TO THE EDITOR:
Hemophagocytic lymphohistiocytosis (HLH) is an aggressive, life-threatening syndrome of excessive immune activation. HLH clinically presents with fever, pancytopenia, splenomegaly, and hemophagocytosis in the bone marrow, lymph nodes, or liver. A proposed mechanism for HLH is a paradoxical downregulation of various aspects of the immune response, including B-cell development and function, Toll-like receptor expression and signaling, and apoptosis induction.1 HLH can be familial or secondary. Secondary HLH is usually associated with infections, rheumatologic disorders, or malignancies. Twenty-seven percent of secondary HLH is related to malignancy, the vast majority being hematologic malignancies (HMs), and associated with overall mortality >90%. Yu et al2 demonstrated that HLH could be an indicator for treatment resistance in patients with lymphoma, especially those of B-cell origin. Chemotherapy may not salvage these patients because of end-organ dysfunction at presentation.3 B-cell lymphoma–associated HLH may constitute a distinct biological and clinical disease entity that includes specific chromosomal abnormalities.4 The purpose of this study was to describe the genetic landscape of HM-associated HLH to identify potential targets for clinical trials.
We used the National Cancer Institute’s Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer to identify patients with HM-associated HLH. We identified 16 patients with B-cell lymphoma–associated HLH. All identified references were reviewed and patient data were extracted regarding clinical and pathologic details along with therapy and outcomes. Data were analyzed using Microsoft Excel and GraphPad Prism. All P values were 2-sided and results ≤.05 were considered significant.
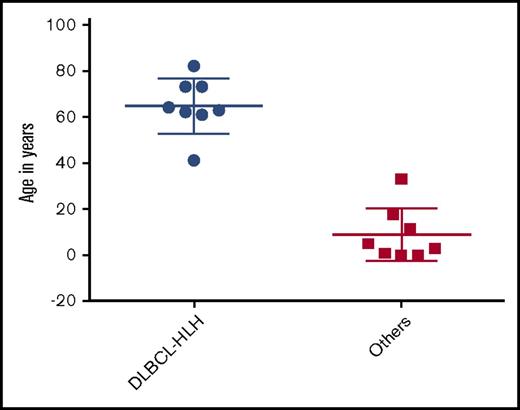
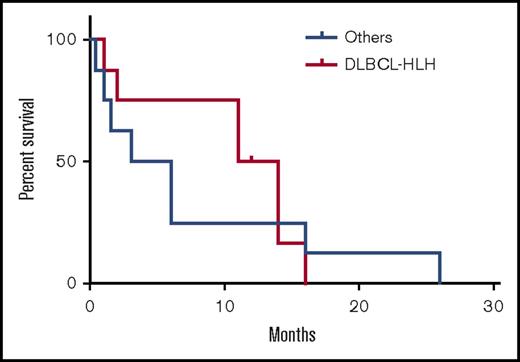
Of these 16 patients, 8 had diffuse large B-cell lymphoma (DLBCL)-associated HLH (DLBCL-HLH), 5 had EBV-associated B-cell lymphomas, and 3 had B-cell lymphomas not otherwise specified. Non–DLBCL-HLH was categorized as a single group and compared with DLBCL-HLH. The diagnosis of DLBCL and non-DLBCL was made based on histopathology; 8 of the 16 cases were CD20+. These cases were before 2001, which antedates the Choi update5 and the Hans algorithm.6 All cases were CD10−, and immunohistochemistry for BCL-6 and MUM-1 was not available. Patients with DLBCL-HLH were significantly older (mean, 64.9 years [range, 41-82]) than the non–DLBCL-HLH patients (8.9 years [0.3-33], P < .0001) (Figure 1). Seventy-five percent (6/8) of DLBCL-HLH patients were male, similar to 63% (5/8) of non–DLBCL-HLH patients. Median lactate dehydrogenase, ferritin, and interleukin-2R were 1117 U/L, 1073 ng/mL, and 13 323 pg/mL, respectively, in the DLBCL-HLH patients; these were not reported in the non–DLBCL-HLH patients. Last, 88% (7/8) of patients with DLBCL-HLH received rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone (R-CHOP), whereas 100% (8/8) of the non–DLBCL-HLH patients received etoposide and corticosteroids with or without other agents. Median overall survival was 12.5 months in DLBCL-HLH vs 4.5 months in non–DLBCL-HLH patients (log rank, P = .23) (Figure 2).
DLBCL-HLH patients were more likely to harbor unbalanced rearrangements (75% vs 13%; relative risk [RR] 3.9; 95% confidence interval [CI], 1.1-13.6; P = .04) and rearrangements in immune-related loci (9p24: PDL1/2 [2]; 19q13: interferon-λ [IFN-λ] 1,2,3 [2]; and 12p11: DDX11 [1]) vs none in the non–DLBCL-HLH patients (63% vs 0%; RR, 3.7; 95% CI, 1.4-9.6; P = .03). Both cases of 9p24 and the case of 12p11 were additions, and both cases of 19q13 were complex translocations with 3p23 der(19)t(3;19)(p23;q13). One patient each with DLBCL-HLH was rearranged at MYC and BCL2. None of the non–DLBCL-HLH had rearrangements in MYC, or BCL2. No patient in either group had a rearrangement of BCL6. Compared with DLBCL without associated HLH within Mitelman, the incidence of rearrangements and additions in the immune-related loci 9p24 (25% [2/8] vs 3% [37/1413]; P = .02), 19q13 (25% [2/8] vs 4% [61/1413]; P < .05), and 12p11 (13% [1/8] vs <1% [6/1413; P = .04) were significantly higher in DLBCL-HLH patients. Within the 61 cases of 19q13 abnormalities in DLBCL without HLH, there were no cases of der(19)t(3;19)(p23;q13).
Our data, which suggests correlation between DLBCL-HLH and rearrangements of genes associated with immune system evasion, encourages investigation of immunomodulatory treatment strategies such as PD-1–blocking drugs. Because 9p24 loci include PD-L1, significant recurrence of 9p24 rearrangements and additions found in DLBCL-HLH may result in high tumor expression of PD-L1 to activate its survival pathway. Roemer and colleagues showed that almost all patients with classical Hodgkin lymphoma (cHL) had 9p24.1 amplification. The patients with the highest expression of 9p24.1 were those with advanced-stage disease. These findings suggest that increased 9p24.1 amplification leads to more PD-L1 expression and therefore increased tumor evasion of the immune system and disease spread. This supports the finding that patients with cHL show great response to PD-1 blockade with drugs such as nivolumab.7
Moreover, rearrangements in 19q13, which includes IFN-λ, may represent downregulation of IFN-λ, favoring cancer activity. One role of IFN-λ is upregulation of major histocompatibility complex class I, which helps T cells monitor malignant cells.8 HLH phenomenon may be due to IFN-λ amplification resulting in killer T cells overidentifying tumor cells. Also, 19q13 locus has been shown to include Cbl gene. Cbl protein is an E3 ubiquitin protein ligase, which ultimately serves as a regulator of several proliferative signals including c-kit, macrophage colony-stimulating factor, and STAT5.9,10 A 19q13 rearrangement causing inhibition or amplification of IFN-λ and inhibition of Cbl gene function may result in HLH because of hyperresponsiveness causing proliferation and destruction by sensitized T cells. DDX11 on 12p11 loci has been shown to play a major role in stabilizing telomere length and therefore causing apoptosis when inhibited.11 Georgiou et al demonstrated the genetic basis of PD-L1 overexpression in DLBCL and suggests that treatments targeting the PD-1–PD-L1/PD-L2 axis might benefit DLBCL patients, especially those belonging to the more aggressive non–germinal center B-cell subtype, but further studies have been disappointing.12
Currently, R-CHOP is the standard-of-care first-line chemotherapy regimen for DLBCL-HLH. The survival benefit for DLBCL-HLH compared with non–DLBCL-HLH could be a result of better chemotherapy sensitivity in DLBCL as well as inclusion of rituximab as a treatment of DLBCL. Yu et al also demonstrated that patients with B-cell lymphoma–associated HLH showed a trend toward better survival compared with patients with T-cell lymphoma–associated HLH because of better chemotherapy and the addition of rituximab.3 Retrospective analysis of malignancy-associated HLH has shown that DLBCL-HLH had a poor response with both first- and second-line treatment regimens.13 With this, patients often received only a temporary therapy because their symptoms could not be controlled between chemotherapy cycles.14 To overcome this, early intensive chemotherapy was suggested.14 We believe our data may point to more targeted therapy.
Several phase 1/2 clinical trials, including other PD-1 inhibitors such as atezolizumab and durvalumab in combination with other drugs for treatment of non-Hodgkin lymphoma, are ongoing (JCAR014 and Durvalumab in Treating Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma [NCT02706405], A Safety and Pharmacology Study of Atezolizumab (MPDL3280A) Administered With Obinutuzumab in Patients With Relapsed/Refractory Follicular Lymphoma and Diffuse Large B-cell Lymphoma [NCT02220842], A Study of Obinutuzumab, Polatuzumab Vedotin, and Atezolizumab in Relapsed or Refractory Follicular Lymphoma (FL) or Diffuse Large B-Cell Lymphoma (DLBCL) [NCT02729896], and A Phase 1b Study of Atezolizumab in Combination With Either Obinutuzumab Plus Bendamustine or Obinutuzumab Plus CHOP in Patients With Follicular Lymphoma or Diffuse Large B-Cell Lymphoma [NCT02596971]). A study done in 2015 showed that a PD-1 blockade using nivolumab has low activity in unselected patients.15 Targeting patients with specific genetic alterations is necessary to avoid unnecessary toxicity of chemotherapy and to maximize benefit of PD-1 inhibitors.7,16
In conclusion, DLBCL-HLH showed more frequent rearrangements of immune-related loci in contrast to non–DLBCL-HLH and DLBCL not associated with HLH. Novel strategies evaluating immunomodulatory agents, such as PD-1 inhibitors to more specifically target these genes, need to be investigated.
This study’s primary limitation is a small sample size of hematologic malignancy–associated HLH patients based on Mitelman database. HLH is a rare condition with variable, nonspecific presentation, which makes the diagnosis challenging. Underrecognition of HLH in nonteaching and rural hospitals also may play a role in lacking patient data.17
Authorship
Contribution: S.S.L., K.P., P.V., N.R.V., and M.G.M. were involved in the conceptual design and analysis planning of the project; S.S.L., K.P., P.V., N.R.V., and M.G.M. were involved in acquisition of the data; M.G.M. performed the statistical analysis of the data; S.S.L., P.V., and K.P. drafted the manuscript; S.S.L., K.P., P.P., and M.G.M. were involved in critical revision of the manuscript; and M.G.M. supervised all aspects of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kruti Patel, University of Tennessee Health and Science Center, H314 Coleman College of Medicine Building, 956 Court Ave, Memphis, TN 38163; e-mail: krutip03@gmail.com.
References
Author notes
K.P. and S.S.L. contributed equally to this study.