Key Points
Long-term, once-weekly emicizumab was well tolerated with no thromboembolic adverse events in patients with hemophilia A.
Long-term, once-weekly emicizumab shows encouraging efficacy irrespective of the presence of FVIII inhibitors in patients with hemophilia A.
Abstract
Emicizumab (ACE910), a recombinant humanized bispecific monoclonal antibody, provides factor VIII (FVIII) cofactor bridging function to restore hemostasis in people with hemophilia A. In a phase 1 trial involving 18 Japanese patients with severe hemophilia A, once-weekly subcutaneous administration of emicizumab 0.3, 1, or 3 mg/kg (cohorts 1, 2, and 3, respectively) was well tolerated and substantially reduced annualized bleeding rates (ABRs) in the presence or absence of FVIII inhibitors. The current study represents an open-label, long-term extension of the previously reported 12-week phase 1 study, in which 16 of 18 patients continued to receive emicizumab for up to 33.3 months. Long-term emicizumab treatment was well tolerated, with no thromboembolic events reported and no neutralizing antiemicizumab antibodies developing during the course of the study. Plasma concentrations of emicizumab increased in a dose-proportional manner, with activated partial thromboplastin times remaining short. In cohorts 1, 2, and 3, respectively, median ABRs remained low at 1.4, 0.2, and 0 compared with 4.4, 0, and 0 in the 12-week study. Overall, 8 patients experienced no bleeding events (6 patients with and 2 patients without FVIII inhibitors); dose up-titration resulted in further reduction in ABRs in patients with suboptimal bleeding control; and the episodic use of clotting factors to control bleeding was reduced. In conclusion, long-term emicizumab treatment demonstrated a favorable safety profile with encouraging efficacy, irrespective of the presence of FVIII inhibitors, in patients with hemophilia A. This study was registered at www.clinicaltrials.jp as #JapicCTI-132195.
Introduction
Hemophilia A, a deficiency of clotting factor VIII (FVIII) that results in frequent bleeding, is estimated to develop in 1 in 5000 males worldwide.1 More than half the population of patients with hemophilia A have severe disease characterized by FVIII activity <0.01 IU/mL.2 These patients often experience frequent traumatic or spontaneous bleeding events,2 including joint bleeding,3 which leads to irreversible hemophilic arthropathy, the major cause of morbidity and reduced quality of life in patients with hemophilia.4,5
Prophylactic or episodic IV infusions of plasma-derived or recombinant FVIII are the current options for managing severe hemophilia A.6,7 Prophylactic treatment reduces the risk of joint damage.8 However, the short half-lives of approximately 12 to 15 hours9-13 for currently available agents necessitate frequent (eg, 2-3 times per week), inconvenient, and time-consuming administration to maintain protective FVIII levels.7,14 In infants, the need for frequent IV access can prove particularly difficult, and central venous access devices are often required.15
The development of FVIII inhibitors (anti-FVIII antibodies), which render FVIII replacement therapy ineffective, is 1 of the most challenging complications of treatment.16 Inhibitors develop in approximately 30% of patients with severe hemophilia A and lead to substantial morbidity and decreased quality of life.17 For patients with FVIII inhibitors, treatment may involve inhibitor-eliminating immune tolerance induction therapy, which is burdensome and not successful in every patient. For patients undergoing or refractory to immune tolerance induction therapy, bleeding events are controlled with bypassing agents (eg, recombinant activated FVII [rFVIIa] or activated prothrombin complex concentrate [aPCC]).16 Although these treatments can be efficacious for many patients, neither of these therapies is considered as effective as FVIII replacement.
Emicizumab (ACE910) has been developed to address the burdens associated with current treatment options for patients with hemophilia A, such as frequent infusion, inconvenient IV administration, and FVIII inhibitor development. Emicizumab is a recombinant humanized bispecific monoclonal antibody that acts as an FVIII mimetic by binding simultaneously to activated factor IX (FIXa) and factor X (FX).18,19 Because of its unique structure, emicizumab is not expected to induce FVIII inhibitor development or be affected by existing FVIII inhibitors. Subcutaneously administered emicizumab showed high bioavailability in cynomolgus monkeys,20 and once-weekly administration significantly reduced spontaneous bleeding symptoms in a primate model of acquired hemophilia A.21 In its first-in-human phase 1 study, emicizumab demonstrated a long half-life of 4 to 5 weeks, and single subcutaneous doses of ≤1 mg/kg had favorable safety and tolerability in healthy participants.22 Subsequently, in the first-in-patient 12-week, phase 1 study, once-weekly subcutaneous emicizumab at 0.3, 1, or 3 mg/kg was well tolerated and substantially reduced annualized bleeding rates (ABRs) in patients with severe hemophilia A both with and without inhibitors.23
Currently, an extension of the first-in-patient 12-week study is ongoing. The objective of this long-term study is to investigate safety and, in an exploratory manner, the prophylactic effect of emicizumab on bleeding events in patients with hemophilia A with or without inhibitors. Here we report long-term data up to a cutoff of February 2016 in combination with the complete data from the 12-week study, in which emicizumab treatment was initiated from May 2013.
Methods
This ongoing phase 1/2, open-label, multicenter extension study has been conducted since August 2013 in compliance with the International Conference on Harmonisation Guideline for Good Clinical Practice and was approved by institutional review boards. The 12-week study and this extension study were registered at www.clinicaltrials.jp/user/cteSearch_e.jsp (#JapicCTI-121934 and #JapicCTI-132195, respectively). All patients and/or their legally authorized representatives provided written informed consent for study participation.
Patients
Eighteen Japanese patients aged 12 to 58 years, with severe hemophilia A with or without inhibitors, who enrolled in the 12-week phase 1 study23 were candidates for the extension study. Eligibility criteria for the 12-week study have been described previously.23 Inclusion criteria specific for the extension study included report of ≥3 bleeding episodes in the 6 months before the 12-week study enrollment (for patients without inhibitors). On the basis of inclusion and exclusion criteria for the extension study, investigators determined patient eligibility. The efficacy and safety evaluation committee for the study also determined patients’ eligibility for the extension study based on their individual clinical findings, including laboratory tests, vital signs, 12-lead electrocardiogram (ECG) results, adverse events (AEs), pharmacokinetics (PK), pharmacodynamics (PD), and serum cytokine concentrations.
Study design
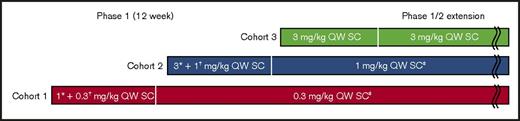
Patients started once-weekly subcutaneous emicizumab at 0.3 (cohort 1) or 1 mg/kg (cohort 2) from 1 week after receiving a loading dose of 1 (cohort 1) or 3 mg/kg (cohort 2) on the first day of the 12-week study (day 1); patients in cohort 3 received 3 mg/kg once weekly from day 1.23 Each cohort included 6 patients. In the extension study, eligible patients continued on the assigned dose for their respective cohort from the 12-week study, with potential dose up-titration to 1 (cohort 1) or 3 mg/kg (cohorts 1 and 2; Figure 1). In cohort 3, patients remained on 3 mg/kg once weekly.
Study design schematic of the extension study following the first-in-patient study. SC, subcutaneous; QW, once weekly. *Loading dose. †Maintenance dose. ‡Dose escalation within patient may be allowed (decided by efficacy and safety evaluation committee).
Study design schematic of the extension study following the first-in-patient study. SC, subcutaneous; QW, once weekly. *Loading dose. †Maintenance dose. ‡Dose escalation within patient may be allowed (decided by efficacy and safety evaluation committee).
Decisions on dose up-titration during the extension study were made by the efficacy and safety evaluation committee on an individual patient basis after reviewing all patient data from the phase 1 study at a dose considered for up-titration. If a bleeding event occurred, the committee determined the feasibility of dose up-titration within the dose range considered acceptable for the extension study based on clinical findings, including laboratory tests, vital signs, 12-lead ECG, AEs, PK/PD responses, serum cytokine concentrations, and the number of bleeding episodes over ≥12 consecutive emicizumab administrations in the patient. Approval of self-injection by a patient or caregiver was also made by the committee on a dose-level basis, similar to the assessments made for dose up-titration. For dose up-titration from 0.3 to 1 mg/kg, the initial subcutaneous dose was 3 mg/kg, followed by subsequent weekly doses of 1 mg/kg. At the discretion of the investigator, individual administrations of emicizumab could be suspended.
If a patient’s reduction in bleeding rate from pre- to postemicizumab treatment in the 12-week study was <50%, the emicizumab dose was considered suboptimal. Such patients did not initially participate in the extension study, but instead transitioned to the postemicizumab follow-up observation period, during which reinitiation of prior treatment was allowed. Restarting emicizumab treatment with dose up-titration was at the discretion of the investigators. Once the efficacy and safety evaluation committee approved initiation of emicizumab treatment at a higher dose in the extension study, the patient could be enrolled in the extension study to restart emicizumab treatment at this up-titrated dose (supplemental methods).
Breakthrough bleeding episodes that occurred during the study were treated with FVIII or a bypassing agent according to standard clinical practice.
Outcome measures
The primary end point of the extension study was safety, including AEs and treatment-related AEs, laboratory tests, clinical symptoms, vital signs, 12-lead ECG and chest X-ray, and immunogenicity (ie, plasma antiemicizumab (drug) antibodies [ADAs]). Secondary end points included PK profiles assessed with plasma concentrations of emicizumab, FIX and FX, and PD responses of activated partial thromboplastin time (aPTT) and activated factor XI (FXIa)–triggered thrombin generation (TG). The assay methods for the PK, PD, and ADAs have been described previously.22 Coagulation factor use was allowed to treat breakthrough bleeding or bleeding related to procedure/surgery. The number of breakthrough bleeding episodes requiring coagulation factor treatment was an exploratory efficacy end point of the study.
Statistical analysis
All analyses were conducted using the combined data from the 12-week and extension studies. Although this study had no preplanned analysis, a data cutoff of February 2016 was chosen because the median follow-up time of the population was >2 years and considered long enough to assess long-term safety and exploratory efficacy. Summary statistics were calculated for demographic, PK, PD, and safety and tolerability outcomes. The ABRs during emicizumab treatment were compared with those during the 6 months before emicizumab treatment as captured from the patients’ medical records. The ABR was calculated as 365.25 times the number of bleeding episodes divided by the number of days treated. The relationship between emicizumab dose and ABR was explored within each patient who had dose up-titration. Data after dose up-titration were excluded from summary statistical calculation of PK, PD, and ABR data.
Results
As of February 2016, 16 of 18 Japanese patients who enrolled in the 12-week study continued into the extension study: 6 patients in cohort 1, and 5 patients each in cohorts 2 and 3. Two patients from the 12-week study were not eligible for the extension study. One patient (inhibitor; cohort 2) discontinued emicizumab on day 29 of the 12-week study because of mild injection-site erythema, and the second patient (noninhibitor; cohort 3) had no bleeding event before emicizumab administration. One patient (noninhibitor; cohort 1) restarted emicizumab in the extension study at a higher dose (1 mg/kg) than in the 12-week study (0.3 mg/kg) after the postemicizumab follow-up observation.
Demographics and baseline characteristics of patients enrolled in the initial 12-week study have been reported previously.23 In brief, characteristics were similarly distributed across cohorts with the exception of ABRs in the 6 months before the study (ABRs for patients in cohort 1, particularly patients without inhibitors, were higher than for patients in cohorts 2 and 3) and prior bypassing agent use (episodic for cohorts 1 and 2 and prophylactic for cohort 3).
Across the 12-week and extension studies, at a median follow-up of 32.6 (range, 32.2-33.3), 27.0 (range, 8.2-28.5), and 21.5 months (range, 11.1-22.6) for cohorts 1, 2, and 3, respectively, 16 patients had continued study treatment. Four patients had dose up-titration: 2 patients required up-titration from 0.3 to 1 mg/kg and then 3 mg/kg; 1 patient required up-titration from 0.3 to 1 mg/kg; and the fourth patient required up-titration from 1 to 3 mg/kg. Most patients self-administered emicizumab during the study; some patients received treatment from a caregiver or health care professional.
PK/PD
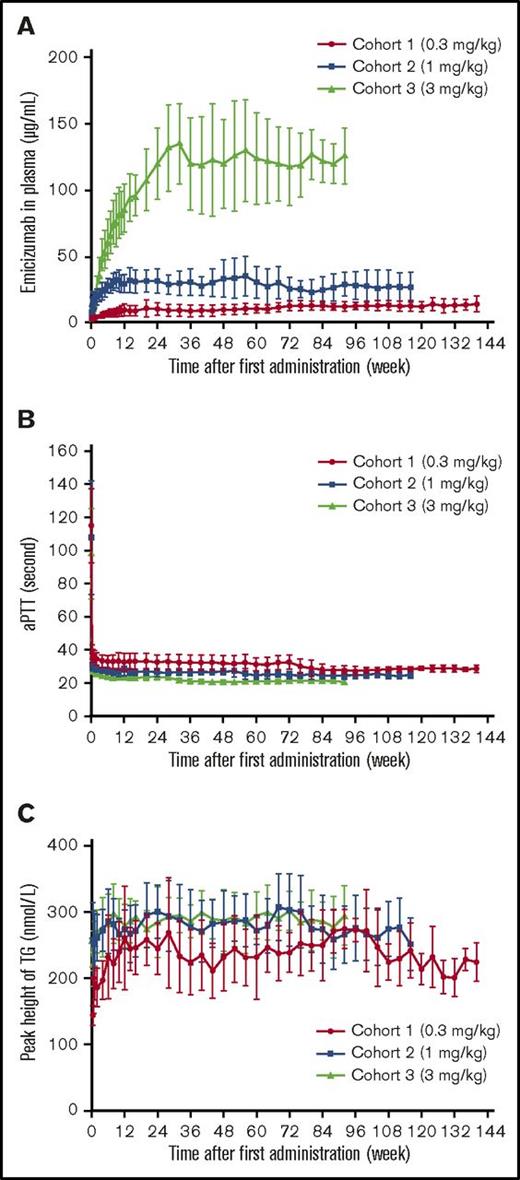
Plasma emicizumab concentrations reached steady state ∼12 weeks after treatment initiation in cohorts 1 and 2, where an initial loading dose was administered, and after ∼24 weeks in cohort 3, where no loading dose was administered (Figure 2A). Mean (± standard deviation) steady-state trough levels increased in a dose-proportional manner at 10.3 (± 4.54), 29.9 (± 6.88), and 120 (± 26.8) μg/mL in cohorts 1, 2 and 3, respectively. In the 4 patients with dose up-titrations, plasma emicizumab increased with increased doses (data not shown).
PK and PD of emicizumab during once-weekly subcutaneous administrations of emicizumab. Mean (± standard deviation) time courses of plasma emicizumab concentration (A), aPTT (B), peak height of FXIa-triggered TG (C). The reference ranges for aPTT and peak height of FXIa-triggered TG are 25.4 to 37.2 seconds and 341 to 489 nmol/L, respectively, which were derived from healthy Japanese participants.22 All data collected after dose up-titrations were excluded; the number of patients for this summary was 6 per cohort. Data points where the number of patients with quantifiable measurement was ≥2 and not less than half of the number of observed patients were plotted.
PK and PD of emicizumab during once-weekly subcutaneous administrations of emicizumab. Mean (± standard deviation) time courses of plasma emicizumab concentration (A), aPTT (B), peak height of FXIa-triggered TG (C). The reference ranges for aPTT and peak height of FXIa-triggered TG are 25.4 to 37.2 seconds and 341 to 489 nmol/L, respectively, which were derived from healthy Japanese participants.22 All data collected after dose up-titrations were excluded; the number of patients for this summary was 6 per cohort. Data points where the number of patients with quantifiable measurement was ≥2 and not less than half of the number of observed patients were plotted.
Shortening of aPTT and promotion of FXIa-triggered TG were maintained with weekly emicizumab treatment; a slight dose dependency was observed (Figure 2B-C). At steady state, mean (± standard deviation) aPTT was 32.5 (± 5.5), 27.7 (± 3.5), and 24.0 (± 2.3) seconds in cohorts 1, 2, and 3, respectively. No obvious changes in plasma concentrations of FIX or FX were observed (data not shown). Dose up-titration resulted in slight or negligible shortening of aPTT and promotion of FXIa-triggered TG (data not shown).
Safety
It was not possible to conduct a comparison of different dose groups, because some patients experienced dose up-titration, and patients were not randomly assigned to dose groups. Therefore, all 18 patients were included in the safety analysis.
Once-weekly subcutaneous emicizumab administration for a maximum of 33.3 months was well tolerated, even at steady state for the highest dose (3 mg/kg). In this patient population, no thromboembolic AEs were observed, even when FVIII or a bypassing agent was administered to treat breakthrough bleeding episodes. No deaths have been reported during the study.
AEs that occurred in ≥2 patients are listed in Table 1. All patients experienced ≥1 AE, with 150 AEs reported overall. The most frequently reported AEs were local injection-site reactions (erythema, hematoma, pruritus, discomfort, pain, or rash); 12 events, all mild, were reported in 7 patients (38.9%). All injection-site reactions occurred after >2 emicizumab doses. One patient in cohort 2 withdrew from treatment on day 29 of the 12-week study because of repeated mild injection-site erythema. The AE required approximately 2 weeks of oral/topical antihistamine and resolved after 36 to 41 days; however, because of the patient’s history of urticaria and concerns over potential future injection-related erythema, the study investigator discontinued emicizumab. At the time of data cutoff, with the exception of this patient, injection-site hematoma, erythema, pain, and discomfort had resolved without treatment. One occurrence of rash and 1 of pruritus resolved after approximately 2 weeks of oral/topical antihistamine or combined topical steroid/antibiotic treatment. Two other occurrences of pruritus resolved without treatment. Fourteen AEs in 7 patients were considered emicizumab related and included injection-site reactions, malaise, diarrhea, nausea, increased blood creatinine phosphokinase, and increased C-reactive protein (supplemental Table 1). Treatment-related injection-site reactions were reported in 6 patients (33.3%).
AEs reported in at least 2 patients
| . | All cohorts (N = 18) . |
|---|---|
| Total patients with ≥1 AE, n (%) | 18 (100.0) |
| Total number of AEs | 150 |
| AEs reported in ≥2 patients, n (%) | |
| Nasopharyngitis | 8 (44.4) |
| Contusion | 7 (38.9) |
| Dental caries | 6 (33.3) |
| Pharyngitis | 5 (27.8) |
| Headache | 5 (27.8) |
| Excoriation | 4 (22.2) |
| Upper respiratory tract infection | 3 (16.7) |
| Tongue injury | 3 (16.7) |
| Wound | 3 (16.7) |
| Diarrhea | 3 (16.7) |
| Injection-site erythema | 3 (16.7) |
| Injection-site hematoma | 3 (16.7) |
| Injection-site pruritus | 3 (16.7) |
| Erythema | 3 (16.7) |
| Myalgia | 3 (16.7) |
| Infected dermal cyst | 2 (11.1) |
| Nausea | 2 (11.1) |
| Malaise | 2 (11.1) |
| Dizziness | 2 (11.1) |
| Back pain | 2 (11.1) |
| Rhinitis allergic | 2 (11.1) |
| Hemophilia | 2 (11.1)* |
| Calculus ureteric | 2 (11.1) |
| . | All cohorts (N = 18) . |
|---|---|
| Total patients with ≥1 AE, n (%) | 18 (100.0) |
| Total number of AEs | 150 |
| AEs reported in ≥2 patients, n (%) | |
| Nasopharyngitis | 8 (44.4) |
| Contusion | 7 (38.9) |
| Dental caries | 6 (33.3) |
| Pharyngitis | 5 (27.8) |
| Headache | 5 (27.8) |
| Excoriation | 4 (22.2) |
| Upper respiratory tract infection | 3 (16.7) |
| Tongue injury | 3 (16.7) |
| Wound | 3 (16.7) |
| Diarrhea | 3 (16.7) |
| Injection-site erythema | 3 (16.7) |
| Injection-site hematoma | 3 (16.7) |
| Injection-site pruritus | 3 (16.7) |
| Erythema | 3 (16.7) |
| Myalgia | 3 (16.7) |
| Infected dermal cyst | 2 (11.1) |
| Nausea | 2 (11.1) |
| Malaise | 2 (11.1) |
| Dizziness | 2 (11.1) |
| Back pain | 2 (11.1) |
| Rhinitis allergic | 2 (11.1) |
| Hemophilia | 2 (11.1)* |
| Calculus ureteric | 2 (11.1) |
All data collected after dose up-titrations were included.
Serious adverse event (SAE; left hip joint bleeding and subcutaneous hemorrhage of the proglossis).
SAEs reported in 4 patients (2 with and 2 without inhibitors) were all considered unrelated to emicizumab by the study investigators. One patient (inhibitor) experienced left hip joint bleeding resulting from hemophilia 22 weeks after the last emicizumab dose. The second patient (noninhibitor) had experienced abdominal pain from day 440. The patient was diagnosed with appendicitis after 2 days and was hospitalized and underwent surgery on the same day. The appendicitis resolved on day 458 without any interruption to emicizumab treatment, and the patient remained on emicizumab at the time of data cutoff. The third patient (noninhibitor) was hospitalized with mesenteric hematoma on day 477. Hemorrhage resulting from thrombosis was considered unlikely because the patient had experienced no acute abdominal condition, the hematoma developed slowly, there was no evidence of intestinal edema, and there were no clinically significant changes in laboratory coagulation markers to suggest thrombosis. Relevant computed tomography images are shown in supplemental Figure 1. After an interruption of emicizumab from weeks 69 to 75 (equating to 7 missed doses), when episodic FVIII therapy was administered, the event resolved, with no further bleeding reported; the patient remained on emicizumab at the time of data cutoff. The fourth patient (inhibitor) required hospitalization for control of a subcutaneous hemorrhage of the proglossis on day 860 and also experienced a nosebleed (also on day 860). The nosebleed resolved by the time of hospitalization. Without any interruption to emicizumab treatment, the patient was administered a bypassing agent twice daily and was discharged the following day. The hemorrhage event resolved 9 days after onset, and the patient remained on emicizumab at the time of data cutoff.
Efficacy
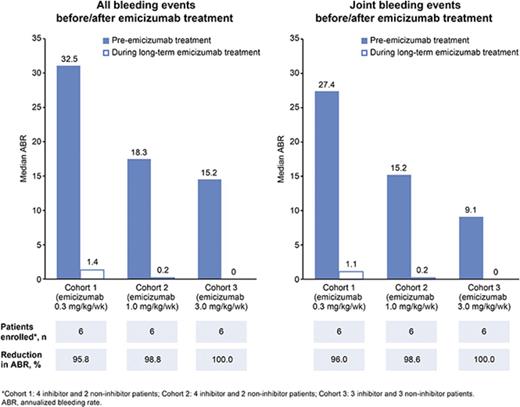
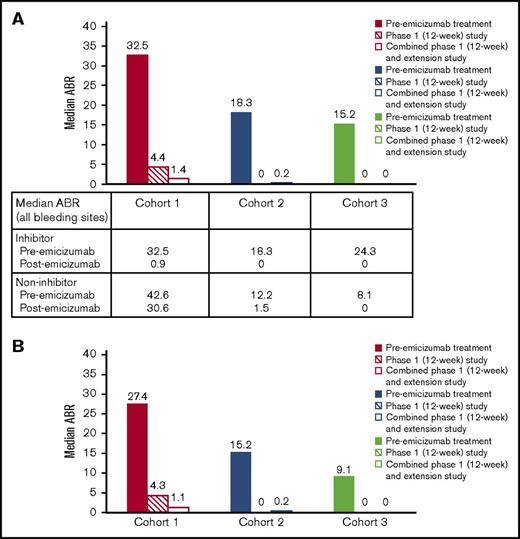
Median ABRs for each cohort 6 months before starting emicizumab prophylaxis, during the 12-week phase 1 study, and during combined phase 1 and extension studies up to the data cutoff are shown in Figure 3. The reduction in median ABRs (bleeding events at any site) from the prior 6 months to the 12-week study has been reported previously23 ; this reduction continued in the extension study. In cohorts 1, 2, and 3, respectively, median (range) ABRs remained low at 1.4 (0.0-59.5), 0.2 (0.0-2.7), and 0.0 (0.0-0.6), vs 4.4 (0.0-59.5), 0.0 (0.0-4.3), and 0.0 (0.0-4.2) in the 12-week study. The low ABR overall in the extension study was reflected in the median (range) frequency of joint bleeding events vs the 12-week study: 1.1 (0.0-59.5) vs 4.3 (0.0-59.5) in cohort 1, 0.2 (0.0-1.9) vs 0.0 (0.0-0.0) in cohort 2, and maintained at 0 (0.0-0.0 for both periods) in cohort 3. Overall, 8 patients experienced no bleeding events with weekly emicizumab as of the data cutoff. These patients included 1 with inhibitors in cohort 1, 3 with inhibitors in cohort 2, and 2 with and 2 without inhibitors in cohort 3. The decrease in ABRs did not differ for patients with or without inhibitors (supplemental Figure 2). In all emicizumab dose cohorts, patients with inhibitors had a reduction in ABRs to nearly 0 after receiving treatment.
Median ABR for each dose cohort during once-weekly subcutaneous administration of emicizumab. (A) Bleeding events at any site. (B) Joint bleeding events. All data collected after dose up-titrations were excluded; the number of patients for this summary was 6 per cohort. Cohort 1 included 4 patients with and 2 without inhibitors at study enrollment and data cutoff; cohort 2 included 4 patients with and 2 without inhibitors at study enrollment, and 3 patients with and 2 without inhibitors at data cutoff; cohort 3 included 3 patients with and 3 without inhibitors at study enrollment, and 3 patients with and 2 without inhibitors at data cutoff.
Median ABR for each dose cohort during once-weekly subcutaneous administration of emicizumab. (A) Bleeding events at any site. (B) Joint bleeding events. All data collected after dose up-titrations were excluded; the number of patients for this summary was 6 per cohort. Cohort 1 included 4 patients with and 2 without inhibitors at study enrollment and data cutoff; cohort 2 included 4 patients with and 2 without inhibitors at study enrollment, and 3 patients with and 2 without inhibitors at data cutoff; cohort 3 included 3 patients with and 3 without inhibitors at study enrollment, and 3 patients with and 2 without inhibitors at data cutoff.
The efficacy and safety evaluation committee allowed a small subset of 4 patients to have emicizumab dose up-titration because of suboptimal bleeding control. In each patient who had emicizumab dose up-titration, a further reduction in ABR was observed (supplemental Figure 3). These individual reductions in ABR ranged from 85% to 100% after dose up-titration.
All breakthrough bleeding episodes were successfully treated with episodic treatment (FVIII or bypassing agents) according to standard clinical practice. The daily amounts of the various coagulation factors required to control breakthrough bleeding or bleeding related to a procedure postemicizumab are summarized in Table 2. There were 83 FVIII treatment episodes, with a dose range of 12 to 98 IU/kg per day for breakthrough bleeding during emicizumab treatment in 5 of 7 noninhibitor patients. Most FVIII treatment episodes were for 1 day (63 episodes) or at ≤50 IU/kg per day (69 episodes). Among 11 inhibitor patients, 2, 1, and 3 patients used aPCC only, rFVIIa only, or both bypassing agents, respectively, with a dose range of 38 to 203 U/kg per day for aPCC and 86 to 504 μg/kg per day for rFVIIa. One patient used aPCC only for bleeding related to a procedure (ie, he experienced no breakthrough bleeding that required coagulation factors). No patient administered aPCC and rFVIIa on the same day. There were 23 aPCC and 35 rFVIIa treatment events. Most aPCC treatment episodes were for 1 day (20 episodes) or at ≤100 U/kg per day (21 episodes). Most rFVIIa treatment episodes were for 1 or 2 days (25 or 9 episodes, respectively) or at ≤270 μg/kg per day (33 episodes). The amount of coagulation factor required per breakthrough bleeding episode, pre- and postemicizumab, is shown in supplemental Figure 4. The range of percent change in the intrapatient mean amount of each coagulation factor per bleeding episode from pre- to postemicizumab administration was −37.5% to +70.0% (for 4 of 5 patients) for FVIII products, −41.0% to +30.0% (n = 4) for aPCC, and −20.0% to +21.4% (n = 4) for rFVIIa. One patient required a large amount of FVIII products because of repeated treatments for a single SAE (mesenteric hematoma). None of the remaining patients required coagulation factor products during emicizumab administration (ie, the percent change was −100%). A majority of patients, including those with no bleeding event, experienced a decrease in the mean use of coagulation factor products during emicizumab administration (supplemental Figure 4).
Coagulation factor products required during emicizumab treatment
| Duration of treatment, days . | Range of dose . | Total number of treatments . | |||
|---|---|---|---|---|---|
| FVIII, IU/kg per day (n = 5) | <25 | 25-50 | 51-75 | >75 | |
| 1 | 10 | 40 | 12 | 1* | 63 |
| 2 | 4 | 4 | 0 | 0 | 8 |
| 3 | 1 | 4 | 1 | 0 | 6 |
| 4 | 0 | 1 | 0 | 0 | 1 |
| >4 | 3 | 2 | 0 | 0 | 5 |
| Total number of treatments per dose category | 18 | 51 | 13 | 1 | 83 |
| aPCC, U/kg per day (n = 5) | <50 | 50-100 | 101-150 | >150 | |
| 1 | 3 | 16 | 1† | 0 | 20 |
| 2 | 0 | 2 | 0 | 1‡ | 3 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 |
| >4 | 0 | 0 | 0 | 0 | 0 |
| Total number of treatments per dose category | 3 | 18 | 1 | 1 | 23 |
| rFVIIa, μg/kg per day (n = 4) | <90 | 90-180 | 181-270 | >270 | |
| 1 | 6 | 4 | 13 | 2§ | 25 |
| 2 | 0 | 0 | 9 | 0 | 9 |
| 3 | 0 | 0 | 1 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0 |
| >4 | 0 | 0 | 0 | 0 | 0 |
| Total number of treatments per dose category | 6 | 4 | 23 | 2 | 35 |
| Duration of treatment, days . | Range of dose . | Total number of treatments . | |||
|---|---|---|---|---|---|
| FVIII, IU/kg per day (n = 5) | <25 | 25-50 | 51-75 | >75 | |
| 1 | 10 | 40 | 12 | 1* | 63 |
| 2 | 4 | 4 | 0 | 0 | 8 |
| 3 | 1 | 4 | 1 | 0 | 6 |
| 4 | 0 | 1 | 0 | 0 | 1 |
| >4 | 3 | 2 | 0 | 0 | 5 |
| Total number of treatments per dose category | 18 | 51 | 13 | 1 | 83 |
| aPCC, U/kg per day (n = 5) | <50 | 50-100 | 101-150 | >150 | |
| 1 | 3 | 16 | 1† | 0 | 20 |
| 2 | 0 | 2 | 0 | 1‡ | 3 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 |
| >4 | 0 | 0 | 0 | 0 | 0 |
| Total number of treatments per dose category | 3 | 18 | 1 | 1 | 23 |
| rFVIIa, μg/kg per day (n = 4) | <90 | 90-180 | 181-270 | >270 | |
| 1 | 6 | 4 | 13 | 2§ | 25 |
| 2 | 0 | 0 | 9 | 0 | 9 |
| 3 | 0 | 0 | 1 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0 |
| >4 | 0 | 0 | 0 | 0 | 0 |
| Total number of treatments per dose category | 6 | 4 | 23 | 2 | 35 |
As of February 2016, 16 of 18 Japanese patients enrolled in the 12-week study continued into the extension study: 6 patients in cohort 1, and 5 patients each in cohorts 2 and 3. All data collected after dose up-titrations were included.
The patient 1-6 used 2000 IU of FVIII 3 times per day (98 IU/kg per day) to treat breakthrough bleeding during treatment with 3 mg/kg of emicizumab. This patient had 59 treatment events in total and was the only 1 who used >50 IU/kg per day of FVIII.
The patient 2-1 used 3000 U of aPCC 3 times per day (115 U/kg per day) to treat bleeding related to a procedure (not counted as breakthrough bleeding) during treatment with 1 mg/kg of emicizumab.
The patient 1-2 used 7000 U of aPCC twice per day (203 U/kg per day) for 2 days to treat breakthrough bleeding during treatment with 0.3 mg/kg of emicizumab.
The patient 1-3 used 15 mg of rFVIIa twice per day (504 μg/kg per day) to treat breakthrough bleeds during treatment with 0.3 and 1 mg/kg of emicizumab.
Immunogenicity
Four patients tested positive for ADAs: 1 tested positive before emicizumab initiation (baseline), and 3 tested positive only after emicizumab initiation. Two of the 4 patients underwent dose up-titration. Because the presence of ADAs had no effect on plasma concentrations of emicizumab, FIX or FX, PD markers (aPTT or TG), or reduction in ABR in any of the 4 patients (data not shown), the ADAs were considered nonneutralizing. One patient who received 0.3 mg/kg of emicizumab up to week 96, and 1 mg/kg thereafter, developed anti-emicizumab immunoglobulin E (IgE). This patient tested negative for ADAs at baseline and positive for ADAs at weeks 36, 48, 60, 68, 72, and 84, which included positive IgE tests at weeks 36 and 48. The patient also tested negative for ADAs at weeks 12, 24, 56, 64, 76, and 80 and every 4 weeks from week 88 until week 136. This patient experienced mild injection-site reactions (discomfort, erythema) and several other AEs that were reported by >1 patient (Table 1). Overall, no specific AE occurrence patterns for the patient were observed, and no hypersensitivity, other than local injection-site reactions, was reported.
Discussion
This ongoing extension of the 12-week phase 1 study23 was conducted to assess the long-term safety and efficacy of emicizumab in the management of patients with severe hemophilia A. Once-weekly subcutaneous emicizumab was well tolerated for up to 33.3 months, with tolerability of the highest dose of 3 mg/kg once weekly confirmed under steady-state conditions (mean trough level, 120 μg/mL). Treatment-related AEs were manageable and did not occur more often than AEs unrelated to study treatment (ie, nasopharyngitis, headache, and dizziness); treatment-related AEs were successfully treated with commonly available drugs or resolved without treatment. Injection-site reactions were the most frequently reported treatment-related AEs. A total of 4 patients experienced SAEs, all of which resolved and none of which were considered related to emicizumab treatment by the study investigators.
Long-term emicizumab treatment showed encouraging efficacy in patients with hemophilia A, irrespective of the presence of FVIII inhibitors, as demonstrated by the substantial decrease in ABRs across dose cohorts. The low ABRs achieved during the first 12 weeks of emicizumab administration were maintained with long-term treatment. Joint bleeding was well controlled, especially in cohort 3, where an ABR of 0 was maintained in all 6 patients. Eight of 18 patients who enrolled in the 12-week study achieved 0 bleeding episodes; 1 additional patient achieved 0 bleeding episodes at an up-titrated dose.
aPTT is a commonly used biomarker to monitor hemostatic activity.24 The postemicizumab aPTT at steady state averaged 24.0 to 32.5 seconds and was within or shorter than the reference range of 25.4 to 37.2 seconds derived from healthy Japanese participants under the same assay conditions.22 Results from the current study, where a majority of patients experienced bleeding events even with such apparently normalized aPTT, suggest that aPTT measurements overestimate the hemostatic activity of emicizumab. Nevertheless, monitoring aPTT may allow for the detection of neutralizing ADA development, as evidenced by data from 1 healthy participant in the first-in-human study.22 In contrast to aPTT, there may be a potential relationship between plasma emicizumab concentrations and reductions in bleeding rate, as suggested by the PK and ABR findings in patients who had emicizumab dose up-titration.
A linear PK profile of emicizumab has been demonstrated in patients with hemophilia A, as well as in healthy participants.22 Within the tested dose range of 0.3 to 3 mg/kg, steady-state trough levels of plasma emicizumab concentrations increased in a dose-proportional manner. In addition, emicizumab-dependent accumulation or depletion of FIX or FX was not observed, even at steady state for the highest dose (3 mg/kg). These findings suggest that antigen-antibody complex formation in plasma, which may affect the clearance of the antibody-based drugs and their antigens and may disturb the function of the antigen,25 is unlikely to occur with emicizumab. This is probably because of the weak antigen-binding affinity of emicizumab to each factor.26
Episodic coagulation factors were effectively administered during this study and treated breakthrough bleeding. The daily amount of coagulation factor administered to treat breakthrough bleeding was considered within the standard therapeutic dose range, and the amount of coagulation factor required per bleeding episode decreased in 5 of 10 patients with bleeding events during emicizumab administration. Four of 10 patients required increased amounts of coagulation factor concentrate to resolve their bleeding events vs before starting the study. An additional patient required many episodic FVIII administrations to treat a mesenteric hematoma. It was demonstrated previously that emicizumab in combination with bypassing agents, particularly aPCC, results in enhanced TG in vitro and in vivo.27 This suggests that episodic bypassing agents, especially aPCC, concomitant with emicizumab prophylaxis have a potential to induce thromboembolic events. In an ongoing phase 3 study in patients with inhibitors, thrombotic microangiopathy and thromboembolic events occurred after aPCC treatment, with doses averaging >100 U/kg per day for >1 day, during emicizumab prophylaxis.28 However, no such events were observed in the current study. This may be because 22 of 23 aPCC administrations were at a lower dose than >100 U/kg per day for >1 day.
Although 4 patients had ADAs, there was no impact on their PK or PD profiles of emicizumab, indicating that the ADAs were nonneutralizing and did not affect the efficacy of emicizumab. One patient did develop antiemicizumab IgE, but it was not persistently detected. AEs reported in this patient did not seem to include any specific events and were not unique to this patient. Moreover, because no hypersensitivity, other than local injection-site reactions, occurred and the development of ADAs did not result in treatment discontinuation, no clinical relevance of ADAs was evident. Of note, although neutralizing ADAs developed in 1 healthy participant in the first-in-human study, no AEs related to either abnormal coagulability or allergic reaction were observed.22
Limitations to the current study include that it was not randomized or controlled, and the small patient numbers in each dose cohort restricted assessment of the incidence and characteristics of AEs. Disparity in the baseline severity of disease, based on ABRs in the 6 months before the study, hindered the assessment of a dose-response relationship with emicizumab treatment in terms of bleeding prophylactic efficacy. Moreover, each patient’s physical activity was not documented during the course of the study, which may have affected the interpretation of reported bleeding events. Considering that hemophilia A is a lifelong disease, further long-term assessment of the safety and efficacy of emicizumab is required.
Nonetheless, the available clinical data highlight the potential for emicizumab to shift the treatment paradigm in hemophilia A. The long half-life of emicizumab has been predicted to result in minimal variation in peak/trough plasma concentrations at repeated subcutaneous injections,22 which may result in fewer joint bleeds than with treatments with more fluctuating PK profiles. It also suggests potential for less frequent administration than once weekly, for more dosing convenience, which will be explored in future studies. The subcutaneous administration of emicizumab (vs IV administration for current agents) has the potential to further decrease the treatment burden for patients. Subcutaneous administration simplifies the initiation and introduction of regular prophylaxis and may in turn improve compliance with prophylaxis regimens, which could also improve efficacy and long-term health outcomes. Additionally, because emicizumab showed promising efficacy in patients with inhibitors and is not expected to induce inhibitors, it represents a potential solution to the unmet need in patients with hemophilia A who develop inhibitors to FVIII therapy.
In conclusion, long-term emicizumab treatment demonstrated a favorable safety profile with encouraging efficacy, irrespective of the presence of FVIII inhibitors, in patients with hemophilia A. Emicizumab has the potential to provide an effective and convenient prophylactic therapeutic option with a lower treatment burden vs current prophylactic agents, including in patients with inhibitors and/or with venous access difficulty.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all the patients and their family members, Hoyu Takahashi at Niigata Prefectural Kamo Hospital, investigators and staff at participating medical institutions, Chiai Nagae at the Department of Pediatrics and Kunihisa Miyakawa at the Department of Radiology, St Marianna University School of Medicine, and project team members at Chugai Pharmaceutical Co., Ltd, especially Naoki Fukazawa, Shingo Maisawa, and Mariko Hoshiba.
Editorial assistance for this manuscript was provided by Corin Wing, Envision Scientific Solutions, and funded by Chugai Pharmaceutical Co., Ltd.
Authorship
Contribution: M.S. and R.K. wrote the manuscript; M.S., R.K., K.Y., and H.Y. designed the study; H.H., T.M., M.T., T.S., K.F., and K.N. conducted the study; K.Y. and H.Y. analyzed the data; all authors had access to the data and analysis and approved the final manuscript; and M.S. is the guarantor.
Conflict-of-interest disclosure: This work was supported by Chugai Pharmaceutical Co., Ltd (honoraria [M.S., K.N.], board of directors or advisory committee membership [M.S., M.T., T.M., K.F.]; research funding [M.S., H.H., M.T., T.M., T.S., K.F., K.N.]; employment [R.K., K.Y., H.Y.]), and F. Hoffmann-La Roche Ltd (consultancy [M.S.]); M.S., K.Y., and K.N. are listed on patents for anti-FIXa/X bispecific antibodies.
Correspondence: Midori Shima, Department of Pediatrics, Nara Medical University, 840 Shijo-cho Kashihara, Nara 634-8522, Japan; e-mail: mshima@naramed-u.ac.jp.