Key Points
Carfilzomib is associated with reversible cardiotoxicity in 12% of consecutive myeloma patients.
Transient renal toxicity is common, but carfilzomib may improve renal function in myeloma-related renal impairment.
Abstract
Clinical trials with carfilzomib have indicated a low but reproducible incidence of cardiovascular and renal toxicities. Among 60 consecutive myeloma patients treated with carfilzomib-based regimens who were thoroughly evaluated for cardiovascular risk factors, 12% (95% confidence interval, 3.8%-20%) experienced a reversible reduction of left ventricular ejection fraction (LVEF) by ≥20%, an objective measure of cardiac dysfunction. The incidence of LVEF reduction was 5% at 3 months, 8% at 6 months, 10% at 12 months, and 12% at 15 months, whereas the respective carfilzomib discontinuation rate unrelated to toxicity was 17%, 35%, 41%, and 49%. The presence of any previously known cardiovascular disease was associated with an increased incidence of cardiac events (23.5% vs 7%; P = .07), but there was no association with the dose of carfilzomib or the duration of infusion. Re-treatment with carfilzomib at lower doses was possible. Carfilzomib was commonly associated with a transient reduction of estimated glomerular filtration rate (eGFR) but also improved renal function in 55% of patients with baseline eGFR <60 mL/min/1.73 m2. Further investigation is needed to elucidate the underlying mechanisms of carfilzomib-related cardiorenal toxicity.
Introduction
Carfilzomib is an epoxyketone proteasome inhibitor (PI) that binds selectively and irreversibly to the chymotrypsin catalytic subunit of the 20S proteasome,1,2 but it is structurally different and has shown less off-target activity compared with bortezomib.2 Notably, a low but reproducible incidence of cardiovascular toxicities, including hypertension, congestive heart failure (CHF), coronary artery disease (CAD), and renal toxicities have been associated with carfilzomib.3-5 In a pooled safety analysis5 and in the recently reported ASPIRE study6 (in which patients were treated with carfilzomib 27 mg/m2 intravenously over 10 minutes with lenalidomide and dexamethasone) and the ENDEAVOR study7 (in which carfilzomib was administered at 56 mg/m2 intravenously over 30 minutes with dexamethasone), hypertension was recorded in 4% to 14%, acute renal failure in 3.3% to 5.3%, and cardiac events in 7% to 10.6% of patients, including CHF in 3.8% to 7.2% of patients. The etiology of these cardiovascular and renal complications has not been clearly defined. Few studies have evaluated possible associations with baseline risk factors, the outcome of patients experiencing these events has seldom been described in detail, and data on retreatment with carfilzomib are scarce. We report a detailed analysis of 60 consecutive myeloma patients who received carfilzomib-based regimens in our center.
Patients and methods
For all patients, established cardiovascular and renal risk factors and all medications were recorded in detail. Blood pressure was measured before every carfilzomib infusion; electrocardiograms and echocardiography were performed at baseline, and patients were assessed with echocardiography and serum N-terminal prohormone brain natriuretic peptide (NT-proBNP) and troponins when there was a suspicion of a cardiac complication, such as when symptoms of dyspnea, chest pain, new onset fatigue, and palpitations were reported. All patients had baseline left ventricular ejection fraction (LVEF) ≥40%. To have an objective measure of cardiotoxicity, a cardiac event was defined as a relative reduction of the LVEF by at least 20%. Patients who developed a cardiac event were observed and received serial echocardiograms every 2 weeks for the first month and then once per month thereafter. Before and after each carfilzomib infusion, 250 mL of saline (NaCl 0.9%) was given; after the initial cycles of carfilzomib, hydration was reduced. Doses of carfilzomib 27 mg/m2 were given over 10 minutes and doses of 36 mg/m2 or 56 mg/m2 were given over 30 minutes. Dexamethasone dose was 20 to 40 mg once per week, except for patients who received carfilzomib with melphalan and prednisone who were given prednisone at 60 mg/m2 for 4 consecutive days every 6 weeks. The cardiovascular and renal events were defined and rated according to US National Institutes of Health, National Cancer Institute Common Terminology Criteria for Adverse Events, v4.03. An informed consent for collection of data and analysis was obtained from the patients per the Declaration of Helsinki. Approval from the institutional review board of Alexandra Hospital was obtained for data collection and publication. Time-to-event curves were plotted with cardiac events. Discontinuation of carfilzomib as a result of progression of disease or for other reasons was treated as a competing event, according to the method of Fine and Gray.8 Analysis was performed using R software as proposed by Scrucca et al.9
Results
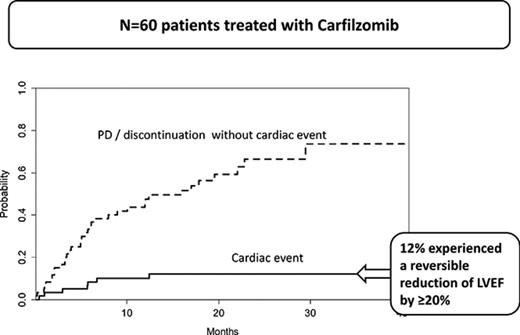
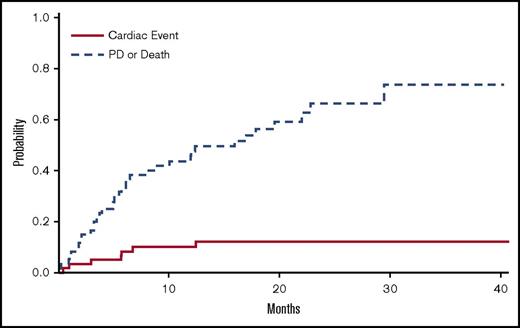
Table 1 shows patient characteristics, including baseline cardiovascular and renal risk factors. Table 2 shows the characteristics of patients treated with different carfilzomib-based regimens. Median duration of carfilzomib therapy was 9.3 months (range, 0.5-40.1+ months). Four patients (2 with normal baseline blood pressure and 2 with prior hypertension) developed grade 3 hypertension which was controlled with anti-hypertensives. Cardiac events were defined on the basis of clinically significant relative reduction in the LVEF by at least 20% from baseline, obtained before the initiation of therapy with carfilzomib. Thus, during carfilzomib therapy, 7 (12%; 95% confidence interval [CI], 3.7%-20%) patients had a relative LVEF reduction ≥20%, an objective measure of cardiotoxicity, within a median of 6 months (range, 1-13 months) from initiation of therapy. The incidence of relative LVEF reduction ≥20% was 5%, 8%, 10%, and 12% at 3, 6, 12, and 15 months, whereas carfilzomib discontinuation rate unrelated to cardiac toxicity (mostly for disease progression) was 17%, 35%, 41%, and 49%, respectively (Figure 1). In all patients with ejection fraction reduction, NT-proBNP increased concomitantly with LVEF decrease (median increase, 2412 pg/mL; range, 2219-10 162 pg/mL) but without increase in serum troponins. The presence of any previous known cardiovascular disease (ie, CAD, peripheral artery disease, or stroke) was associated with an increased incidence of cardiac events (23.5% vs 7%; P = .07). We found no significant association between the dose of carfilzomib (ie, 27 mg m2 vs 36 mg/m2 vs 56 mg/m2) and the incidence or time to occurrence of cardiac events. There was also no difference between previously treated (2 of 12 [17% developed ejection fraction reduction]) and untreated patients (5 [10%] of 48; P = .546) and the difference remained nonsignificant, even after adjustment for prior therapy and dose of carfilzomib. We did not find a clear association between the duration of infusion and the frequency (7.4% vs 15.2% for 10 vs 30 minutes of infusion; P = .353) or time to LVEF reduction, even after adjustment for the different dose levels.
Patient characteristics
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, y (range) | 72 (39-86) | |
| Sex | ||
| Male | 39 | 58 |
| Female | 21 | 42 |
| Median No. of prior treatments (range) | 2 (0-7) | |
| NDMM | 12 | 20 |
| RRMM | 48 | 80 |
| Prior high-dose therapy | 33 | 55 |
| Prior anthracyclines | 22 | 37 |
| Bortezomib | 39 | 65 |
| Immunomodulatory imide drugs | 40 | 67 |
| Cardiovascular risk factors | ||
| Smoking | 26 | 43 |
| Hypertension | 22 | 36 |
| Coronary artery disease | 5 | 8 |
| Peripheral artery disease | 13 | 22 |
| Diabetes mellitus | 10 | 16 |
| Hyperlipidemia | 12 | 20 |
| Stroke | 3 | 5 |
| Arrhythmias | 4 | 6 |
| Cardiovascular disease (coronary artery disease, peripheral artery disease, stroke) | 17 | 28 |
| LVEF, % (range) | 60 (40-70) | |
| Baseline electrocardiogram | ||
| Any abnormality | 19 | 32 |
| Atrial fibrillation | 3 | 5 |
| ST-T wave abnormalities | 6 | 10 |
| Right bundle branch block | 8 | 13 |
| Left bundle branch block | 1 | 1.5 |
| Left ventricle strain | 1 | 1.5 |
| Medication | ||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 18 | 30 |
| Calcium channel blockers | 10 | 17 |
| Beta blockers | 10 | 17 |
| Diuretics | 11 | 18 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 (range) | 88 (15 to >150) | |
| Carfilzomib dose,* mg/m2 | ||
| 20/27 | 27 | 45 |
| 20/36 | 12 | 20 |
| 20/56 | 21 | 35 |
| Carfilzomib regimen | ||
| Kd | 31 | 52 |
| KRd | 17 | 28 |
| KMP | 12 | 20 |
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, y (range) | 72 (39-86) | |
| Sex | ||
| Male | 39 | 58 |
| Female | 21 | 42 |
| Median No. of prior treatments (range) | 2 (0-7) | |
| NDMM | 12 | 20 |
| RRMM | 48 | 80 |
| Prior high-dose therapy | 33 | 55 |
| Prior anthracyclines | 22 | 37 |
| Bortezomib | 39 | 65 |
| Immunomodulatory imide drugs | 40 | 67 |
| Cardiovascular risk factors | ||
| Smoking | 26 | 43 |
| Hypertension | 22 | 36 |
| Coronary artery disease | 5 | 8 |
| Peripheral artery disease | 13 | 22 |
| Diabetes mellitus | 10 | 16 |
| Hyperlipidemia | 12 | 20 |
| Stroke | 3 | 5 |
| Arrhythmias | 4 | 6 |
| Cardiovascular disease (coronary artery disease, peripheral artery disease, stroke) | 17 | 28 |
| LVEF, % (range) | 60 (40-70) | |
| Baseline electrocardiogram | ||
| Any abnormality | 19 | 32 |
| Atrial fibrillation | 3 | 5 |
| ST-T wave abnormalities | 6 | 10 |
| Right bundle branch block | 8 | 13 |
| Left bundle branch block | 1 | 1.5 |
| Left ventricle strain | 1 | 1.5 |
| Medication | ||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 18 | 30 |
| Calcium channel blockers | 10 | 17 |
| Beta blockers | 10 | 17 |
| Diuretics | 11 | 18 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 (range) | 88 (15 to >150) | |
| Carfilzomib dose,* mg/m2 | ||
| 20/27 | 27 | 45 |
| 20/36 | 12 | 20 |
| 20/56 | 21 | 35 |
| Carfilzomib regimen | ||
| Kd | 31 | 52 |
| KRd | 17 | 28 |
| KMP | 12 | 20 |
Kd, carfilzomib with dexamethasone; KMP, carfilzomib with melphalan and prednisone; KRd, carfilzomib with lenalidomide and dexamethasone; NDMM, newly diagnosed multiple myeloma; RRMM, relapsed or refractory multiple myeloma.
Carfilzomib dosing: 20 mg/m2 on first 2 infusions followed by infusions at doses of 27 mg/m2 (20/27), 36 mg/m2 (20/36), or 56 mg/m2 (20/56).
Patient characteristics and incidence of ejection fraction reduction ≥20% for different carfilzomib-based regimens
| Characteristic . | KRd (%)(n = 17) . | Kd (%)(n = 31) . | KMP (%)(n = 12) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Ejection fraction reduction ≤20% | 6 | 13 | 17 | |||
| Age >65 y | 82 | 68 | 100 | |||
| Sex | ||||||
| Male | 35 | 87 | 50 | |||
| Female | 65 | 13 | 50 | |||
| No. of prior treatments (range) | 2 (1-3) | 2 (1-7) | 0 | |||
| NDMM | 0 | 0 | 100 | |||
| RRMM | 100 | 100 | 0 | |||
| Prior high-dose therapy | 53 | 71 | 0 | |||
| Prior anthracyclines | 41 | 45 | 0 | |||
| Bortezomib | 65 | 84 | 0 | |||
| Immunomodulatory imide drugs | 77 | 87 | 0 | |||
| Cardiovascular risk factors | ||||||
| Smoking | 29 | 55 | 42 | |||
| Hypertension | 29 | 32 | 58 | |||
| Coronary artery disease | 6 | 6.5 | 8 | |||
| Peripheral artery disease | 29 | 23 | 33 | |||
| Diabetes mellitus | 18 | 23 | 25 | |||
| Hyperlipidemia | 13 | 12 | 50 | |||
| Stroke | 6 | 3 | 17 | |||
| Arrhythmias | 0 | 10 | 8 | |||
| Cardiovascular disease (coronary artery disease, peripheral artery disease, stroke) | 29 | 26 | 33 | |||
| Medication | ||||||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 29 | 19 | 58 | |||
| Calcium channel blockers | 12 | 13 | 33 | |||
| Beta blockers | 12 | 16 | 25 | |||
| Diuretics | 24 | 13 | 25 | |||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 91 | 91 | 58 | |||
| Cafilzomib dose,* mg/m2 | ||||||
| 20/27 | 100 | 32 | 0 | |||
| 20/36 | 0 | 0 | 100 | |||
| 20/56 | 0 | 68 | 0 | |||
| Characteristic . | KRd (%)(n = 17) . | Kd (%)(n = 31) . | KMP (%)(n = 12) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Ejection fraction reduction ≤20% | 6 | 13 | 17 | |||
| Age >65 y | 82 | 68 | 100 | |||
| Sex | ||||||
| Male | 35 | 87 | 50 | |||
| Female | 65 | 13 | 50 | |||
| No. of prior treatments (range) | 2 (1-3) | 2 (1-7) | 0 | |||
| NDMM | 0 | 0 | 100 | |||
| RRMM | 100 | 100 | 0 | |||
| Prior high-dose therapy | 53 | 71 | 0 | |||
| Prior anthracyclines | 41 | 45 | 0 | |||
| Bortezomib | 65 | 84 | 0 | |||
| Immunomodulatory imide drugs | 77 | 87 | 0 | |||
| Cardiovascular risk factors | ||||||
| Smoking | 29 | 55 | 42 | |||
| Hypertension | 29 | 32 | 58 | |||
| Coronary artery disease | 6 | 6.5 | 8 | |||
| Peripheral artery disease | 29 | 23 | 33 | |||
| Diabetes mellitus | 18 | 23 | 25 | |||
| Hyperlipidemia | 13 | 12 | 50 | |||
| Stroke | 6 | 3 | 17 | |||
| Arrhythmias | 0 | 10 | 8 | |||
| Cardiovascular disease (coronary artery disease, peripheral artery disease, stroke) | 29 | 26 | 33 | |||
| Medication | ||||||
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 29 | 19 | 58 | |||
| Calcium channel blockers | 12 | 13 | 33 | |||
| Beta blockers | 12 | 16 | 25 | |||
| Diuretics | 24 | 13 | 25 | |||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 91 | 91 | 58 | |||
| Cafilzomib dose,* mg/m2 | ||||||
| 20/27 | 100 | 32 | 0 | |||
| 20/36 | 0 | 0 | 100 | |||
| 20/56 | 0 | 68 | 0 | |||
Carfilzomib dosing: 20 mg/m2 on first 2 infusions followed by infusions at doses of 27 mg/m2 (20/27), 36 mg/m2 (20/36), or 56 mg/m2 (20/56).
Incidence of cardiac events in patients treated with carfilzomib. Cumulative incidence function estimates of cardiac events and discontinuation because of progressive disease or for other (nontoxicity) reasons.
Incidence of cardiac events in patients treated with carfilzomib. Cumulative incidence function estimates of cardiac events and discontinuation because of progressive disease or for other (nontoxicity) reasons.
The use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers was not associated with cardiac or renal events, although use of beta blockers was more common in patients who developed LVEF reduction (30% vs 8%; P = .048); however, 70% of those with known cardiovascular disease used such drugs. Baseline parameters that were assessed by echocardiography did not correlate with cardiac events. There was no association with prior bortezomib-based therapy, anthracycline exposure, high-dose therapy, or radiotherapy to thoracic spine.
All patients who experienced a cardiac event (n = 7) were evaluated further: 3 were diagnosed with CAD, 2 had angioplasty, and 1 had coronary artery bypass surgery. Thus, appropriate investigation for CAD should be performed in patients receiving carfilzomib who develop LVEF reduction. For all patients with cardiac events, carfilzomib was temporarily discontinued, supportive treatment was administered, and serial echocardiograms were performed. LVEF improved to baseline in all patients after a median of 60 days (range, 15-180 days). Only 1 of the patients who had an LVEF decrease of ≥20% required hospitalization for acute heart failure. Of the other 6 patients, 5 were hospitalized for scheduled investigations related to a cardiac event, such as coronary angiography or treatment of the diagnosed CAD with percutaneous angioplasty or coronary artery graft surgery. During the period of carfilzomib discontinuation, 1 patient experienced myeloma progression; in the other 6 patients, carfilzomib was reintroduced with dose reduction and longer infusion time5,10,11 ; the dose of dexamethasone remained the same. One patient had repeated reduction of LVEF after reintroduction of carfilzomib, which again returned to baseline after permanent discontinuation. Thus, similar to other reports,5,11-13 carfilzomib-related cardiotoxicity was reversible within a short period, and patients could be re-challenged with carfilzomib.
We also evaluated the effects of carfilzomib on renal functions, excluding renal dysfunction, which were associated with disease progression: 22 patients (37%) had grade ≥ 1 creatinine increase (grade 1, 18%; grade 2, 17%; grade 3, 2%), 17 (28%) had acute kidney injury grade ≥ 1 (grade 1, 25%; grade 3, 3%), and 21 (35%) had a reduction of estimated glomerular filtration rate (eGFR) ≥25%. Similar to previous reports,14 this decrease was transient in 13 (62%) of 21 patients and occurred within the first cycle of carfilzomib in 9 (43%) of 21 patients. Median time until return of eGFR to baseline was 15 days (range, 7-28 days). With higher doses of carfilzomib, eGFR reduction was more common: 22%, 33%, and 52% for doses of 27 mg/m2, 36 mg/m2, and 56 mg/m2, respectively (P = .093). Hydration amount has been correlated with the risk of renal insufficiency, and increased fluid intake has been suggested to be protective.14 No baseline factor, including age, diabetes, or medication (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, calcium channel blockers or diuretics) correlated with eGFR reduction. However, in 11 (55%) of 20 patients with baseline eGFR <60 mL/min, eGFR improved to >60 mL/min; thus, carfilzomib is a treatment option for patients with myeloma-related renal dysfunction.
Discussion
In our series of 60 consecutive patients with myeloma who were treated with carfilzomib-based regimens, we found that 12% developed clinically significant cardiac toxicity defined as a relative decrease of the LVEF by at least 20%. Carfilzomib-related cardiotoxicity may have some distinct features: LVEF reduction is modest and increased NT-proBNP invariably occurs, but serum troponins are usually normal, indicating lack of myocardial necrosis. Occurrence or timing of the cardiac toxicity related to carfilzomib is unpredictable and, as the prospective substudy of the ENDEAVOR trial showed, serial echocardiograms are of no practical use in predicting cardiac events.15 NT-proBNP levels are an important marker for the diagnosis of acute heart failure and monitoring CHF, including conditions such as amyloid light-chain amyloidosis. Serial measurement of NT-proBNP could be explored as a monitoring tool, but because its levels are affected by many factors, including hydration status, steroid use, and renal function, it may be difficult to predict development of clinically significant cardiac dysfunction only on the basis of modest increases in NT-proBNP.
In our analysis, we found no statistically significant differences in the incidence of cardiac events among patients who received different doses of carfilzomib or correlation with cumulative dose, but cardiotoxicity may be more frequent in patients with underlying cardiovascular disease. There may be many factors that influence toxicity such as peak drug levels and duration of exposure, differences in the degree the inhibition of proteasome function in the heart or endothelium, or differences in the distribution or metabolism of the drug. The mechanisms underlying carfilzomib cardiotoxicity have not been clearly recognized and are only speculative.11,13 Cardiomyocytes depend on increased proteasome activity16 to cope with the production of misfolded proteins that result from various forms of cardiac stress,17 and failure or insufficiency of proteasome activity can have detrimental effects on cellular function.18 Proteasome activity also affects, in a biphasic manner, the levels and activity of endothelial nitric acid synthase and nitric oxide levels.19 Loss of nitric oxide bioavailability leads to endothelial dysfunction which, in turn, is associated with impaired vasodilatation, oxidative and inflammatory stress, and thromogenicity20 and has been associated with incident hypertension, and cardiac and renal dysfunction.21-23 PIs may inhibit nuclear factor κB (NF-κB) signaling in some cells but may also induce NF-κB via the canonical pathway, depending on the cell type and the conditions.24 Depending on the environment, dose and dose duration, cell type, and organ studied, PIs can have diverse and broad effects and can act as poisons or remedies25 in several pathophysiologic processes, including vascular function and atherosclerosis.26-28 Under certain conditions, PIs may actually induce the activation of NF-κB which, in turn, promotes cardiovascular events.29 Irreversible proteasome inhibition and higher potency of carfilzomib1,2 may partially explain the higher frequency of cardiotoxicity with carfilzomib than with bortezomib.30,31 An analysis of bortezomib studies found no significant increase in the risk of cardiac complications, but the retrospective nature of this report did not allow vigorous evaluation of potential cardiac effects.31 Thus, there is still a question of whether cardiotoxicity is carfilzomib-related or a PI class effect.
Even with our data and published data from other investigators, it remains difficult to predict which patients will develop cardiac toxicity from carfilzomib. A prudent strategy would include a careful evaluation of cardiovascular risk factors before initiation of therapy. We believe that the presence of cardiovascular disease alone is not an exclusion criterion for carfilzomib therapy; however, patients should be observed carefully on clinical grounds, and targeted evaluation should be used if symptoms develop that suggest cardiac dysfunction. It is notable that toxicity was reversible and almost all of our patients could be re-challenged with carfilzomib. Although we did not find a clear correlation with the rate of infusion, on the basis of other reports,11,12,32 slower infusion rates may be safer in high-risk patients. Importantly, no dose effect was found in our patients, so dosing should follow study data regarding the approved combinations.
In our study, we also evaluated the effects of carfilzomib therapy in renal function after carefully excluding disease progression as the cause of renal dysfunction. As in previous reports,5 we found that transient and mild reduction of eGFR was common. However, in our analysis, we found that the doses of carfilzomib may be related to these effects. Importantly, carfilzomib therapy improved renal function in patients who presented with moderate renal dysfunction. Thus, carfilzomib therapy may be an option for patients with myeloma-related renal dysfunction. Whether common mechanisms may link the cardiac and renal effects of carfilzomib is also an intriguing hypothesis and should be further investigated.
In conclusion, 12% of our myeloma patients who were treated with carfilzomib had reversible LVEF reduction. Prior or underlying cardiovascular disease may be involved in this effect, at least in some patients. A transient eGFR reduction was common, but carfilzomib therapy improved renal function in patients with myeloma-related renal dysfunction. Further investigation is needed to elucidate the underlying mechanisms of toxicity and identify predictive markers of cardiovascular complications.
Authorship
Contribution: M.A.D., M.R., E.T., and E.K. collected and analyzed data and wrote the manuscript; and M.G., E.P., D.Z., E.E.P., D.F., M.M., N.K., I.P., A.N., E.P., K.S., E.M., C.P., and S.K. collected and analyzed data and reviewed the manuscript.
Conflict-of-interest disclosure: M.A.D. received honoraria from Celgene, Janssen, Takeda, and Amgen. E.T. received honoraria from Medtronic, Celgene, Janssen, and Amgen. E.K. received honoraria from Janssen, Takeda, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Meletios A. Dimopoulos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, 80 Vasilissis Sofias, 11258 Athens, Greece; e-mail: mdimop@med.uoa.gr.