Key Points
Race, income, and partnered status were associated with anticoagulant use but clinical factors had a stronger association.
Abstract
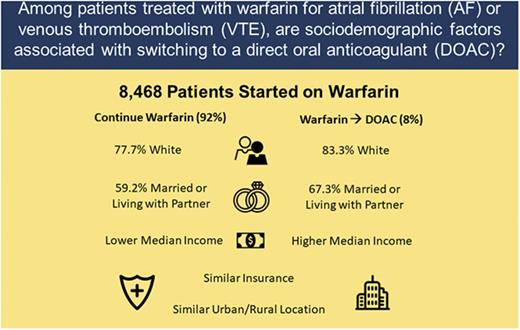
Clinical factors and patient preferences are important for selecting oral anticoagulants for venous thromboembolism (VTE) and atrial fibrillation (AF). The relative association of sociodemographic factors with anticoagulant use is unknown. We evaluated a prospective cohort to compare sociodemographic variables in patients who continued on warfarin for AF or VTE to those who transitioned to 1 of the direct oral anticoagulants (DOACs). Adult patients, newly started on warfarin, were enrolled through 6 anticoagulation clinics across Michigan. Of 8468 patients, 53.3% had AF, 45.6% had VTE, and 1.1% had both. Of these, 696 (8.2%) switched from warfarin to a DOAC. There were no significant differences between switchers and nonswitchers for percentage of time with a therapeutic international normalized ratio on warfarin, urban-rural residence status, or health insurance. Switchers were more often white (83.3% vs 77.7%; P < .001), partnered (67.3% vs 59.2%; P < .001), or resided in a zip code with a higher median household income (P < .001). The results show that sociodemographic factors, such as race, partnered status, and income are associated with a patient’s likelihood of switching to a DOAC vs remaining on warfarin therapy. Although clinical factors predominate, the reason for, and impact of, these observed variations in care requires further investigation.
Introduction
The regulatory approval and growing clinical embrace of the direct oral anticoagulants (DOACs: apixaban, dabigatran, edoxaban, or rivaroxaban), has challenged warfarin as the mainstay of oral anticoagulation for the treatment of venous thromboembolism (VTE) and stroke prevention in nonvalvular atrial fibrillation (AF). Although clinical factors, including renal function or comorbidities, are associated with warfarin vs DOAC use, it is unknown how socioeconomic variables such as income, race, sex, health insurance, or partnered status may influence anticoagulant use.
A history of labile international normalized ratio (INR) tests among otherwise compliant patients is hypothesized to affect anticoagulant use, favoring the DOACs, which do not require routine monitoring or have as many drug or dietary interactions.1 The quality of INR control on warfarin can be reflected by the percentage of time in the therapeutic range (TTR), a measure associated with morbidity and mortality.2 The influence of TTR on choice of anticoagulant use has not been well studied. However, studies in AF have shown that socially disadvantaged patients spend less TTR2 and are at increased risk of bleeding from warfarin.3
We sought to investigate the association between clinical factors, TTR, and sociodemographic variables with anticoagulant use for patients with AF and/or VTE. We hypothesized that socioeconomically advantaged patients (as reflected by insurance status, income, race, and partnered status) would be more likely to transition from warfarin to a DOAC. Furthermore, we predicted that patients from rural areas would be more likely to switch given that monitoring is less accessible. We also expected that clinical factors, such as renal or hepatic dysfunction, extreme obesity (body mass index [BMI] > 40),4 a low TTR, or recent bleeding would be associated with continued warfarin use.
Methods
The study is a retrospective analysis of a prospectively collected data set. The study included all consecutive, adult patients newly initiated on warfarin anticoagulation for nonvalvular AF or VTE recruited through Blue Cross Blue Shield of Michigan’s Anticoagulation Quality Improvement Initiative over the study period. This collaborative of 6 outpatient anticoagulation clinics throughout the state of Michigan represents both academic and community practices that enroll patients with all forms of health insurance.5 Institutional review board approval was obtained through the University of Michigan, along with an institutional review board–approved waiver of consent. Patients were enrolled and followed prospectively from January 2009 to July 2016. The “warfarin” group consisted of patients treated exclusively with warfarin; those who transitioned to a DOAC comprised the “DOAC switch” group.
Data were abstracted from enrollment through the end of the study period or anticoagulant discontinuation by trained data abstractors with random audits to ensure the accuracy of the data. Collected data included demographics, comorbid conditions, medications, stroke and VTE risk factors, creatinine clearance, INR values, and episodes of hemorrhage, thrombosis, or blood transfusion. TTR was calculated using Rosendaal linear interpolation.6 A modified Charlson Comorbidity Index7 and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score8 were calculated for each patient. The primary outcome was anticoagulant status (continue on warfarin vs switch to DOAC). Independent variables included race, partnered status, health insurance, income, and household degree of urbanization. Income was derived from the 2014 US Census Bureau’s American Community Survey9 based on the median income of the patient’s zip code of residence; the 2010 rural-urban commuting area codes (RUCAs) were used to determine the degree of urbanization corresponding to the patient’s zip code of residence.10
The Student t test and Wilcoxon rank-sum test were used for continuous variables, and χ2 and Fisher exact tests were used for categorical variables. Univariate analyses were performed for each of the factors felt to a priori potentially influence anticoagulant selection. We then performed multivariable logistic regression and reduced the model with only significant variables included. All authors had access to the primary study data.
Results
A total of 8468 patients met the study inclusion criteria; 53.3% were anticoagulated for stroke prevention with nonvalvular AF, 45.6% for VTE, and 1.1% for both. Over the study period, 8.2% switched from warfarin to a DOAC. Given that the DOACs were not approved until 2010, no patients switched in 2009 and a trivial number switched in 2010. From 2011 to mid-2016, the rate of switching averaged 4% (range, 2.2%-5.4%) without a clinically significant difference by year. The demographics of the warfarin group were similar to the DOAC switch group with respect to age, sex, and weight (Table 1). The modified Charlson Comorbidity Index, HAS-BLED scores, and percentage TTR on warfarin were also comparable between the 2 groups.
Clinical characteristics of patients remaining on warfarin compared with those switching to a DOAC
| . | Warfarin, N = 7772 . | DOAC switch, N = 696 . | P . |
|---|---|---|---|
| Age, mean (SD), y | 66 (16) | 67 (14) | .24 |
| Male sex, N (%) | 4003 (51) | 378 (54) | .15 |
| Weight, <50 kg, N (%) | 218 (3) | 14 (2) | .24 |
| Weight, >120 kg, N (%) | 785 (10) | 74 (11) | .68 |
| BMI >40, N (%) | 816 (11) | 74 (11) | .98 |
| Length of treatment, mean (SD), d | 470 (589) | 406 (495) | <.001 |
| Current tobacco user, N (%) | 616 (8) | 51 (7) | .57 |
| Primary indication, N (%) | |||
| DVT/PE | 3683 (47) | 179 (26) | <.001 |
| AF/flutter | 4002 (52) | 510 (73) | <.001 |
| Both | 87 (1) | 7 (1) | .78 |
| TTR, % of time (SD) | 57 (0.2) | 56 (0.2) | .89 |
| Comorbidities, N (%) | |||
| Diabetes mellitus | 1932 (25) | 153 (22) | .09 |
| Coronary artery disease | 1975 (25) | 205 (30) | .02 |
| Recent myocardial infarction, ≤6 mo | 248 (3) | 23 (3) | .87 |
| Remote myocardial infarction, >6 mo | 621 (8) | 49 (7) | .37 |
| Hypertension | 5026 (65) | 508 (73) | <.001 |
| Congestive heart failure | 1357 (18) | 136 (20) | .17 |
| Hypercoagulable state | 202 (3) | 25 (4) | .14 |
| Liver disease | 148 (2) | 15 (2) | .64 |
| Chronic kidney disease | 1009 (13) | 45 (7) | <.001 |
| Cancer | 1630 (21) | 129 (19) | .13 |
| Prior stroke or transient ischemic attack | 850 (11) | 91 (13) | .09 |
| Prior gastrointestinal bleed | 356 (5) | 22 (3) | .08 |
| Prior DVT/PE | 1197 (15) | 85 (12) | .02 |
| Any recent bleeding, ≤30 d | 150 (2) | 17 (2) | .35 |
| Any remote bleeding, >30 d | 166 (2) | 7 (1) | .04 |
| Bleeding diathesis | 54 (1) | 3 (0) | .63 |
| History of embolism, not DVT/PE | 50 (1) | 6 (1) | .46 |
| Heavy alcohol or drug use | 371 (5) | 46 (7) | .03 |
| Former tobacco use | 2281 (29) | 239 (34) | .006 |
| Falls | 223 (3) | 13 (2) | .12 |
| Seizure disorder | 101 (1) | 12 (2) | .35 |
| Peripheral arterial disease | 414 (5) | 37 (5) | .99 |
| Chemotherapy | 174 (2) | 18 (3) | .56 |
| HAS-BLED, score (SD) | 2.4 (1.4) | 2.5 (1.3) | .6 |
| Modified Charlson Comorbidity Index, score (SD) | 4.3 (2.2) | 4.3 (1) | .33 |
| Antiplatelet medications, N (%) | |||
| Aspirin (any dose) | 3093 (40) | 311 (45) | .01 |
| NSAIDs | 327 (4) | 35 (5) | .3 |
| Clopidogrel, ticlopidine | 411 (5) | 47 (7) | .1 |
| Prasugrel, ticagrelor or other antiplatelet | 25 (0.3) | 2 (0.3) | .88 |
| One antiplatelet agent | 2927 (38) | 294 (42) | .02 |
| >1 antiplatelet agent | 307 (4) | 34 (5) | .23 |
| . | Warfarin, N = 7772 . | DOAC switch, N = 696 . | P . |
|---|---|---|---|
| Age, mean (SD), y | 66 (16) | 67 (14) | .24 |
| Male sex, N (%) | 4003 (51) | 378 (54) | .15 |
| Weight, <50 kg, N (%) | 218 (3) | 14 (2) | .24 |
| Weight, >120 kg, N (%) | 785 (10) | 74 (11) | .68 |
| BMI >40, N (%) | 816 (11) | 74 (11) | .98 |
| Length of treatment, mean (SD), d | 470 (589) | 406 (495) | <.001 |
| Current tobacco user, N (%) | 616 (8) | 51 (7) | .57 |
| Primary indication, N (%) | |||
| DVT/PE | 3683 (47) | 179 (26) | <.001 |
| AF/flutter | 4002 (52) | 510 (73) | <.001 |
| Both | 87 (1) | 7 (1) | .78 |
| TTR, % of time (SD) | 57 (0.2) | 56 (0.2) | .89 |
| Comorbidities, N (%) | |||
| Diabetes mellitus | 1932 (25) | 153 (22) | .09 |
| Coronary artery disease | 1975 (25) | 205 (30) | .02 |
| Recent myocardial infarction, ≤6 mo | 248 (3) | 23 (3) | .87 |
| Remote myocardial infarction, >6 mo | 621 (8) | 49 (7) | .37 |
| Hypertension | 5026 (65) | 508 (73) | <.001 |
| Congestive heart failure | 1357 (18) | 136 (20) | .17 |
| Hypercoagulable state | 202 (3) | 25 (4) | .14 |
| Liver disease | 148 (2) | 15 (2) | .64 |
| Chronic kidney disease | 1009 (13) | 45 (7) | <.001 |
| Cancer | 1630 (21) | 129 (19) | .13 |
| Prior stroke or transient ischemic attack | 850 (11) | 91 (13) | .09 |
| Prior gastrointestinal bleed | 356 (5) | 22 (3) | .08 |
| Prior DVT/PE | 1197 (15) | 85 (12) | .02 |
| Any recent bleeding, ≤30 d | 150 (2) | 17 (2) | .35 |
| Any remote bleeding, >30 d | 166 (2) | 7 (1) | .04 |
| Bleeding diathesis | 54 (1) | 3 (0) | .63 |
| History of embolism, not DVT/PE | 50 (1) | 6 (1) | .46 |
| Heavy alcohol or drug use | 371 (5) | 46 (7) | .03 |
| Former tobacco use | 2281 (29) | 239 (34) | .006 |
| Falls | 223 (3) | 13 (2) | .12 |
| Seizure disorder | 101 (1) | 12 (2) | .35 |
| Peripheral arterial disease | 414 (5) | 37 (5) | .99 |
| Chemotherapy | 174 (2) | 18 (3) | .56 |
| HAS-BLED, score (SD) | 2.4 (1.4) | 2.5 (1.3) | .6 |
| Modified Charlson Comorbidity Index, score (SD) | 4.3 (2.2) | 4.3 (1) | .33 |
| Antiplatelet medications, N (%) | |||
| Aspirin (any dose) | 3093 (40) | 311 (45) | .01 |
| NSAIDs | 327 (4) | 35 (5) | .3 |
| Clopidogrel, ticlopidine | 411 (5) | 47 (7) | .1 |
| Prasugrel, ticagrelor or other antiplatelet | 25 (0.3) | 2 (0.3) | .88 |
| One antiplatelet agent | 2927 (38) | 294 (42) | .02 |
| >1 antiplatelet agent | 307 (4) | 34 (5) | .23 |
DVT, deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation.
The DOAC switch group had a greater representation of patients with AF (73.3% vs 51.5%, P < .001; Table 1). Accordingly, this group had more patients with AF risk factors, such as coronary artery disease, hypertension, prior tobacco use, or heavy alcohol use. This group was more likely to be on antiplatelet therapy. The warfarin group was more likely to have a history of bleeding, a history of VTE, or chronic kidney disease. A greater proportion of switchers had experienced a new thromboembolic event (3% vs 0.8%, P < .001) while followed on warfarin. This was true among those anticoagulated for both VTE (8.9% of switchers vs 1.1% of patients remaining on warfarin) and AF (1% of switchers vs 4% of patients remaining on warfarin), however, it was only significantly increased for VTE (P < .001).
With regard to sociodemographic variables (Table 2), patients in the DOAC switch group were more often white (83.3% vs 77.7%, P < .001), married/living with a partner (67.3% vs 59.2%, P < .001), and more often resided in a zip code with a higher median household income, compared with patients remaining on warfarin. The degree of urbanization as reflected by RUCA codes revealed no significant differences between the groups, but over 90% of the study population lived in a metropolitan area.
Comparison of socioeconomic variables between patients remaining on warfarin and those transitioning to a DOAC
| . | Warfarin, N = 7772 . | DOAC switch, N = 696 . | P . |
|---|---|---|---|
| Median income, USD | |||
| First quartile (lowest) | $34 757 | $36 404 | <.001 |
| Second quartile | $51 265 | $51 443 | |
| Third quartile | $65 338 | $65 626 | |
| Fourth quartile (highest) | $90 609 | $93 152 | |
| Partnered status, N (%) | |||
| Married/living with partner | 4562 (59) | 465 (67) | <.001 |
| Race, N (%) | |||
| White | 5986 (78) | 574 (83) | <.001 |
| Black | 1136 (15) | 47 (7) | |
| Other | 585 (8) | 68 (10) | |
| Insurance, N (%) | |||
| Medicare | 3973 (52) | 388 (57) | .08 |
| Commercial | 3345 (44) | 275 (40) | |
| Medicaid | 237 (3) | 20 (3) | |
| Uninsured | 64 (1) | 2 (0) | |
| RUCA code,*N (%) | |||
| Metropolitan area: 1 | 6383 (82) | 571 (82) | .78 |
| Metropolitan area: 2-3 | 884 (11) | 75 (11) | |
| Micropolitan area: 4-6 | 235 (3) | 20 (3) | |
| Small town: 7-9 | 112 (1) | 12 (2) | |
| Rural area: 10 | 155 (2) | 17 (2) | |
| Other: 11 | 21 (0) | 1 (0) |
| . | Warfarin, N = 7772 . | DOAC switch, N = 696 . | P . |
|---|---|---|---|
| Median income, USD | |||
| First quartile (lowest) | $34 757 | $36 404 | <.001 |
| Second quartile | $51 265 | $51 443 | |
| Third quartile | $65 338 | $65 626 | |
| Fourth quartile (highest) | $90 609 | $93 152 | |
| Partnered status, N (%) | |||
| Married/living with partner | 4562 (59) | 465 (67) | <.001 |
| Race, N (%) | |||
| White | 5986 (78) | 574 (83) | <.001 |
| Black | 1136 (15) | 47 (7) | |
| Other | 585 (8) | 68 (10) | |
| Insurance, N (%) | |||
| Medicare | 3973 (52) | 388 (57) | .08 |
| Commercial | 3345 (44) | 275 (40) | |
| Medicaid | 237 (3) | 20 (3) | |
| Uninsured | 64 (1) | 2 (0) | |
| RUCA code,*N (%) | |||
| Metropolitan area: 1 | 6383 (82) | 571 (82) | .78 |
| Metropolitan area: 2-3 | 884 (11) | 75 (11) | |
| Micropolitan area: 4-6 | 235 (3) | 20 (3) | |
| Small town: 7-9 | 112 (1) | 12 (2) | |
| Rural area: 10 | 155 (2) | 17 (2) | |
| Other: 11 | 21 (0) | 1 (0) |
RUCA, rural-urban commuting area code; USD, US dollar.
Per the United States Department of Agriculture Economic Research Service.10
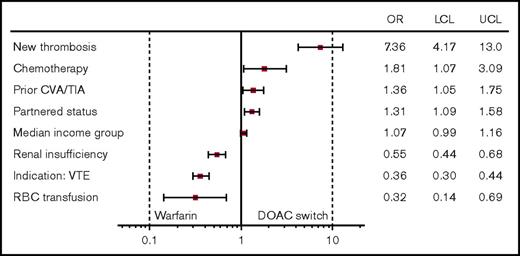
In multivariable logistic regression analysis, a new thromboembolic event while on warfarin was the strongest predictor of DOAC switching status (odds ratio [OR], 7.03; 95% confidence interval [CI], 3.99-12.37). Patients with higher predicted income showed a nonsignificant increase in the rate of switching to a DOAC (OR, 1.07; 95% CI, 0.99-1.16), whereas partnered status remained significant (OR, 1.31; 95% CI, 1.09-1.58) when adjusting for other factors. Renal insufficiency, having received a blood transfusion, and VTE as the indication for anticoagulation were all associated with remaining on warfarin (Figure 1).
Patient factors associated with warfarin to DOAC switch: OR and 95% CI. CVA, cerebrovascular accident; LCL, lower control limit; RBC, red blood cell; TIA, transient ischemic attack; UCL, upper control limit.
Patient factors associated with warfarin to DOAC switch: OR and 95% CI. CVA, cerebrovascular accident; LCL, lower control limit; RBC, red blood cell; TIA, transient ischemic attack; UCL, upper control limit.
Discussion
Our study suggests that sociodemographic factors are associated with which patients transition from warfarin to DOACs. However, clinical factors demonstrated a much stronger association. Such sociodemographic variation raises concerns that patients who are vulnerable, whether by virtue of race, income, or unpartnered status, may not have equitable access to the DOACs. Furthermore, patients who belong to >1 of these groups may face even greater barriers to the DOACs; further attention to these findings is warranted. Warfarin management, as reflected by the TTR, was similar between the 2 groups, suggesting it is not a common impetus to change anticoagulants.
Socially disadvantaged patients may have more difficulty with INR monitoring for warfarin due to barriers of cost or transportation; they otherwise may be lacking in necessary social support or agency.11-13 Therefore, it could be anticipated that such patients could benefit from transitioning to 1 of the DOACs. However, the direct costs of obtaining the DOACs pose a barrier for many of these patients. Indeed, a large Canadian study suggested that higher-socioeconomic-status patients with AF were more likely to switch to dabigatran through private means, until the drug was publicly available, and this disparity resolved.14 Patients who identified themselves as married or living with a partner were more likely to transition to a DOAC. Although there could be social reasons to explain this finding, it is likely also a reflection of income.15 Partnered status was a question asked directly in this study whereas income was approximated, which likely explains our findings. Interestingly, health insurance type was not associated with who transitioned to a DOAC. In our clinical experience, many of our insured patients have comparable copays with the DOACs and warfarin; uninsured patients are often able to access DOACs through prescription assistance programs. Therefore, providers should be cautious not to assume that insurance status precludes DOAC use for an appropriately selected patient without fully exploring this option.
Although informative on how sociodemographic factors could relate to anticoagulant use, the data are intriguing on how DOACs are being used clinically for AF and VTE. For example, recent guidance advocates not using DOACs for VTE in patients with a BMI > 40 kg m−2 given the paucity of data in this population.4 Despite this, 11% of switchers had BMIs in this range, similar to the number observed in the warfarin group. Patients with chronic kidney disease more commonly stayed on warfarin (13.0% vs 6.5%; P < .001). This is likely appropriate due to the pharmacokinetics of DOACs and the limited data available for DOACs in this population. Although our analysis did not assess DOAC use by the degree of renal impairment, it does show that the drugs are being used for this group. Both groups had ∼2% of patients identified as having chronic liver disease. This is again a population where there are limited data to support the use of the DOACs, and caution is necessary. Further research is needed to better understand both safety and efficacy outcomes for these special populations being treated with DOACs.
We observed that patients with AF were more likely to switch to a DOAC than patients with VTE. This could be related to the difference in anticoagulation duration between the 2 groups. Patients with AF will often be on long-term anticoagulation and thus may be more inclined to change therapies. Our study included patients on short-term anticoagulation for VTE, who may be less likely to change anticoagulants once started on warfarin. Another contributor may be that patients with AF are often being managed by specialists compared with VTE, which may be managed by primary care providers. Specialists may have different prescribing patterns compared with general practitioners. It is also noteworthy that DOACs were approved for stroke prevention for AF starting in 2010, years prior to their respective approvals for the treatment and prevention of VTE starting in 2012. Patients with AF and their providers thus had more time to consider switching anticoagulants, potentially contributing to the increased proportion of AF patients who switched anticoagulants in our study.
Patients remaining on warfarin were more likely to have a history of bleeding (2.1% vs 1%; P = .04). This is likely related to the potential to reverse warfarin-based anticoagulant effects with clotting factors, vitamin K, or plasma. As antidotes to DOACs become more widely available, it is possible that this difference in practice patterns will change. The warfarin group also had a slightly higher percentage of patients with a history of VTE (15.4% vs 12.2%; P = .02). The reason for this observation is not clear but could reflect the time frame of DOAC approval for VTE relative to the study, a patient preference for an agent they may have been treated with in the past successfully, or a clinician desire for an agent that can be monitored, especially if there were concerns for noncompliance.
Strengths of the study include the large size and prospective follow-up. Limitations of this study include limited geographical area, with a mostly metropolitan (>90%) population. Our measure of income was approximated based on zip code of residence, and there was a low number of uninsured or Medicaid patients. The study may have insufficient statistical power to determine differences in anticoagulant use for these insurance statuses and based on degree of urbanization. The study did not include patients started directly on DOACs, and there could be selection bias among those patients started on warfarin. However, many patients were enrolled prior to DOACs becoming commercially available. All patients were also followed at anticoagulation centers, where referral patterns or management practices could affect the results. Additionally, anticoagulation clinics may favor warfarin relative to DOACs, given that DOACs may not require the services they provide.
In conclusion, sociodemographic variables may influence which patients transition from warfarin to a DOAC for AF or VTE, but clinical factors predominate. Further research is needed to understand the reason for these variations in care.
Authorship
Contribution: G.D.B., J.K.S., and S.L.S. were the principal authors and designed the study; X.G., X.K., G.D.B., J.K.S., and S.L.S. conducted the statistical analysis; and all authors assisted in data acquisition, analysis, and interpretation, and reviewed the manuscript.
Conflict-of-interest disclosure: E.K.-R. reports serving on the board of Anticoagulation Forum and consulting for Janssen and American College of Physicians. S.A. reports grant support from Boston Scientific Watchman and the Abbott Absorb Trial, consulting for Kona, Trice Orthopedics, and Micardia, and ownership in Biostar Ventures and Ablative Solutions. S.K. reports receiving speaker’s fees from Janssen, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, CSL Behring, and Daiichi Sankyo, and has been a consultant for Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Janssen, Daiichi Sankyo, Portola, and Roche. J.B.F. reports consulting for Merck, Janssen, and Novartis, grant support from Blue Cross Blue Shield of Michigan and the Fibromuscular Disease Society of America, and serving on the Advisory Committees of Boehringer Ingelheim and Pfizer. G.D.B. reports consulting for Aralez and grant support from Bristol-Myers Squibb/Pfizer and Blue Cross Blue Shield of Michigan. The remaining authors declare no competing financial interests.
Correspondence: Geoffrey D. Barnes, University of Michigan Frankel Cardiovascular Center, 2800 Plymouth Rd, B14 G101, Ann Arbor, MI 48109; e-mail: gbarnes@med.umich.edu.