TO THE EDITOR:
Severe combined immunodeficiency (SCID) may be diagnosed via newborn screening (NBS) by measuring T-cell receptor excision circles.1,2 The incidence of SCID has risen, and this is rise attributed to the identification of patients who would have previously died of infections without being diagnosed.1 Patients with SCID who undergo allogeneic hematopoietic cell transplant (HCT) at <3.5 months of age have excellent overall survival (OS),3 and a positive family history also improves OS.4,5 Surprisingly, preliminary data from the Primary Immunodeficiency Treatment Consortium showed equivalent 1-year OS for patients diagnosed by NBS compared with those clinically diagnosed due to infections.6 However, OS does not capture long-term quality of life. Novel end points have been proposed to address this limitation, including graft-versus-host disease–free survival7 and activities of daily living–compromise-free survival.8 We have observed that some survivors of HCT for SCID have long-term impairment due to infection-related neurologic insults. We hypothesized that patients diagnosed by NBS, who received prompt isolation, infection prophylaxis, and HCT would avoid long-term neurologic impairment (NI) with excellent outcomes using the novel end point of neurologic event–free survival (NEFS).
From 1 January 1990 to 31 January 2016 (ensuring >1 year of follow-up for survivors), 91 patients with SCID (either typical or leaky/Omenn per Primary Immunodeficiency Treatment Consortium criteria9 ) underwent allogeneic HCT at the University of California San Francisco and were analyzed retrospectively for factors associated with long-term severe NI. Eight patients were excluded due to incomplete follow-up regarding NI (n = 3) or potential for NI from underlying deficiencies in ADA (n = 3),10 AK2 (n = 1),11 or BCL11B (n = 1).12 A SCID genotype was established for 84% of patients (Table 1). We obtained institutional review board approval for this retrospective data analysis. All patients consented to data collection for research in accordance with the Declaration of Helsinki.
Patient-related characteristics and outcomes for 83 patients with SCID
| . | N . | Deaths . | 5-Year OS, % (95% CI) . | P . | Neurologic events . | 5-Year cumulative incidence of neurologic events, % (95% CI) . | P . | Neurologic event–free survivors . | 5-Year NEFS, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 83 | 21 | 78 (69-87) | 8 | 11 (4-18) | 54 | 68 (58-78) | |||
| Sex | .06 | |||||||||
| Male | 49 | 16 | 69 (56-82) | 6 | 15 (7-31) | .28 | 27 | 57 (43-71) | .03 | |
| Female | 34 | 5 | 91 (82-100) | 2 | 7 (2-24) | 27 | 85 (73-97) | |||
| Trigger for diagnosis | ||||||||||
| Infection/autoimmunity | 49 | 18 | 67 (54-80) | .02 | 8 | 20 (7-23) | .03 | 23 | 51 (37-65) | <.001 |
| FH | 13 | 1 | 100 (73-100) | 0 | 0 (0-27) | 12 | 100 (72-100) | |||
| NBS | 21 | 2 | 90 (77-100) | 0 | 0 (0-18) | 19 | 90 (77-100) | |||
| Pre-HCT infection | .004 | |||||||||
| No | 34 | 3 | 97 (91-100) | 0 | 0 (0-12) | .006 | 31 | 97 (91-100) | <.001 | |
| Yes | 49 | 18 | 63 (49-76) | 8 | 21 (8-24) | 23 | 49 (32-60) | |||
| Age at HCT | .002 | |||||||||
| <3.5 mo | 39 | 4 | 92 (84-100) | 1 | 3 (1-7) | .02 | 34 | 90 (80-99) | <.001 | |
| >3.5 mo | 44 | 17 | 66 (52-70) | 7 | 20 (7-34) | 20 | 50 (35-65) | |||
| Genotype | .007 | |||||||||
| IL2RG/JAK3 | 20 | 2 | 90 (85-100) | 3 | 16 (6-46) | .73 | 15 | 75 (56-94) | .03 | |
| RAG1/2 | 13 | 2 | 85 (65-100) | 1 | 9 (1-59) | 10 | 77 (54-99) | |||
| DCLRE1C | 23 | 7 | 83 (67-98) | 1 | 4 (1-30) | 15 | 78 (61-95) | |||
| IL7R/CD3D | 12 | 1 | 92 (76-100) | 2 | 17 (5-59) | 9 | 75 (51-99) | |||
| Rare/unknown* | 15 | 9 | 40 (15-65) | 1 | 11 (2-71) | 5 | 33 (10-57) | |||
| SCID classification | .05 | |||||||||
| Typical | 71 | 16 | 82 (72-91) | 8 | 13 (4-21) | .26 | 47 | 70 (59-81) | .36 | |
| Leaky/Omenn | 12 | 5 | 53 (22-85) | 0 | 0 (0-28) | 7 | 53 (22-85) |
| . | N . | Deaths . | 5-Year OS, % (95% CI) . | P . | Neurologic events . | 5-Year cumulative incidence of neurologic events, % (95% CI) . | P . | Neurologic event–free survivors . | 5-Year NEFS, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 83 | 21 | 78 (69-87) | 8 | 11 (4-18) | 54 | 68 (58-78) | |||
| Sex | .06 | |||||||||
| Male | 49 | 16 | 69 (56-82) | 6 | 15 (7-31) | .28 | 27 | 57 (43-71) | .03 | |
| Female | 34 | 5 | 91 (82-100) | 2 | 7 (2-24) | 27 | 85 (73-97) | |||
| Trigger for diagnosis | ||||||||||
| Infection/autoimmunity | 49 | 18 | 67 (54-80) | .02 | 8 | 20 (7-23) | .03 | 23 | 51 (37-65) | <.001 |
| FH | 13 | 1 | 100 (73-100) | 0 | 0 (0-27) | 12 | 100 (72-100) | |||
| NBS | 21 | 2 | 90 (77-100) | 0 | 0 (0-18) | 19 | 90 (77-100) | |||
| Pre-HCT infection | .004 | |||||||||
| No | 34 | 3 | 97 (91-100) | 0 | 0 (0-12) | .006 | 31 | 97 (91-100) | <.001 | |
| Yes | 49 | 18 | 63 (49-76) | 8 | 21 (8-24) | 23 | 49 (32-60) | |||
| Age at HCT | .002 | |||||||||
| <3.5 mo | 39 | 4 | 92 (84-100) | 1 | 3 (1-7) | .02 | 34 | 90 (80-99) | <.001 | |
| >3.5 mo | 44 | 17 | 66 (52-70) | 7 | 20 (7-34) | 20 | 50 (35-65) | |||
| Genotype | .007 | |||||||||
| IL2RG/JAK3 | 20 | 2 | 90 (85-100) | 3 | 16 (6-46) | .73 | 15 | 75 (56-94) | .03 | |
| RAG1/2 | 13 | 2 | 85 (65-100) | 1 | 9 (1-59) | 10 | 77 (54-99) | |||
| DCLRE1C | 23 | 7 | 83 (67-98) | 1 | 4 (1-30) | 15 | 78 (61-95) | |||
| IL7R/CD3D | 12 | 1 | 92 (76-100) | 2 | 17 (5-59) | 9 | 75 (51-99) | |||
| Rare/unknown* | 15 | 9 | 40 (15-65) | 1 | 11 (2-71) | 5 | 33 (10-57) | |||
| SCID classification | .05 | |||||||||
| Typical | 71 | 16 | 82 (72-91) | 8 | 13 (4-21) | .26 | 47 | 70 (59-81) | .36 | |
| Leaky/Omenn | 12 | 5 | 53 (22-85) | 0 | 0 (0-28) | 7 | 53 (22-85) |
Other genotypes included: LIG4 (n = 1) and RMRP (n=1). Thirteen were unknown.
Severe NI was defined as the presence of ≥1 of: cerebral palsy/hemiplegia, blindness, severe developmental delay by Diagnostic and Statistical Manual of Mental Disorders, 5th edition criteria13 (eg, understands speech, but has little ability to communicate; able to learn daily routines; may learn very simple self-care; or needs direct supervision in social situations), or a chronic seizure disorder. NBS began in California in the middle of 2010. Patients were classified as having been diagnosed by family history (FH) if immunologic testing was done in the first weeks of life in the absence of clinical signs of infection or autoimmunity. Patients underwent HCT from a HLA-matched related donor, well-matched, unrelated adult or umbilical cord blood donor, or HLA-mismatched related donor. Patients received either no conditioning; immunosuppression (IS) only (fludarabine, cyclophosphamide, or serotherapy) or reduced intensity or myeloablative conditioning (RIC/MAC).14 If receiving >1 HCT, patients were classified according to their final type of donor cells and most-intense regimen. Patients with an HLA-matched related donor or unrelated adult or umbilical cord blood donor received graft-versus-host disease prophylaxis with a calcineurin inhibitor and methotrexate, whereas patients with an HLA-mismatched related donor underwent ex vivo T-cell depletion.15,16
Univariate comparisons of survival or competing risks outcomes were conducted by using the log-rank test for categorical variables and the Kruskal-Wallis test for continuous variables on SPSS Statistics 24 software (IBM, Armonk, NY). Cox multivariate regression models examining risk factors for NEFS were built by using stepwise forward selection, considering all factors with P < .05 on univariate analysis. Cohen’s κ was run to determine the agreement between the method of diagnosis (combining NBS/FH) and the presence of pre-HCT infection.
The median year of HCT was 2005. The median follow-up of survivors was 9.5 years (range, 1.1-26.8 years). The estimated 5-year OS of the entire cohort was 78% (95% confidence interval [CI], 69%-87%; Table 1). Three deaths >5 years post-HCT, at 7.4, 14.1, and 21.5 years, occurred in patients with DCLRE1C mutations; 1 patient was diagnosed by FH, and 2 patients were diagnosed by infection. The 5-year OS was 88% for patients transplanted after 2005 vs 65% for those transplanted in an earlier era (P = .02). Compared with patients with well-recognized genotypes, those with rare/unknown genotypes had lower 5-year OS, but had a median age at HCT of 309 days (range, 49-1826 days) vs 101 days (range, 13-707 days), suggesting that the identification of a genotype may have facilitated faster HCT.
Patient- and transplant-related factors associated with outcomes are listed in Tables 1 and 2, respectively. HCT at <3.5 months of age was associated with superior 5-year OS (92% vs 66%; P = .002). Patients diagnosed by FH underwent HCT at a median age of 22 days (range, 13-38 days) vs 61 days for those diagnosed by NBS (range, 32-217 days) and 238 days for those diagnosed clinically (range, 53-1826 days; P < .001). The 5-year OS for patients diagnosed by NBS (90%; 95% CI, 77%-99%) or FH (100%; 95% CI, 73%-100%) was superior to those diagnosed clinically (67%; 95% CI, 54%-80%; P = .02). The median year of HCT was 1998, 2013, and 2001 for those diagnosed by FH, NBS, and by clinical signs, respectively (P < .001).
Transplant-related characteristics and outcomes for 83 patients with SCID
| . | N . | Deaths . | 5-Year OS, % (95% CI) . | P . | Neurologic events . | 5-Year cumulative incidence of neurologic events, % (95% CI) . | P . | Neurologic event–free survivors . | 5-Year NEFS, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 83 | 21 | 78 (69-87) | 8 | 11 (4-18) | 54 | 68 (58-78) | |||
| Year of HCT | .02 | |||||||||
| 1990-2004 | 40 | 16 | 65 (50-80) | 4 | 12 (1-22) | .81 | 20 | 57 (42-73) | .04 | |
| 2005-2016 | 43 | 5 | 88 (79-98) | 4 | 10 (1-20) | 34 | 79 (67-91) | |||
| Donor | .31 | |||||||||
| Matched related | 11 | 1 | 100 (70-100) | 0 | 0 (0-30) | .39 | 10 | 100 (70-100) | .11 | |
| Mismatched related | 58 | 17 | 74 (62-83) | 7 | 14 (4-23) | 34 | 62 (49-74) | |||
| Unrelated | 14 | 3 | 79 (57-100) | 1 | 10 (1-29) | 10 | 70 (47-95) | |||
| Conditioning | .27 | |||||||||
| None | 41 | 7 | 83 (71-94) | 7 | 19 (10-37) | .06 | 27 | 66 (51-80) | .98 | |
| Immunosuppression | 25 | 9 | 75 (58-93) | 0 | 0 (0-16) | 16 | 75 (58-93) | |||
| RIC/MAC | 17 | 5 | 68 (44-91) | 1 | 8 (2-51) | 11 | 62 (37-87) | |||
| GVHD prophylaxis | .24 | |||||||||
| T-cell depletion | 58 | 17 | 74 (62-85) | 7 | 14 (4-23) | .21 | 34 | 62 (49-74) | .07 | |
| CNI+/− others | 25 | 4 | 88 (75-100) | 1 | 5 (1-14) | 20 | 84 (69-98) | |||
| Acute GVHD grade II-IV* | .22 | |||||||||
| No | 51 | 9 | 85 (80-90) | 6 | 13 (8-18) | .55 | 36 | 74 (68-80) | .51 | |
| Yes | 28 | 8 | 73 (64-82) | 2 | 8 (3-13) | 18 | 67 (58-76) | |||
| >1 HCT | .09 | |||||||||
| No | 52 | 10 | 82 (71-93) | 5 | 10 (2-19) | .92 | 37 | 72 (60-85) | .14 | |
| Yes | 31 | 11 | 71 (55-87) | 3 | 12 (1-25) | 17 | 61 (44-78) |
| . | N . | Deaths . | 5-Year OS, % (95% CI) . | P . | Neurologic events . | 5-Year cumulative incidence of neurologic events, % (95% CI) . | P . | Neurologic event–free survivors . | 5-Year NEFS, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 83 | 21 | 78 (69-87) | 8 | 11 (4-18) | 54 | 68 (58-78) | |||
| Year of HCT | .02 | |||||||||
| 1990-2004 | 40 | 16 | 65 (50-80) | 4 | 12 (1-22) | .81 | 20 | 57 (42-73) | .04 | |
| 2005-2016 | 43 | 5 | 88 (79-98) | 4 | 10 (1-20) | 34 | 79 (67-91) | |||
| Donor | .31 | |||||||||
| Matched related | 11 | 1 | 100 (70-100) | 0 | 0 (0-30) | .39 | 10 | 100 (70-100) | .11 | |
| Mismatched related | 58 | 17 | 74 (62-83) | 7 | 14 (4-23) | 34 | 62 (49-74) | |||
| Unrelated | 14 | 3 | 79 (57-100) | 1 | 10 (1-29) | 10 | 70 (47-95) | |||
| Conditioning | .27 | |||||||||
| None | 41 | 7 | 83 (71-94) | 7 | 19 (10-37) | .06 | 27 | 66 (51-80) | .98 | |
| Immunosuppression | 25 | 9 | 75 (58-93) | 0 | 0 (0-16) | 16 | 75 (58-93) | |||
| RIC/MAC | 17 | 5 | 68 (44-91) | 1 | 8 (2-51) | 11 | 62 (37-87) | |||
| GVHD prophylaxis | .24 | |||||||||
| T-cell depletion | 58 | 17 | 74 (62-85) | 7 | 14 (4-23) | .21 | 34 | 62 (49-74) | .07 | |
| CNI+/− others | 25 | 4 | 88 (75-100) | 1 | 5 (1-14) | 20 | 84 (69-98) | |||
| Acute GVHD grade II-IV* | .22 | |||||||||
| No | 51 | 9 | 85 (80-90) | 6 | 13 (8-18) | .55 | 36 | 74 (68-80) | .51 | |
| Yes | 28 | 8 | 73 (64-82) | 2 | 8 (3-13) | 18 | 67 (58-76) | |||
| >1 HCT | .09 | |||||||||
| No | 52 | 10 | 82 (71-93) | 5 | 10 (2-19) | .92 | 37 | 72 (60-85) | .14 | |
| Yes | 31 | 11 | 71 (55-87) | 3 | 12 (1-25) | 17 | 61 (44-78) |
CNI, calcineurin inhibitor; GVHD, graft-versus-host disease.
Of 79 engrafted patients.
The 5-year cumulative incidence of severe NI for the entire cohort was 11% (95% CI, 4%-18%). Events were diagnosed at a median of 0.5 years post-HCT (range, 0.1-1.8 years). In the clinically diagnosed group, the 5-year cumulative incidence of severe NI was 20%, attributable to direct (ie, encephalitis/meningitis) or indirect (ie, anoxic brain injury) damage associated with pre-HCT infections with cytomegalovirus (n = 2), respiratory syncytial virus (n = 3), Candida (n = 2), or polybacterial pneumonia (n = 1). No surviving patient diagnosed by FH or NBS has developed evidence of NI, with a median follow-up of 18.4 and 3.5 years, respectively (P = .03). There was a trend toward more NI in patients who received no conditioning prior to HCT. However, because chemotherapy was clinically contraindicated in severely infected patients, selection bias may explain this finding. Preparative chemotherapy was not associated with NI, although formal neurocognitive testing was not performed in the majority of patients, such that subtle differences may have been missed.17
Five-year NEFS was better for patients transplanted since 2005 compared with those transplanted earlier (79% vs 57%; P = .04). Surprisingly, girls had a superior 5-year NEFS compared with boys (85% vs 57%; P = .03). Boys were not more likely to be infected pre-HCT than girls (66% vs 50%, P = .18). However, 10 of the 15 patients with unknown/rare genotypes were boys, and this group did exceptionally poorly (9 of 10 patients died, and the only survivor is extremely developmentally delayed from an anoxic brain injury following polymicrobial pneumonia). Conversely, the 5 girls with unknown/rare genotypes all survived without neurocognitive impairment. Therefore, when restricted solely to patients with classic genotypes, there was no difference in NEFS by sex (P = .43).
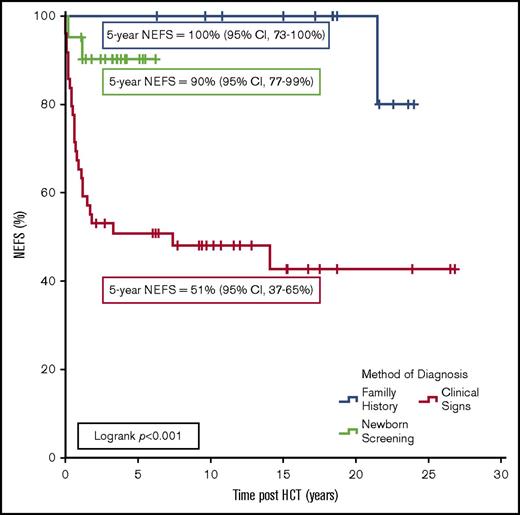
The most significant predictors of NEFS were HCT at <3.5 months of age (90% vs 50%, P < .001), absence of pre-HCT infection (97% vs 49%, P < .001), and the method of diagnosis, with a 5-year NEFS of 51%, 100%, or 90% for patients diagnosed clinically versus by FH or NBS, respectively (P < .001; Figure 1). Multivariate analysis demonstrated that only pre-HCT infection was significantly associated with poor NEFS (hazard ratio, 8.23; 95% CI, 2.84-27.26, P < .001). However, there was significant agreement between the method of diagnosis and infection status pre-HCT (κ = 0.9; 95% CI, 0.85-0.95; P < .001). To attempt to isolate whether this result can be explained solely due to temporal improvements in supportive care and HCT techniques,18 a subgroup analysis of only the 43 patients diagnosed since 2005 was performed. In 19 clinically diagnosed patients, the 5-year NEFS was 63% compared with 92% in the 24 patients diagnosed by FH/NBS (P = .02).
NEFS following allogeneic HCT in 83 patients with SCID. Events were death, cerebral palsy/hemiplegia, blindness, severe developmental delay (by Diagnostic and Statistical Manual of Mental Disorders, 5th edition criteria13 ), or a seizure disorder requiring long-term pharmacologic therapy.
NEFS following allogeneic HCT in 83 patients with SCID. Events were death, cerebral palsy/hemiplegia, blindness, severe developmental delay (by Diagnostic and Statistical Manual of Mental Disorders, 5th edition criteria13 ), or a seizure disorder requiring long-term pharmacologic therapy.
Future efforts in this area should be broadened beyond the recognition of patients with only the most severe NI, because additional subtle findings may be identified with dedicated neurocognitive evaluation.17,19 There may be factors other than pre-HCT infection (ie, the use of high-dose chemotherapy capable of crossing the blood-brain barrier in very young infants) that impact the risk of developing less severe neurocognitive problems, and those factors will be important to identify. Furthermore, there may be other late effects of HCT for SCID that also significantly impact quality of life, and ultimately a composite end point, such as long-term-side-effect–free survival, may be the best way to identify the optimal HCT approach for these patients. We hope that these data on NEFS are the first step in highlighting the fact that, in the modern era, simple OS is no longer a sufficient goal for patients with SCID.
In conclusion, NEFS is a novel end point, informing both post-HCT survival and neurologic status in patients with SCID. With modern antimicrobial and supportive care, many seriously infected SCID patients have survived, but have sustained severe NI unmeasured by analyses of only OS. Because neonatally diagnosed patients typically receive isolation, prophylactic antimicrobials, and typically proceed to HCT without developing serious infection, NEFS analysis demonstrates a significant benefit for patients diagnosed via NBS. Some of the benefits in our single-center cohort may be explained by recent advances in supportive care; however, the excellent NEFS in the group of patients diagnosed neonatally by FH over the same time period as those who were clinically diagnosed supports this conclusion. These results require validation in multicenter, contemporaneous cohorts, but should promote the implementation of universal NBS for SCID to facilitate HCT before the onset of infection.
Authorship
Acknowledgments: C.C.D., J.M.P., and M.J.C. received support from the National Institute of Allergy and Infectious Diseases and the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health, Public Health Service grant/cooperative agreements U54-AI082973 and R13-AI094943 (principal investigator: M.J.C.). J.M.P. and M.J.C. received support from California Institute of Regenerative Medicine grant CLIN1-08363 (principal investigator: J.M.P.). J.M.P. received support for this work from the University of California San Francisco Jeffrey Modell Diagnostic Center for Primary Immunodeficiencies, the Michelle Platt Ross Foundation, Lisa & Douglas Goldman Fund, and National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R01-AI105776.
Contribution: C.C.D. designed the study, performed the data abstraction and analyses, and wrote the paper; and J.M.P., J.T.W., M.D., A.M., and M.J.C. contributed to patient management, study design, and data interpretation, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher C. Dvorak, Division of Pediatric Allergy, Immunology and Bone Marrow Transplantation, Benioff Children’s Hospital, University of California San Francisco, 550 16th St, 4th Floor, Box 0434, San Francisco, CA 94143; e-mail: christopher.dvorak@ucsf.edu.