Key Points
The optimal cutoff for soluble interleukin-2 receptor in this study was 2515 U/mL (sensitivity, 100%; specificity, 72.5%).
sIL-2r ≤2400 U/mL is helpful for ruling out HLH (sensitivity, 100%) and >10 000 U/mL is helpful for ruling it in (specificity, 93%).
Abstract
Serum soluble interleukin-2 receptor (sIL-2r) is an important disease marker in hemophagocytic lymphohistiocytosis (HLH), but there are no published data on its diagnostic value in adults. We conducted a single-center retrospective study of 78 consecutive adults who had sIL-2r measured for suspected HLH. Serum sIL-2r levels were measured by enzyme-linked immunosorbent assay (adult reference range, 241-846 U/mL). There were 38 patients with HLH and 40 with a non-HLH diagnosis (such as sepsis, liver disease, histiocyte disorders, autoimmune disease, leukemia, or lymphoma). The receiver operating characteristic curve demonstrated that sIL-2r is a good to excellent diagnostic test for adult HLH, with an area under the curve (AUC) of 0.90 (95% confidence interval, 0.83-0.97) compared with AUC 0.78 (95% confidence interval, 0.67-0.88) for ferritin. The optimal threshold for sIL-2r was 2515 U/mL (sensitivity, 100%; specificity, 72.5%). Although there was a large indeterminate range for sIL-2r, a level of 2400 U/mL or less was helpful for ruling out HLH (sensitivity, 100%), and more than 10 000 U/mL was helpful for ruling in HLH (specificity, 93%). Higher mean sIL-2r levels were seen in malignancy-associated HLH (20 241 U/mL) compared with infection-associated HLH and macrophage activation syndrome (9720 and 5008 U/mL, respectively; P < .05). Levels above 10 000 U/mL were not associated with worse prognosis in patients with HLH. Serum sIL-2r is a sensitive test for diagnosis of adult HLH, but is not as specific as previously reported in children. Additional studies enriched with patients without HLH who have conditions associated with T-cell activation, such as lymphoma and autoimmune lymphoproliferative syndrome, are needed.
Introduction
Hemophagocytic syndromes (HPS), including hemophagocytic lymphohistiocytosis (HLH), are life-threatening conditions caused by pathological overactivation of the immune system resulting in hypercytokinemia and multiorgan failure.1 HLH is difficult to diagnose, as it can mimic sepsis, liver failure, autoimmune disease, malignancy, and other inflammatory/immune-activating conditions.2 The HLH-2004 criteria were developed for pediatric HLH but are commonly used to diagnose adult HLH as well.3 These criteria are composed of 6 easily measured clinical and laboratory parameters (fever, splenomegaly, cytopenias, hyperferritinemia, hypertriglyceridemia/hypofibrinogenemia, and hemophagocytosis), as well as 3 more specialized tests (genetic testing, NK cell function, and serum soluble interleukin-2 [sIL-2r]). The 6 easily measured criteria are each sensitive for pathologic immune activation in adults, but very nonspecific in and of themselves. For example, extreme hyperferritinemia has traditionally been thought to be quite specific for HLH,4 but recent studies have shown that ferritin levels of 3000 µg/L or higher,5 and even of 50 000 µg/L or higher,6 are not predictive for adult HLH. The most common conditions associated with extreme hyperferritinemia in these studies included liver disease, renal failure, and transfusional iron overload, whereas HPS/HLH made up only 7% (6/83)5 and 20% (22/111)6 of the respective cohorts. Taken as a whole, the HLH-2004 criteria are thought to be specific for HLH in children. However, in adults, the 3 specialized tests (gene mutations, NK cell function, and sIL-2r) are rarely performed.7
Among these 3 tests, sIL-2r is the most easily implemented in most adult centers. Primary HLH is highly penetrant at a young age, so genetic testing in adults is relatively low yield, very costly, and rarely alters management. NK cell function may be decreased in both primary and secondary HLH, but testing for it requires radioactive reagents and/or specialized flow cytometry techniques that are difficult to implement outside of specialized reference centers.8 In contrast, serum sIL-2r level, also known as soluble CD25 (sCD25), is a test based on a simple, low-cost, commercially available assay, and can be performed on a frozen specimen. The interleukin-2 receptor is a heterotrimeric transmembrane protein that is upregulated on activated T cells, and high sIL-2r levels are found in hemophagocytic syndromes, lymphoma, autoimmune lymphoproliferative syndrome, and other conditions associated with T-cell activation.9 Although HLH is frequently referred to as “cytokine storm,” sIL-2r is in fact the only cytokine or cytokine receptor included in diagnostic criteria for HPS/HLH, and is elevated in all forms on HLH, whether primary or secondary.10 However, there are no published data on its performance characteristics in adults and very limited data in children. In a recent systematic scoping review examining the use of sIL-2r in HLH, sensitivity ranged from 88% to 93% in children.10 Specificity of 100% has been cited in prominent reviews,8,11 but is based on 1 pediatric dataset of 152 children younger than 8 years, on which the HLH-2004 sIL-2r criterion of sIL-2r 2400 U/mL or higher is based. However, specificity is likely overestimated, as this original data set did not include subjects with conditions such as lymphoma or autoimmune lymphoproliferative syndrome.10 Higher levels of sIL-2r in the range of more than 10 000 U/mL have been correlated with poor clinical outcomes in HLH, and numerous reviews and commentaries have reported that sIL-2r levels correlate with disease activity, but primary data, particularly in adults, are lacking. We therefore conducted a retrospective study to examine the diagnostic and prognostic value of sIL-2r in adult HLH.
Methods
Institutional review board approval was obtained. We retrospectively reviewed consecutive adult patients (≥18 years) who had sIL-2r measured for suspected hemophagocytic syndromes or histiocyte disorders at Vancouver General Hospital from March 2012 to April 2017. Diagnosis of HLH was based on HLH-2004 diagnostic criteria as well as expert clinical judgement. Patients were diagnosed with HLH if they met HLH-2004 diagnostic criteria and if it was determined that pathologic immune activation was the most likely cause of their clinical problems. For example, some patients may have been labeled as “non-HLH” even if they had 5 of 8 HLH-2004 criteria if it was determined that an alternative diagnosis, such as liver disease or sepsis, was more likely to be the cause of their findings (supplemental Table 1). Genetic testing and NK cell function are not available in our center and were sent to Cincinnati Children’s Hospital Medical Center at the discretion of the treating physician. Genetic testing was sent in patients being considered for allogeneic stem cell transplant, and NK cell function was sent if the treating physician felt it would influence diagnosis or management. Most of the patients included in this study were assessed with 7 of the 8 HLH-2004 nonmolecular/genetic criteria (fever, splenomegaly, cytopenias, hypertriglyceridemia/hypofibrinogenemia, hemophagocytosis in tissue, hyperferritinemia, serum sIL-2r). Patients with HLH were treated with an etoposide and dexamethasone-based protocol modified from the Histiocyte Society HLH-2004 protocol, or in some cases comfort care. No patients underwent stem cell transplant.
Serum sIL-2r levels were measured by an enzyme-linked immunosorbent assay (Siemens IMMULITE Immunoassay platform; adult reference range, 241-846 U/mL).12
Discrete variables were compared using χ-squared and Fisher’s exact test. Two continuous variables were compared with the Wilcoxon rank-sum test, and more than 2 variables were compared using the Kruskal-Wallis test with PASW Statistics 18. The sIL-2r and ferritin receiver operating characteristic (ROC) curves were produced with PASW Statistics 18, and the Youden Index was determined on the basis of the sensitivities and specificities produced for each variable by this software (sensitivity + [1−specificity]). A binary logistic regression was performed using sIL-2r and ferritin values to create a combined variable/predicted probability of having HLH. The combined ROC curve was then produced using the predicted probability as the test variable.
Results
In total, 78 patients were included: 38 HLH, and 40 non-HLH. Non-HLH diagnoses included sepsis, histiocyte disorders, multiple transfusions, liver disease, cardiac disease, autoimmune disease/vasculitis, and other inflammatory diseases. The mean age was 54 years (range, 19-87 years) and 50 years (range, 18-89 years) in the HLH and non-HLH groups, respectively (P = .46). The mean number of HLH criteria fulfilled in the HLH group was 5.6 vs 2.3 in the non-HLH group (P < .005; Table 1). Among the patients with HLH, 36 (95%) fulfilled at least 5 of the HLH-2004 criteria, with 8 fulfilling 6 and 7 fulfilling 7 criteria (supplemental Table 2). Three patients had genetic testing, all of whom were negative for known primary HLH associated genes, and 5 patients had NK function testing, 2 of whom had low NK cell function.
Clinical characteristics of patients
| Clinical characteristics of patients (n = 78) . | HLH (n = 38) . | Non-HLH (n = 40) . | P . |
|---|---|---|---|
| Age, mean (range), y | 54 (19-87) | 50.3 (18-89) | .46 |
| Women, n (%) | 11 (28.9) | 23 (57.5) | <.01 |
| No. of HLH criteria fulfilled (mean) | 5.6 | 2.3 | <.005 |
| HScore, median (range) | 215 (83-302) | 87.5 (0-204) | <.005 |
| Elevated sIL-2r >2400 U/mL, n (%) | 38 (100) | 15 (37.5) | <.005 |
| Elevated sIL-2r >10 000 U/mL, n (%) | 17 (44.7) | 3 (7.5) | <.005 |
| Peak ferritin >500 μg/L, n (%) | 38 (100) | 27 (67.5) | <.005 |
| Peak ferritin >10 000 μg/L, n (%) | 30 (78.9) | 13 (32.5) | <.005 |
| Overall mortality, n (%) | 23 (60.5) | 11 (27.5) | <.005 |
| Mortality in patients with sIL-2r >10 000 U/mL (n = 20), n (%) | 11/17 (64.7) | 33 (100) | .52 |
| Mortality in patients with sIL-2r <10 000 U/mL (n = 58), n (%) | 12/21 (57.1) | 837 (21.6) | .006 |
| Clinical characteristics of patients (n = 78) . | HLH (n = 38) . | Non-HLH (n = 40) . | P . |
|---|---|---|---|
| Age, mean (range), y | 54 (19-87) | 50.3 (18-89) | .46 |
| Women, n (%) | 11 (28.9) | 23 (57.5) | <.01 |
| No. of HLH criteria fulfilled (mean) | 5.6 | 2.3 | <.005 |
| HScore, median (range) | 215 (83-302) | 87.5 (0-204) | <.005 |
| Elevated sIL-2r >2400 U/mL, n (%) | 38 (100) | 15 (37.5) | <.005 |
| Elevated sIL-2r >10 000 U/mL, n (%) | 17 (44.7) | 3 (7.5) | <.005 |
| Peak ferritin >500 μg/L, n (%) | 38 (100) | 27 (67.5) | <.005 |
| Peak ferritin >10 000 μg/L, n (%) | 30 (78.9) | 13 (32.5) | <.005 |
| Overall mortality, n (%) | 23 (60.5) | 11 (27.5) | <.005 |
| Mortality in patients with sIL-2r >10 000 U/mL (n = 20), n (%) | 11/17 (64.7) | 33 (100) | .52 |
| Mortality in patients with sIL-2r <10 000 U/mL (n = 58), n (%) | 12/21 (57.1) | 837 (21.6) | .006 |
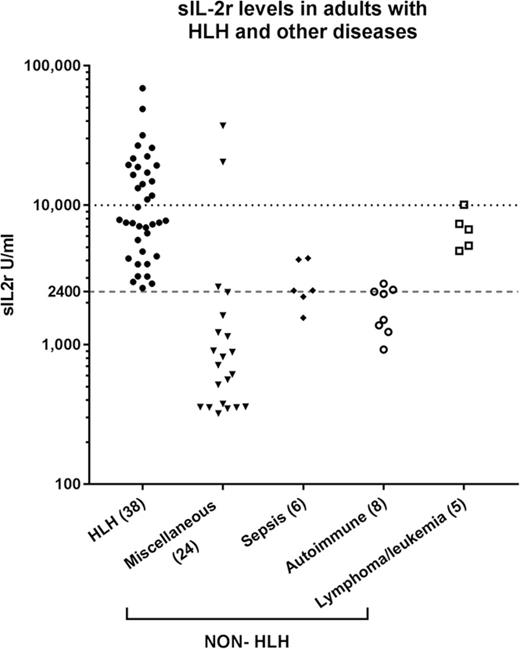
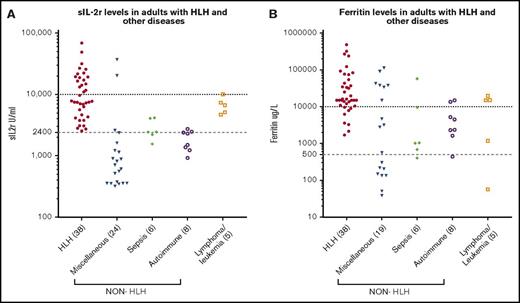
Serum sIL-2r level was higher than 2400 U/mL in 38 (100%) of the patients with HLH, and 17 (44.7%) had levels above 10 000 U/mL compared with 15 (37.5%) and 3 (7.5%), respectively, in the non-HLH patients (P < .005; Figure 1A). Of the non-HLH group, 3 patients had sIL-2r levels above 10 000 U/mL: the first was a 19-year-old woman who presented with fulminant viral myocarditis (sIL-2r, 37 251 U/mL; ferritin, 3234 µg/L; HScore, 204), who died within 24 hours; autopsy findings were consistent with viral myocarditis, with no evidence of lymphoma and no significant hemophagocytosis in any tissue. The second was a 58-year-old woman with ischemic liver postliver transplant (as a result of an occluded hepatic artery; sIL-2r, 20 442 U/mL; ferritin, 26 802 µg/L; HScore, 195). The third was an 83-year-old man who presented with diffuse large B-cell lymphoma (ferritin, 15 050 µg/L; sIL-2r, 10 090 U/mL; HScore, 91). Higher serum ferritin levels were seen in the patients with HLH: 38 patients (100%) with ferritin levels above 500 µg/L and 30 (78.9%) with ferritin levels above 10 000 µg/L vs 27 (67.5%) and 13 (32.5%), respectively, in the non-HLH patients (P < .005; Figure 1B). Mortality was higher in the HLH group (60.5% vs 27.5%; P < .005).
sIL-2r and ferritin levels in HLH and non-HLH. (A) sIL-2r levels in adult HLH and other diseases; (B) serum ferritin in adult HLH and other diseases.
sIL-2r and ferritin levels in HLH and non-HLH. (A) sIL-2r levels in adult HLH and other diseases; (B) serum ferritin in adult HLH and other diseases.
The sensitivity and specificity of sIL-2r and ferritin in diagnosis of adult HLH is summarized in Table 2. The sensitivity of sIL-2r above 2400 U/mL among our patients was 100% (95% confidence interval [CI], 0.89-1.00), and specificity was 63% (95% CI, 0.46-0.77), with a positive likelihood ratio of 2.67 (95% CI, 1.79 - 3.98) and a negative likelihood ratio of 0. Sensitivity was reduced to 45% (95% CI, 0.29-0.62) for sIL-2r higher than 10 000 U/mL, although specificity improved to 93% (95% CI, 0.79-0.98) at this threshold.
Sensitivity and specificity of sIL-2r and ferritin values (n = 78)
| . | sIL-2r >2400 U/mL . | sIL-2r >10 000 U/mL . | Ferritin >500 μg/L . | Ferritin >10 000 μg/L . |
|---|---|---|---|---|
| Sensitivity (95% CI) | 1.00 (0.89-1.00) | 0.45 (0.29-0.62) | 1.0 (0.89-1.0) | 0.79 (0.62-0.89) |
| Specificity (95% CI) | 0.63 (0.46-0.77) | 0.93 (0.79-0.98) | 0.33 (0.19-0.49) | 0.70 (0.53-0.83) |
| PPV* (95% CI) | 0.72 (0.57-0.82) | 0.85 (0.61-0.96) | 0.59 (0.46-0.70) | 0.71 (0.55-0.84) |
| NPV* (95% CI) | 1.00 (0.83-1.00) | 0.64 (0.50-0.75) | 1.0 (0.72-1.0) | 0.78 (0.60-0.89) |
| Positive LR (95% CI) | 2.67 (1.79-3.98) | 5.97 (1.90-18.73) | 1.48 (1.20-1.84) | 2.63 (1.59-4.34) |
| Negative LR (95% CI) | 0 | 0.60 (0.45-0.80) | 0 | 0.30 (0.16-0.57) |
| . | sIL-2r >2400 U/mL . | sIL-2r >10 000 U/mL . | Ferritin >500 μg/L . | Ferritin >10 000 μg/L . |
|---|---|---|---|---|
| Sensitivity (95% CI) | 1.00 (0.89-1.00) | 0.45 (0.29-0.62) | 1.0 (0.89-1.0) | 0.79 (0.62-0.89) |
| Specificity (95% CI) | 0.63 (0.46-0.77) | 0.93 (0.79-0.98) | 0.33 (0.19-0.49) | 0.70 (0.53-0.83) |
| PPV* (95% CI) | 0.72 (0.57-0.82) | 0.85 (0.61-0.96) | 0.59 (0.46-0.70) | 0.71 (0.55-0.84) |
| NPV* (95% CI) | 1.00 (0.83-1.00) | 0.64 (0.50-0.75) | 1.0 (0.72-1.0) | 0.78 (0.60-0.89) |
| Positive LR (95% CI) | 2.67 (1.79-3.98) | 5.97 (1.90-18.73) | 1.48 (1.20-1.84) | 2.63 (1.59-4.34) |
| Negative LR (95% CI) | 0 | 0.60 (0.45-0.80) | 0 | 0.30 (0.16-0.57) |
PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
The cohort-specific disease prevalence in this study is 48.7%.
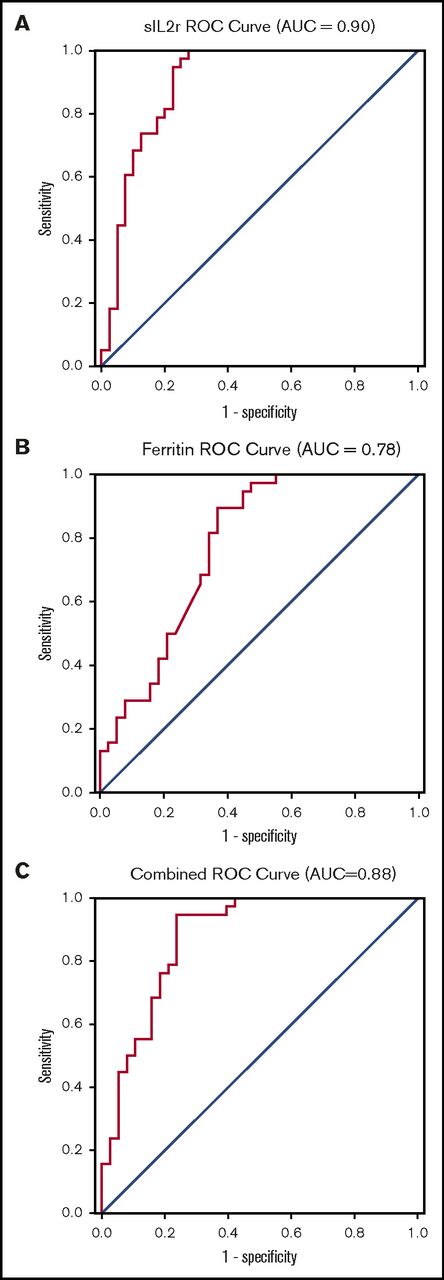
The ROC curve indicates that sIL-2r has good to excellent diagnostic performance in diagnosing HLH, with an area under the curve (AUC) of 0.90 (95% CI, 0.83-0.97; Figure 2A). On the basis of this curve, the cutoff that best optimizes sensitivity and specificity in diagnosing HLH is 2515 U/mL (sensitivity, 100%; specificity, 72.5%). The ROC curve for ferritin suggests this biomarker is fair for diagnosis of HLH, with an AUC of 0.78 (95% CI, 0.67-0.88), and an optimal cutoff is 5775 µg/L (sensitivity, 89.5%; specificity, 63.2%; Figure 2B). The ROC for sIL-2r and ferritin combined was no better than sIL-2r alone, with an AUC of 0.88 (95% CI, 0.80-0.96; Figure 2C)
ROC curves. (A) sIL-2r in diagnosis of adult HLH; (B) ferritin in diagnosis of adult HLH; and (C) sIL-2r and ferritin in diagnosis of HLH.
ROC curves. (A) sIL-2r in diagnosis of adult HLH; (B) ferritin in diagnosis of adult HLH; and (C) sIL-2r and ferritin in diagnosis of HLH.
sIL-2r levels have been correlated with disease activity in previous studies, and recommendations have been made to monitor sIL-2r levels during the course of therapy to evaluate response to therapy. In our center, sIL-2r is primarily used as a diagnostic test, but 7 patients with HLH had multiple sIL-2r levels drawn during their disease course, which generally tracked with their ferritin levels and clinical course (supplemental Figure 1). sIL-2r levels above 10 000 U/mL have been associated with poor prognosis in some HLH studies, but in this study, there was no significant difference in HLH with sIL-2r levels greater than 10 000 U/mL (64.7% vs 57.1% mortality; P = .63).
We subdivided the patients with HLH in our study into malignancy-associated hemophagocytic syndrome (MAHS; n = 16), infection-associated hemophagocytic syndrome (IAHS; n = 14), macrophage activation syndrome associated with autoimmune disease (MAS; n = 3), and unknown (n = 5; supplemental Table 3). There was a statistically significant difference in mean sIL-2r levels between MAHS (20 241 U/mL) in comparison with IAHS (9720 U/mL) and MAS (5008 U/mL; P < .05), but no difference between MAHS and unknown (unknown, 8788 U/mL; P = .058), IAHS and MAS (P = .61), IAHS and unknown (P = .58), or MAS and unknown (P = .10). The ratio of sIL-2r to ferritin levels above 2.0 has been described as a tool to distinguish lymphoma-associated hemophagocytic syndrome from other etiologies of HLH,13 but in this study, only 5 of 16 patients with MAHS had an sIL-2r/ferritin level above 2.0.
Discussion
This is the first study to evaluate sensitivity and specificity of sIL-2r in diagnosing adult HLH. Our results confirm that sIL-2r is a sensitive test and should be used, as per the HLH-2004 diagnostic criteria, in combination with other diagnostic tests and clinical expertise to diagnose HLH. The threshold of sIL-2r of at least 2400 U/mL used in HLH-2004 was 100% sensitive but only 63% specific in our case series. Our patient data suggest a similar optimal threshold of 2515 U/mL for diagnosing HLH, which confers a sensitivity of 100% and a specificity of 72.5%. There is a large indeterminate range (2400-10 000 U/mL), but this study suggests that 2400 U/mL or less is a reasonable rule out (sensitivity, 100%) and above 10 000 is a reasonable rule in (specificity, 93%) for HLH in adults. Importantly, the historically cited specificity of 100% for sIL-2r of at least 2400 U/mL in pediatric HLH is much too high in this adult population, as non-HLH subjects with lymphoma, leukemia, and other conditions had sIL-2r levels above 2400 U/mL. Among subsets of HLH, higher levels of sIL-2r were seen in patients with MAHS in this study compared with IAHS and MAS, which may reflect in part the elevated sIL-2r seen in lymphoma, even without overt hemophagocytic syndrome. Larger studies enriched with patients who have T-cell activation such as lymphoma, leukemia, and autoimmune lymphoproliferative syndrome are needed to better define the specificity of increased sIL-2r for both adult and pediatric HLH.
Two recent important developments in the diagnosis of HLH are particularly relevant to this study. The first is in secondary HPS/HLH, with the development of criteria such as the HScore and MAS 16, which are based on very large datasets of patients with secondary HPS/HLH.14,15 Although these criteria are an important tool for studying secondary HPS/HLH, they exclude specialized tests of immune activation and hypercytokinemia (such as sIL-2r) and instead focus on easily measured, widely available clinical and laboratory parameters. Because secondary HPS/HLH is defined by pathologic immune activation and hypercytokinemia leading to end organ damage, the exclusion of cytokine measurement is an obvious limitation. The other key development is in primary HLH, where flow cytometry for perforin expression and CD107a have proven superior to NK cell cytotoxicity for screening of genetic HLH.16 However, these specialized flow cytometry assays are only available in research centers and require fresh samples (drawn within 24 hours of testing) to be valid. Given the ease with which most clinical laboratories can implement simple cytokine measurements in-house or send frozen samples to a reference center, and the central role of markers such as sIL-2r, CD163, and interferon γ in HLH,17,18 evaluating the diagnostic and prognostic utility of these markers in adult HLH should be prioritized. The results of this study represent an important step in linking the diagnosis of adult HLH back to its defining feature of hypercytokinemia.
This study has several limitations. Genetic testing and NK cytotoxicity were only performed on a small number of patients. Investigators were not blinded to sIL-2r levels, which were in most cases ordered because HLH was suspected, leading to risk for confirmation bias. Because HLH was suspected in most of the 78 patients analyzed, the sensitivity of sIL-2r is likely higher and the specificity lower than a population with a lower pretest probability of HLH. The use of an absolute cutoff value, such as the optimum cutoff of 2515 U/mL found in this study, or 2400 U/mL used in HLH-2004, is dependent on the laboratory and technique used, and will vary from center to center. However, this is the first study of the diagnostic utility of sIL-2r in adult HLH and demonstrates the superiority of this marker to ferritin. Given the complexity of HLH, no single clinical or laboratory parameter is sufficient to rule it in or out, but future diagnostic criteria should include markers of hypercytokinemia and immune activation, which are a key feature of both primary and secondary HLH. Given the diagnostic value of soluble interleukin-2 receptor levels and the ease with which this test can be implemented in most centers, sIL-2r should be incorporated in future diagnostic, prognostic, and therapeutic studies of adult HLH.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Huiru Dong (Statistician, British Columbia Centre on Substance Use) for statistical advice.
This work was supported by the Hal Kettleson Hematology Research Fund.
Authorship
Contribution: A.H. and M.L. contributed to the study’s conception, design, analysis, and interpretation of the data; S.P., M.P., and A.M. contributed to the study design, data collection, and analysis; M.S. and M.B.J. contributed to interpretation of the data; L.Y.C.C. contributed to the study’s conception, design, data analysis, and interpretation and directed the project; and all authors edited and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luke Y. C. Chen, Vancouver General Hospital, 2775 Laurel St, 10th Floor, Vancouver, BC V5Z 1M9, Canada; e-mail: lchen2@bccancer.bc.ca.
References
Author notes
A.H. and M.L. contributed equally to this study.