenter received 3 months of laboratory training at University Children’s Hospital, Tübingen, Germany, through the American Society of Hematology VTP. The training included stem cell processing techniques like collection, immunomagnetic selection, culturing, cryopreservation, and analysis by flowcytometry, as well as donor chimerism analysis.
Using this training experience, we established a fully functional stem cell research department with facilities like a mesenchymal stem cell (MSC) culture laboratory, a molecular biology laboratory for chimerism studies, a flow cytometry laboratory for CD34 quantification, and a biospecimen bank of transplant patients and their donors.
Outcome
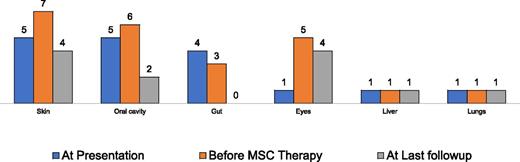
Since February 2014, we have prepared a total of 57 cultured MSC doses for use in 2 human clinical trials carried out at our center for conditions like spinal cord injury (phase 1) and graft-versus-host disease (phase 2) (Figure 1). The former study was published in Cytotherapy, whereas the latter has been submitted for publication.
Outcome of MSC therapy in steroid-resistant graft-versus-host disease.
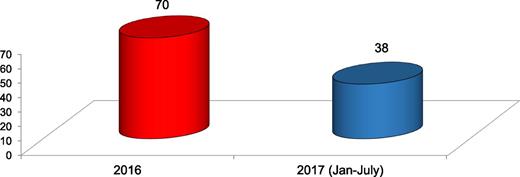
The molecular laboratory has performed chimerism analysis in 108 consecutive transplant patients during the last 2 years (Figure 2). Besides this, we have started testing molecular panels in myeloproliferative neoplasms and acute leukemias.
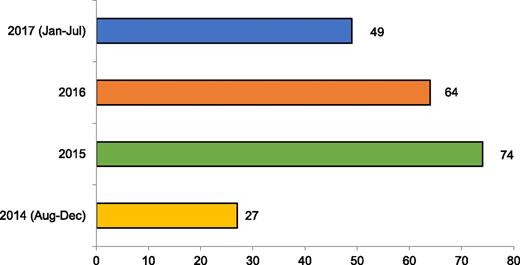
The biospecimen repository currently contains serum, DNA, and nucleated cell specimens for 214 transplanted patients and their donors (Figure 3). We are already using these specimens as a resource in our molecular genetic research projects.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Humayoon Shafique Satti, Armed Forces Bone Marrow Transplant Centre/National Institute of Blood & Marrow Transplant, Rawalpindi, Pakistan; e-mail: humayun225@gmail.com.