Key Points
Autoimmune neutropenia is a rare side effect of nivolumab which may respond to antibody-based therapies.
Nivolumab can lead to durable complete remission in primary mediastinal B-cell lymphoma refractory to multiple lines of therapy.
Introduction
Antibodies against the programmed cell death (PD-1) receptor are rapidly changing the oncologic therapeutic landscape. One such agent, nivolumab, has taken center stage and in <2 years was granted US Food and Drug Administration approval for the treatment of melanoma, non–small cell lung cancer, renal cell cancer, Hodgkin lymphoma, and head and neck cancer, and it will undoubtedly soon find approval in several other malignancies. Oncologists now face a steep learning curve gaining familiarity with nivolumab and its indications, delivery, and unique toxicities.
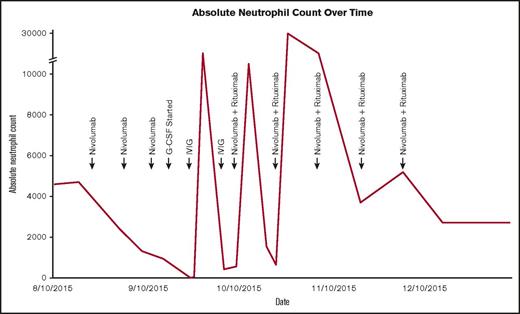
ANC over time following initiation of nivolumab and subsequent interventions.
ANC over time following initiation of nivolumab and subsequent interventions.
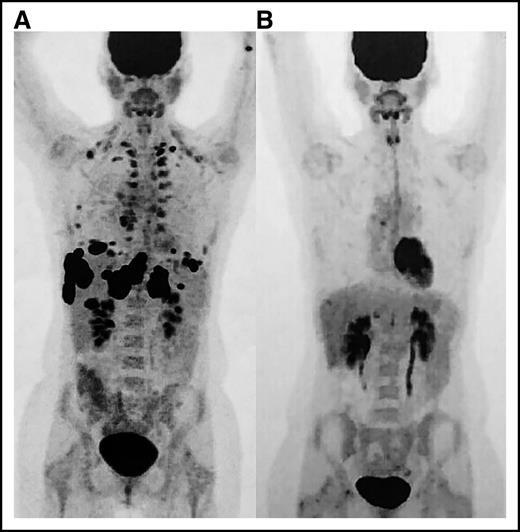
Positron emission tomography (PET) images. (A) Scan prior to initiation of nivolumab and (B) restaging PET after eighth cycle revealing complete remission.
Positron emission tomography (PET) images. (A) Scan prior to initiation of nivolumab and (B) restaging PET after eighth cycle revealing complete remission.
A gap often exists between the toxicities described in medical literature and the toxicities encountered during day-to-day clinical practice. Severe neutropenia, for example, has been reported in only 3 of now thousands of patients who have participated in the numerous studies investigating nivolumab for both solid tumors and hematologic malignancies.1-8 We report a case of grade 4 autoimmune neutropenia in a patient successfully treated with nivolumab for refractory primary mediastinal B-cell lymphoma (PMBCL).
Case description
Our patient, a 38-year-old woman, presented to our center with refractory PMBCL, an uncommon occurrence given remarkable overall survival with multiagent chemotherapy.9 She initially presented with shortness of breath and an anterior mediastinal mass without evidence of extramediastinal disease on positron emission tomography. A subsequent biopsy was performed that revealed large lymphocytes with immunohistochemical stains that were positive for CD20, Pax-5, BCL-2, BCL-6, weakly positive for CD30, MUM-1, and negative for CD10. Ki-67 was 50%, and MYC was not overexpressed. Bone marrow biopsy at the time of diagnosis was negative for malignancy and notably normocellular with trilineage hematopoiesis. She was initially treated with dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin before being transferred to our facility for consideration of salvage therapies and potential high-dose chemotherapy with autologous stem cell transplantation. A repeat biopsy of the anterior mediastinal mass corroborated the diagnosis of PMBCL, was absent chromosomal abnormalities detected by fluorescence in situ hybridization, and additionally was negative for CD15. She initially was treated with salvage gemcitabine, ifosfamide, oxaliplatin, and rituximab. She subsequently developed superior vena cava syndrome with imaging notable for progression of primary disease and new retroperitoneal lymph node involvement. Her mediastinal disease improved with involved field radiotherapy, but her extramediastinal disease continued to advance. She was then started on a second salvage chemotherapy regimen consisting of topotecan, docetaxel, and obinutuzumab, but her disease ultimately progressed. The patient was not a candidate for further salvage chemotherapy or autologous stem cell transplantation given triple-refractory disease. Several encouraging clinical trials at referral centers were closed at the time of evaluation. We were considering immunotherapy but did not pursue PD-L1 analysis because standard testing was not established at the time of the decision. In addition, available nivolumab trial results showed responses in some patients for which expression was unknown2 or occurred independent of PD-L1 expression.3-5 After much discussion with the patient and her family, we pursued treatment with off-label nivolumab.
Methods
The patient maintained normal organ function and performance status at the time of nivolumab initiation despite multiple lines of multiagent chemotherapy, and her starting absolute neutrophil count (ANC) was 4700 cells/mm3. The patient tolerated therapy very well but was noted to have an ANC of 950 cells/mm3 at the time of her third dose. Granulocyte-stimulating colony factor was initiated 1 week later after her ANC decreased to 180 cells/mm3. Pathologist review of the peripheral blood smear showed absolute neutropenia without blasts or signs of dysplasia. Although not previously reported, we were suspicious for autoimmune neutropenia knowing the immune-related effects of nivolumab, temporal association with medication administration, and absence of other etiology. Despite 4 days of granulocyte-stimulating colony factor, her neutrophil count plummeted to 0. We then administered IV immunoglobulin (IVIG) and temporarily held nivolumab. Blood was taken to assess for antineutrophil antibodies prior to initiation of IVIG and ultimately found to be positive.
Results and discussion
The patient’s ANC markedly improved within 3 days, but recovery was transient and required another dose of IVIG 11 days after the first dose (Figure 1). Computed tomography showed a pronounced partial response where no therapy had successfully worked before. It was for this reason that nivolumab was restarted in addition to rituximab to preemptively treat autoimmune neutropenia. The patient never again experienced neutropenia and ultimately developed a complete remission within 4 months (Figure 2). Now 18 months from remission, she has remained relapse free with normal blood cell counts at the time of this article’s submission.
Nivolumab-related autoimmune neutropenia has never been reported. Trials have shown multiple immune-related adverse events to include hepatitis, colitis, thyroiditis, rash, pneumonitis, and hypophysitis.2-8 Only 3 patients reportedly experienced neutropenia in the numerous nivolumab trials.3,5,6 One patient among 135 patients developed low-grade neutropenia in the nivolumab arm of the squamous non–small cell lung cancer phase 3 trial.5 A single patient developed neutropenia among the >600 patients treated with nivolumab in 1 of the phase 3 melanoma trials.3 This patient ultimately died and endured the only grade 5 event noted among patients receiving nivolumab.3 Neither the article or the supplementary appendix offer discussion or evaluation regarding the patient’s neutropenia. High-grade neutropenia was appreciated in 1 of 81 patients in a more recent phase 1 trial investigating nivolumab in various hematologic malignancies, including diffuse large B-cell lymphoma.6 An early phase trial with smaller sample size specific to nivolumab use in Hodgkin lymphoma also noted lymphopenia but did not identify the development of neutropenia.2 The remaining trials did not report a single instance of neutropenia.1,2,4,7,8
Autoimmune neutropenia could be a unique adverse event when using nivolumab in the setting of hematologic malignancy, and a signal may become present as the use of these agents in hematologic cancer continues to evolve. Neutropenia is a very rare side effect only noted 3 times among now thousands of patients in multiple nivolumab trials, and it is uncertain if they were immune-associated side effects. This report shows that rituximab and IVIG successfully treated this patient’s high-grade autoimmune neutropenia and might safely and effectively be used in other patients afflicted by nivolumab-induced autoimmune neutropenia without compromising efficacy of the drug.
A finding of equal importance in this report is the complete remission that occurred in this disease that was otherwise refractory to multiple lines of chemotherapy and radiotherapy. At the time of our decision to treat with nivolumab, there were no studies to show clinical efficacy nor was there evidence of PD-1–mediated immune escape in PMBCL. Nivolumab was pursued given the response appreciated in many of the patients in the phase 1 Hodgkin lymphoma trial2 and absence of other available treatment or active clinical trials. A recent study indeed showed that 36% of patients with PMBCL were positive for PD-L1 expression,10 which opened the feasibility of PD-1 blockade as a potential treatment of this disease. Another PD-1 antibody, pembrolizumab, was recently studied in a phase 1 trial of 18 patients with relapsed/refractory PMBCL, which reported an overall response rate of 41% and interestingly reported a single patient that developed neutropenia as well.11 Our patient’s clinical course supports that nivolumab could be an option for refractory PMBCL and warrants further investigation to potentially salvage the rare patients who fail traditional therapies.
Authorship
Contribution: Z.W. and A.B. contributed equally to the contents of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zachary Wright, Department of Hematology/Oncology, Keesler Medical Center, 301 Fisher St, Keesler AFB, Biloxi, MS 39534; e-mail: zachary. wright.4@us.af.mil; and Alexander Brown, Department of Hematology/Oncology, San Antonio Military Medical Center, 3551 Roger Brooke Dr, Fort Sam Houston, TX 78234; e-mail: abrowns@aol.com.
Acknowledgment
The views expressed in this article are those of the authors and do not reflect the official policy or position of the US government, the Department of Defense, or the Department of the Air Force.
References
Author notes
Z.W. and A.B. contributed equally to this study.